Synthetic Optimizations for Gram-Scale Preparation of 1-O-Methyl d-Glycero-α-d-gluco-heptoside 7-Phosphate from d-Glucose
Abstract
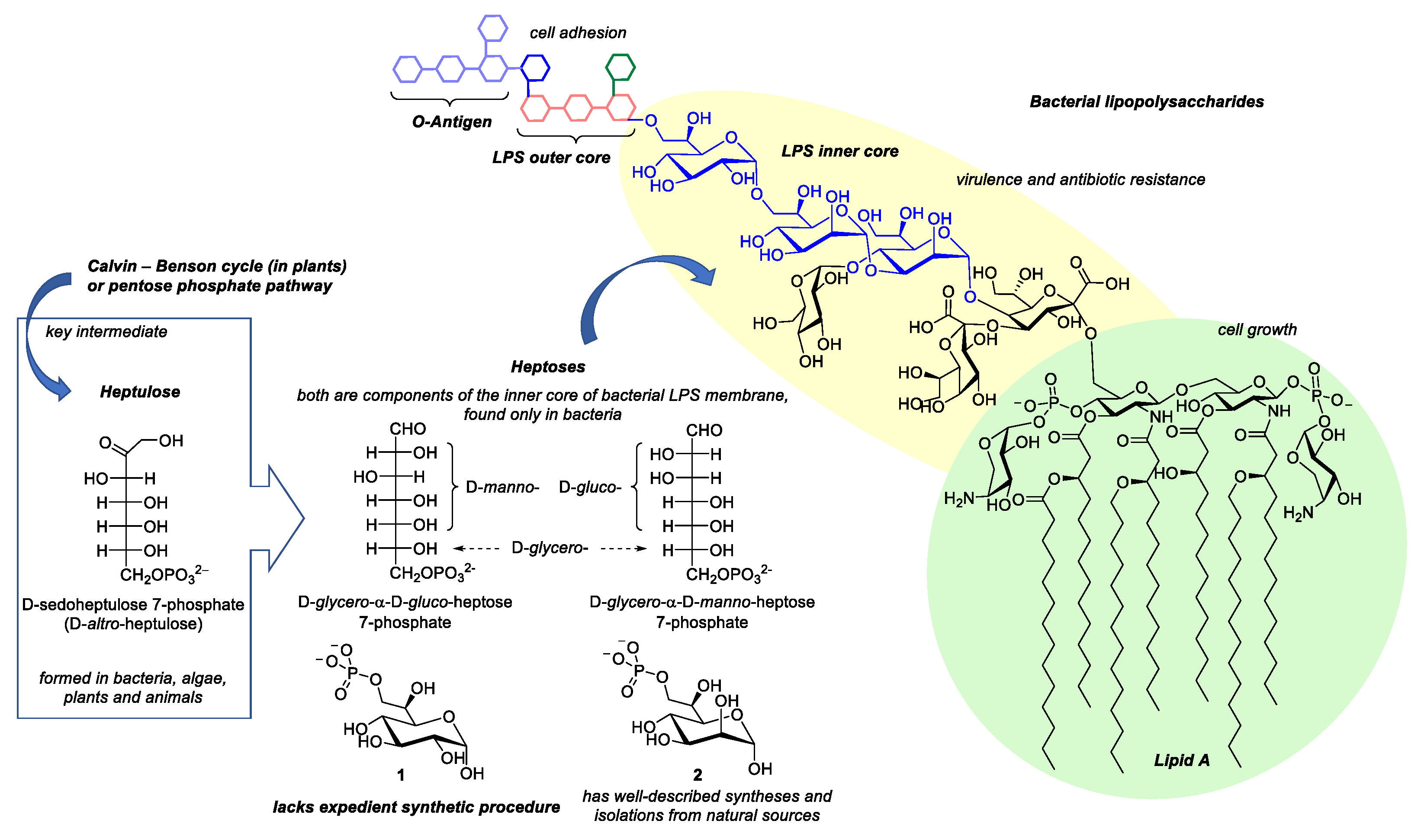
1. Introduction
2. Results and Discussion
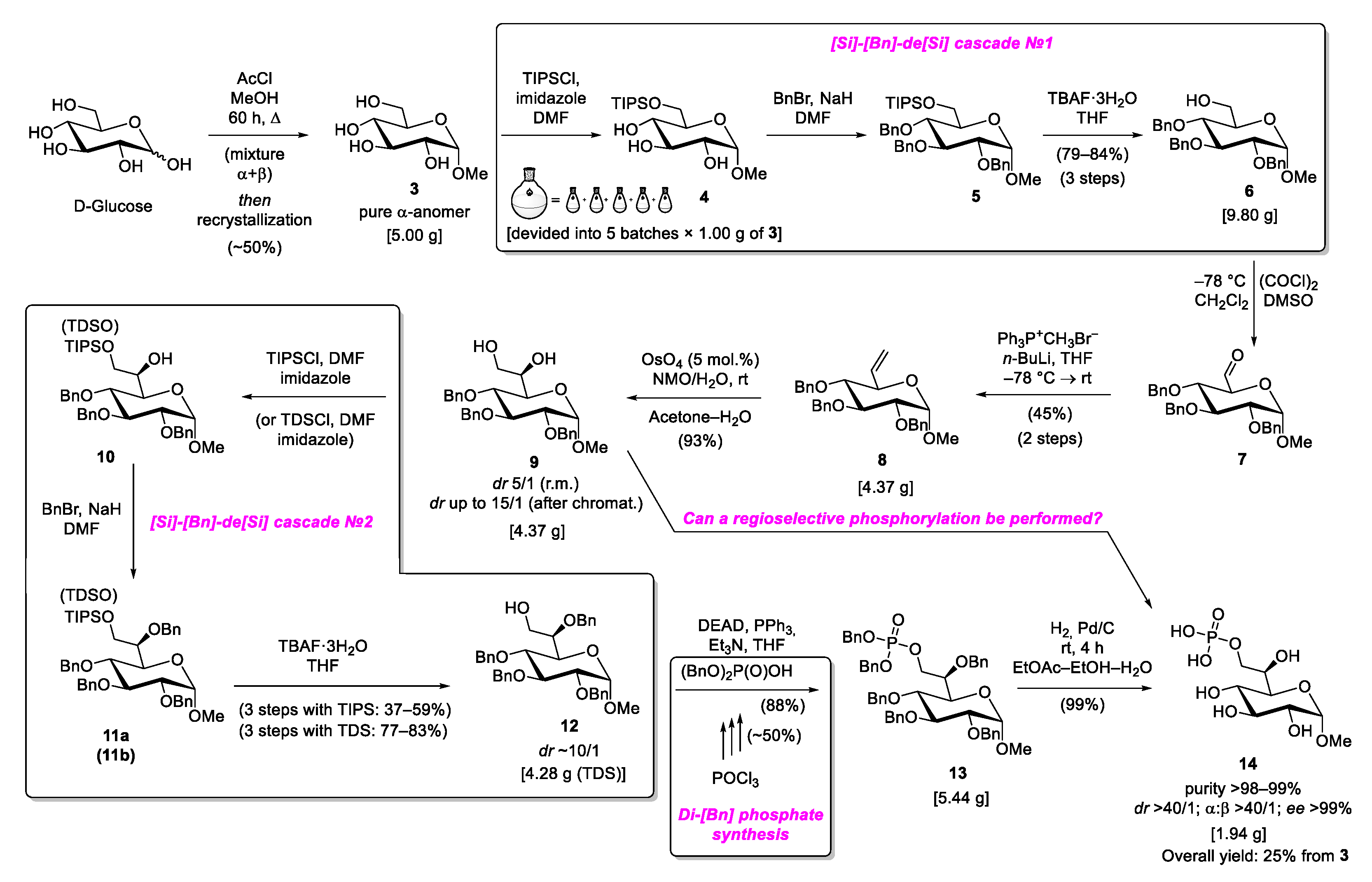
2.1. General Synthetic Route
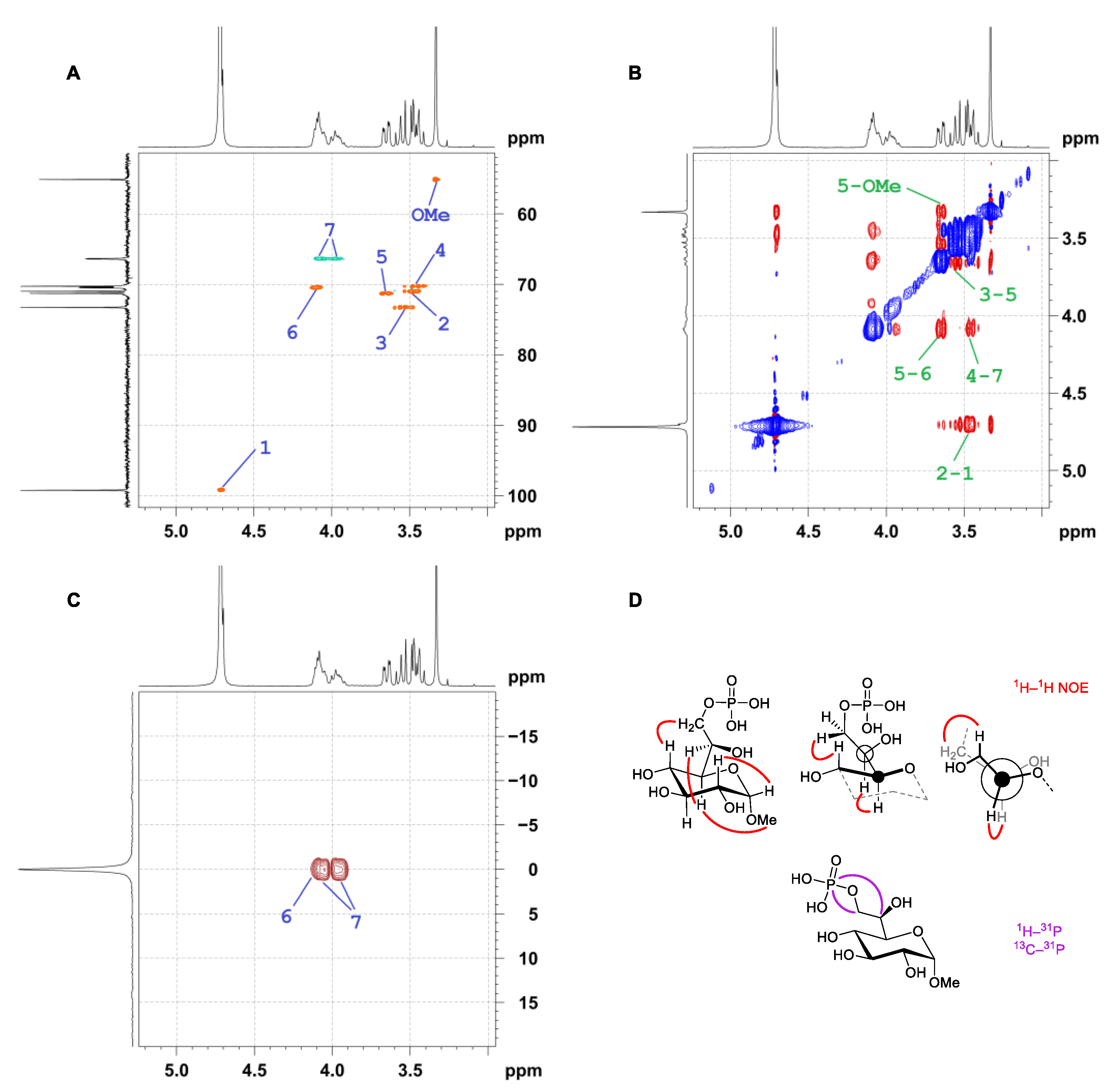
2.2. Structure Elucidation of the Sugar Configuration
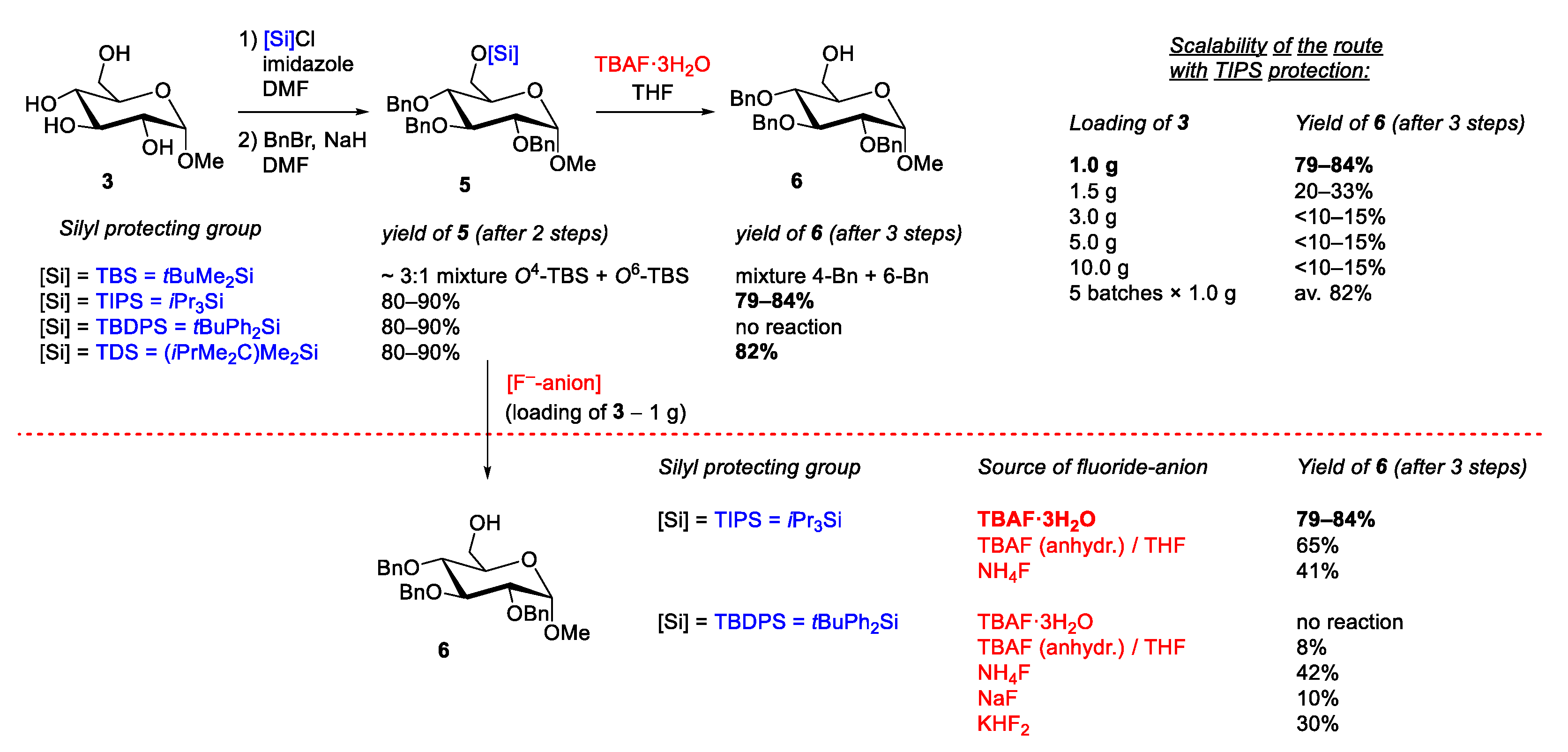
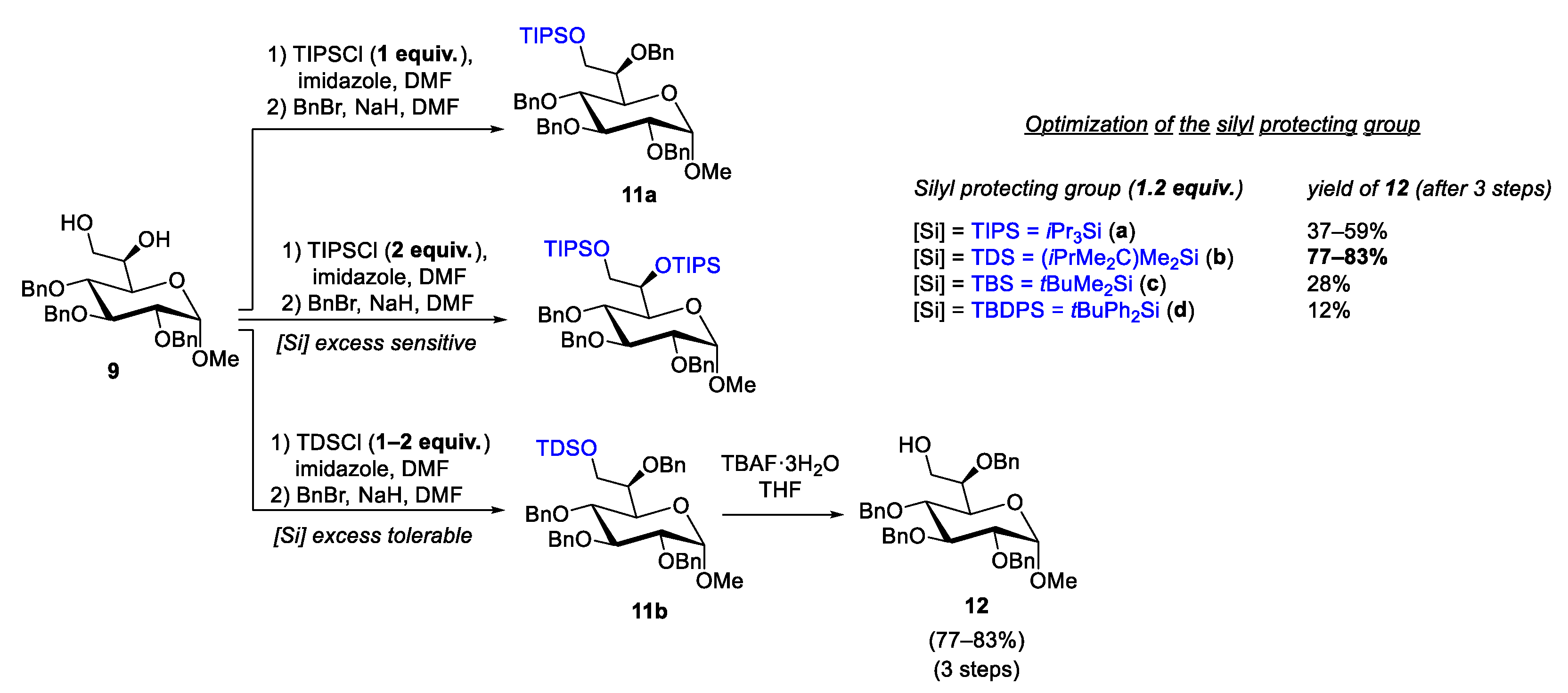
2.3. Silylation Group Challenges and Optimizations
2.4. Phosphorylation Using Dibenzyl Phosphate
2.5. Phosphorylation Using Dibenzyl Phosphoriodidate
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowan, A.K. Occurrence, metabolism, transport and function of seven-carbon sugars. Phytochem. Rev. 2016, 16, 137–157. [Google Scholar] [CrossRef]
- Valvano, M.A. Genetics and Biosynthesis of Lipopolysaccharide. In Molecular Medical Microbiology; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Waltham, MA, USA, 2015; pp. 55–89. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integr. Biol. 2015, 7, 1339–1377. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Jamshidi, M.P.; Sauvageau, J. Pathogen-Associated Molecular Patterns: The Synthesis of Heptose Phosphates and Derivative. Synthesis 2022, 54, 79–91. [Google Scholar] [CrossRef]
- Adekoya, I.A.; Guo, C.X.; Gray-Owen, S.D.; Cox, A.D.; Sauvageau, J. d-Glycero-β-d-Manno-Heptose 1-Phosphate and d-Glycero-β-d-Manno-Heptose 1,7-Biphosphate are Both Innate Immune Agonists. J. Immunol. 2018, 201, 2385–2391. [Google Scholar] [CrossRef]
- Asamizu, S.; Xie, P.; Brumsted, C.J.; Flatt, P.M.; Mahmud, T. Evolutionary Divergence of Sedoheptulose 7-Phosphate Cyclases Leads to Several Distinct Cyclic Products. J. Am. Chem. Soc. 2012, 134, 12219–12229. [Google Scholar] [CrossRef][Green Version]
- Milicaj, J.; Castro, C.D.; Jaunbocus, N.; Taylor, E.A. Extraction of ADP-Heptose and Kdo2-Lipid A from E. coli Deficient in the Heptosyltransferase I Gene. Appl. Sci. 2021, 11, 8314. [Google Scholar] [CrossRef]
- Kosma, P. Chemical synthesis of the core oligosaccharide of bacterial lipopolysaccharide. In Microbial Glycobiology. Structures, Relevance and Applications, 1st ed.; Moran, A.P., Holst, O., Brennan, P.J., von Itzstein, M., Eds.; Academic Press: London, UK, 2009; pp. 429–454. ISBN 9780123745460. [Google Scholar]
- Liang, L.; Cao, J.; Wei, T.-Y.W.; Tsai, M.-D.; Vincent, S.P. Synthesis of a biotinylated heptose 1,7-bisphosphate analogue, a probe to study immunity and inflammation. Org. Biomol. Chem. 2021, 19, 4943–4948. [Google Scholar] [CrossRef]
- Stanetty, C.; Baxendale, I.R. Large-Scale Synthesis of Crystalline 1,2,3,4,6,7-Hexa-O-acetyl-l-glycero-α-d-manno-heptopyranose. Eur. J. Org. Chem. 2015, 2015, 2718–2726. [Google Scholar] [CrossRef]
- Li, T.; Wen, L.; Williams, A.; Wu, B.; Li, L.; Qu, J.; Meisner, J.; Xiao, Z.; Fang, J.; Wang, P.G. Chemoenzymatic synthesis of ADP-d-glycero-β-d-manno-heptose and study of the substrate specificity of HldE. Bioorg. Med. Chem. 2014, 22, 1139–1147. [Google Scholar] [CrossRef][Green Version]
- Guo, Z.; Tang, Y.; Tang, W.; Chen, Y. Heptose-containing bacterial natural products: Structures, bioactivities, and biosynthesis. Nat. Prod. Rep. 2021, 38, 1887–1909. [Google Scholar] [CrossRef]
- Tang, W.; Guo, Z.; Cao, Z.; Wang, M.; Li, P.; Meng, X.; Zhao, X.; Xie, Z.; Wang, W.; Zhou, A.C.; et al. d-Sedoheptulose-7-phosphate is a common precursor for the heptoses of septacidin and hygromycin B. Proc. Natl. Acad. Sci. USA 2018, 115, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Picard, S.; Crich, D. Improved methods for the stereoselective synthesis of mannoheptosyl donors and their glycosides: Toward the synthesis of the trisaccharide repeating unit of the Campylobacter jejuni RM1221 capsular polysaccharide. Tetrahedron 2013, 69, 5501–5510. [Google Scholar] [CrossRef]
- Begbie, R.; Richtmyer, N.K. The isolation of some heptoses, heptuloses, octuloses, and nonuloses from Primula officinalis Jacq. Carbohydr. Res. 1966, 2, 272–288. [Google Scholar] [CrossRef]
- Dong, L.; Roosenberg, J.M.; Miller, M.J. Total Synthesis of Desferrisalmycin B. J. Am. Chem. Soc. 2002, 124, 15001–15005. [Google Scholar] [CrossRef] [PubMed]
- Durka, M.; Tikad, A.; Périon, R.; Bosco, M.; Andaloussi, M.; Floquet, S.; Malacain, E.; Moreau, F.; Oxoby, M.; Gerusz, V.; et al. Systematic Synthesis of Inhibitors of the Two First Enzymes of the Bacterial Heptose Biosynthetic Pathway: Towards Antivirulence Molecules Targeting Lipopolysaccharide Biosynthesis. Chem. Eur. J. 2011, 17, 11305–11313. [Google Scholar] [CrossRef]
- Khare, N.K.; Sood, R.K.; Aspinall, G.O. Diastereoselectivity in the synthesis of d-glycero-d-aldoheptoses by 2-trimethylsilylthiazole homologation from hexodialdo-1,5-pyranose derivatives. Can. J. Chem. 1994, 72, 237–246. [Google Scholar] [CrossRef]
- Kosma, P.; Blaukopf, M.; Atamanyuk, D.; Xavier, N.; Gerusz, V. Synthesis of 1,5-Anhydro-d-glycero-d-gluco-heptitol Derivatives as Potential Inhibitors of Bacterial Heptose Biosynthetic Pathways. Synthesis 2017, 49, 5320–5334. [Google Scholar] [CrossRef]
- Brimacombe, J.S.; Kabir, A.K.M. Convenient syntheses of l-glycero-d-manno-heptose and d-glycero-d-manno-heptose. Carbohydr. Res. 1986, 152, 329–334. [Google Scholar] [CrossRef]
- Inuki, S.; Aiba, T.; Kawakami, S.; Akiyama, T.; Inoue, J.-I.; Fujimoto, Y. Chemical Synthesis of D-glycero-D-manno-Heptose 1,7-Bisphosphate and Evaluation of Its Ability to Modulate NF-κB Activation. Org. Lett. 2017, 19, 3079–3082. [Google Scholar] [CrossRef]
- Sauvageau, J.; Bhasin, M.; Guo, C.X.; Adekoya, I.A.; Gray-Owen, S.D.; Oscarson, S.; Guazzelli, L.; Cox, A. Alternate synthesis to d-glycero-β-d-manno-heptose 1,7-biphosphate. Carbohydr. Res. 2017, 450, 38–43. [Google Scholar] [CrossRef]
- Wang, J.; Rong, J.; Lou, Q.; Zhu, Y.; Yang, Y. Synthesis of l-glycero- and d-glycero-d-manno-Heptose Building Blocks for Stereoselective Assembly of the Lipopolysaccharide Core Trisaccharide of Vibrio parahemolyticus O2. Org. Lett. 2020, 22, 8018–8022. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.K.; Kim, N.-S. Acyclic Stereocontrol Induced by Allylic Alkoxy Groups. Synthetic Applications of Stereoselective Dihydroxylation in Natural Product Synthesis. Chem. Rev. 1995, 95, 1761–1795. [Google Scholar] [CrossRef]
- According to Prices for #M9376. Available online: http://www.sigmaaldrich.com (accessed on 11 October 2022).
- Guilbert, E.; Villermaux, E. Chemical reactions rectify mixtures composition. Phys. Rev. Fluids 2021, 6, L112501. [Google Scholar] [CrossRef]
- Hoffmann, W.; Ess, R.J.; Usinger, R.P., Jr. The Transesterification of Trialkyl Phosphites with Aliphatic Alcohols. J. Am. Chem. Soc. 1956, 78, 5817–5821. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Aghabeydi, S.; Sadjadi, M.A.S. Transesterification of Trimethylphosphate Catalyzed By Sodium Alkoxides. J. Phys. Theor. Chem. IAU Iran 2004, 1, 161–167. [Google Scholar]
- Zervas, L.; Dilaris, I. Dealkylation and Debenzylation of Triesters of Phosphoric Acid. Phosphorylation of Hydroxy and Amino Compounds. J. Am. Chem. Soc. 1955, 77, 5354–5357. [Google Scholar] [CrossRef]
- Kotlarska, J.; Binnemans, K.; Dehaen, W. A convenient two-step synthesis of dialkylphosphate ionic liquids. Tetrahedron 2013, 69, 9947–9950. [Google Scholar] [CrossRef]
- Aitken, R.; Collett, C.; Mesher, S. Convenient Preparation of Long-Chain Dialkyl Phosphates: Synthesis of Dialkyl Phosphates. Synthesis 2012, 44, 2515–2518. [Google Scholar] [CrossRef]
- Pokorny, B.; Müller-Loennies, S.; Kosma, P. Synthesis of α-d-glucosyl substituted methyl glycosides of 3-deoxy-α-d-manno- and d-glycero-α-d-talo-oct-2-ulosonic acid (Kdo/Ko) corresponding to inner core fragments of Acinetobacter lipopolysaccharide. Carbohydrate Res. 2014, 391, 66–81. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Pohl, N.L.B. A Fluorous Phosphate Protecting Group with Applications to Carbohydrate Synthesis. Org. Lett. 2011, 13, 1824–1827. [Google Scholar] [CrossRef]
- Li, T.; Tikad, A.; Pan, W.; Vincent, S.P. β-Stereoselective Phosphorylations Applied to the Synthesis of ADP- and Polyprenyl-β-Mannopyranosides. Org. Lett. 2014, 16, 5628–5631. [Google Scholar] [CrossRef] [PubMed]
- Stowell, J.K.; Widlanski, T.S. A new method for the phosphorylation of alcohols and phenols. Tetrahedron Lett. 1995, 36, 1825–1826. [Google Scholar] [CrossRef]
- Ladame, S.; Claustre, S.; Willson, M. Selective Phosphorylation On Primary Alcohols Of Unprotected Polyols. Phosphorus Sulfur Silicon Relat. Elem. 2001, 174, 37–47. [Google Scholar] [CrossRef]

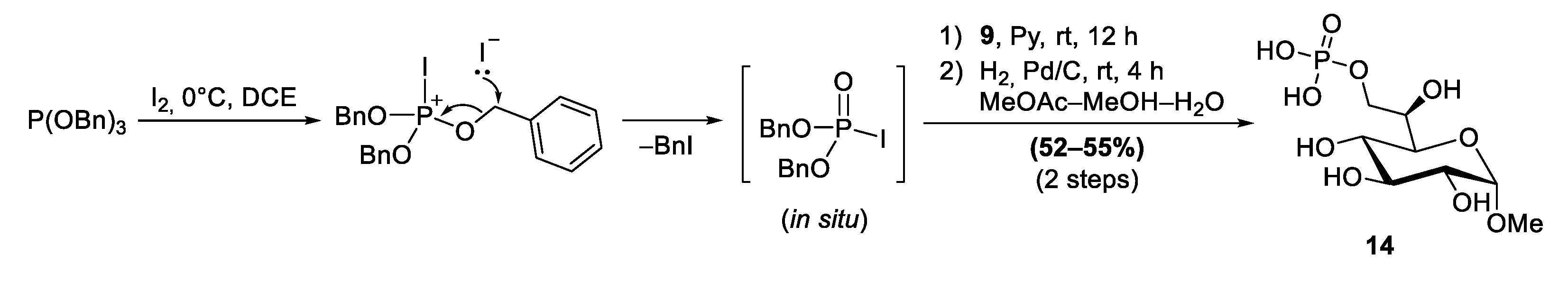
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, K.V.; Novikov, R.A.; Solyev, P.N.; Kochetkov, S.N.; Makarov, A.A.; Mitkevich, V.A. Synthetic Optimizations for Gram-Scale Preparation of 1-O-Methyl d-Glycero-α-d-gluco-heptoside 7-Phosphate from d-Glucose. Molecules 2022, 27, 7534. https://doi.org/10.3390/molecules27217534
Potapov KV, Novikov RA, Solyev PN, Kochetkov SN, Makarov AA, Mitkevich VA. Synthetic Optimizations for Gram-Scale Preparation of 1-O-Methyl d-Glycero-α-d-gluco-heptoside 7-Phosphate from d-Glucose. Molecules. 2022; 27(21):7534. https://doi.org/10.3390/molecules27217534
Chicago/Turabian StylePotapov, Konstantin V., Roman A. Novikov, Pavel N. Solyev, Sergey N. Kochetkov, Alexander A. Makarov, and Vladimir A. Mitkevich. 2022. "Synthetic Optimizations for Gram-Scale Preparation of 1-O-Methyl d-Glycero-α-d-gluco-heptoside 7-Phosphate from d-Glucose" Molecules 27, no. 21: 7534. https://doi.org/10.3390/molecules27217534
APA StylePotapov, K. V., Novikov, R. A., Solyev, P. N., Kochetkov, S. N., Makarov, A. A., & Mitkevich, V. A. (2022). Synthetic Optimizations for Gram-Scale Preparation of 1-O-Methyl d-Glycero-α-d-gluco-heptoside 7-Phosphate from d-Glucose. Molecules, 27(21), 7534. https://doi.org/10.3390/molecules27217534





