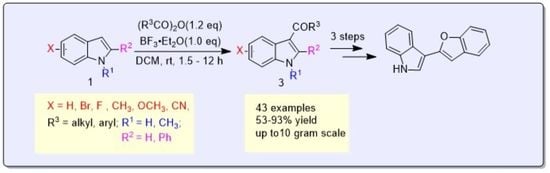
Boron Trifluoride Etherate Promoted Regioselective 3-Acylation of Indoles with Anhydrides
Abstract
1. Introduction
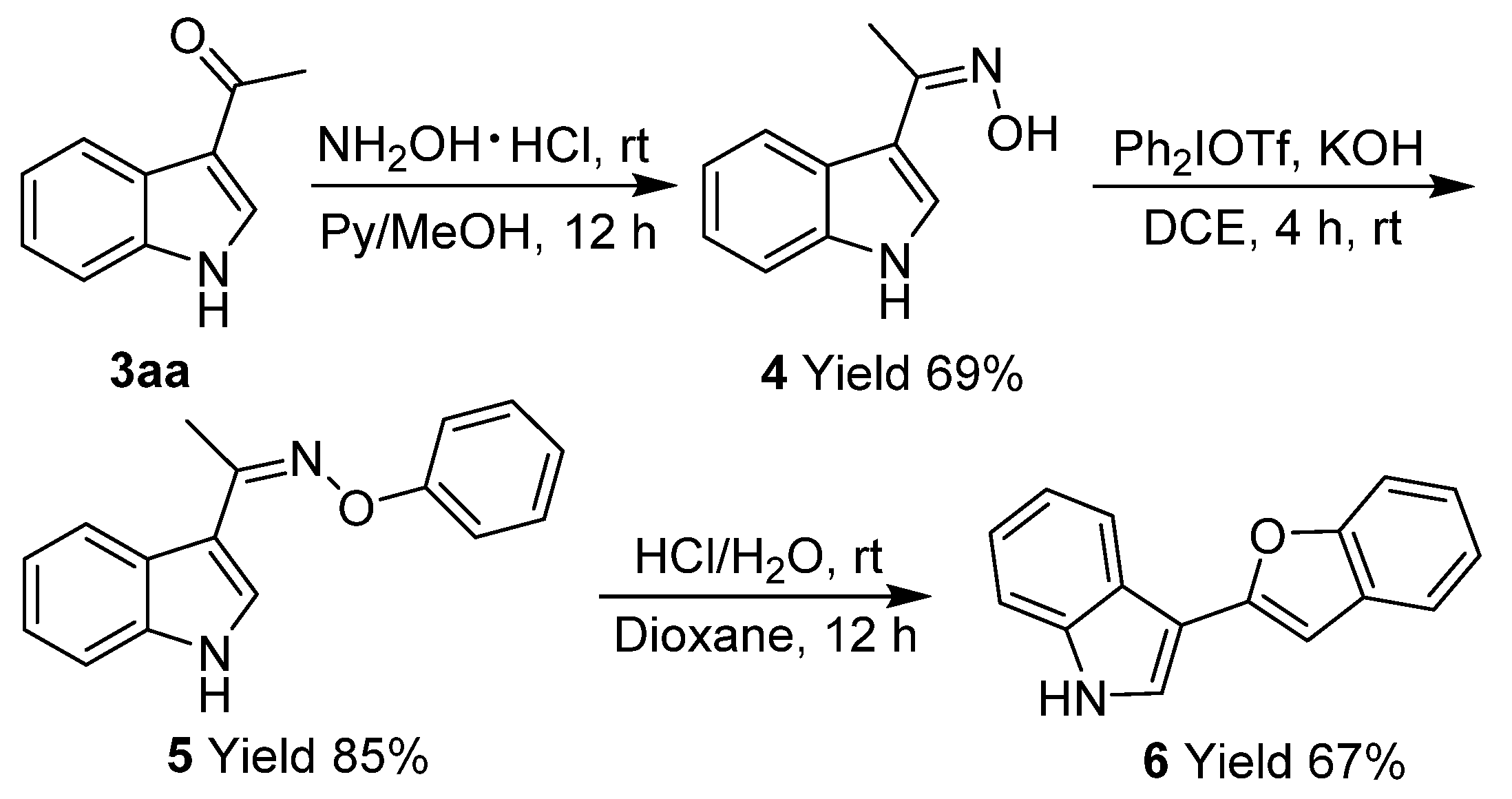
2. Results and Discussion
3. Experimental Section
3.1. General Procedure for the Synthesis of Product (3aa–3jd)
3.2. Up to Ten Scale Synthesis of Selected (3aa)
3.3. Procedure for Synthesis of 4
3.4. Procedure for Synthesis of 5
3.5. Procedure for Synthesis of 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shiri, M. Indoles in Multicomponent Processes (MCPs). Chem. Rev. 2012, 112, 3508–3549. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska, K.; Anna, J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G. Synthesis and Functionalization of Indoles through Palladium-catalyzed Reactions. Chem. Rev. 2005, 105, 2873. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- Platon, M.; Amardeil, R.; Djakovitch, L.; Hierso, J.C. Progress in Palladium-based Catalytic Systems for the Sustainable Synthesis of Annulated Heterocycles: A Focus on Indole Backbones. Chem. Soc. Rev. 2012, 41, 3929–3968. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010, 39, 4449–4465. [Google Scholar] [CrossRef]
- Miller, K.A.; Williams, R.M. Synthetic Approaches to the Bicyclo[2.2.2]diazaoctane Ring System Common to the Paraherquamides, Stephacidins and Related Prenylated Indole Alkaloids. Chem. Soc. Rev. 2009, 38, 3160–3174. [Google Scholar] [CrossRef]
- Jiang, P.; Chen, S.; Huang, H.; Hu, K.; Xia, Y.; Deng, G.J. Metal-free Synthesis of Indolo[2,3-b]indoles through Aerobic Cascade Dehydrogenative Aromatization/Oxidative Annulation. Green Synth. Catal. 2021, 2, 78–81. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Miller, D.D. Solid Phase Synthesis of Biologically Important Indoles. Curr. Med. Chem. 2009, 16, 2531–2565. [Google Scholar] [CrossRef]
- Golantsov, N.E.; Festa, A.A.; Karchava, A.V.; Yurovskaya, M.A. Marine Indole Alkaloids Containing an 1-(indol-3-yl)ethane-1,2-diamine Fragment (Review). Chem. Heterocycl. Compd. 2013, 49, 203–225. [Google Scholar] [CrossRef]
- Ruiz-Sanchis, P.; Savina, S.A.; Albericio, F.; Alvarez, M. Structure, Bioactivity and Synthesis of Natural Products with Hexahydropyrrolo[2,3-b]indole. Chem. Eur. J. 2011, 17, 1388–1408. [Google Scholar] [CrossRef]
- Fontana, G. Current Bioactive Azole-Containing Natural Products. Curr. Bioact. Compd. 2010, 6, 284–308. [Google Scholar] [CrossRef]
- Veale, C.J.L.; Zoraghi, R.; Young, R.M.; Morrison, J.P.; Pretheeban, M.; Lobb, K.A.; Reiner, N.E.; Anderson, R.J.; Davies-Coleman, M.T. Synthetic Analogues of the Marine Bisindole Deoxytopsentin: Potent Selective Inhibitors of MRSA Pyruvate Kinase. J. Nat. Prod. 2015, 78, 355–362. [Google Scholar] [CrossRef]
- Hadimani, M.B.; Macdonough, M.T.; Ghatak, A.; Strecker, T.E.; Lopez, R.; Sriram, M.; Nguyen, B.L.; Hall, J.J.; Kessler, R.J.; Shirali, A.R.; et al. Synthesis of a 2-Aryl-3-aroyl Indole Salt (OXi8007) Resembling Combretastatin A-4 with Application as a Vascular Disrupting Agent. J. Nat. Prod. 2013, 76, 1668–1678. [Google Scholar] [CrossRef]
- Pinney, K.G.; Wang, F.; Del Pilar Mejia, M. Indole-Containing and Combretastatin-Related Anti-Mitotic and Anti-Tubulin Polymerization Agents. WIPO (PCT) Patents WO 2001019794, 22 March 2001. [Google Scholar]
- Chen, H.; Bai, J.; Fang, Z.F.; Yu, S.S.; Ma, S.G.; Xu, S.; Li, Y.; Qu, J.; Ren, J.S.; Li, L.; et al. Indole Alkaloids and Quassinoids from the Stems of Brucea mollis. J. Nat. Prod. 2011, 74, 2438–2445. [Google Scholar] [CrossRef]
- Rago, A.J.; Dong, G.B. Synthesis of Indoles, Indolines, and Carbazoles via Palladium-Catalyzed C–H Activation. Green Synth. Catal. 2021, 2, 216–227. [Google Scholar] [CrossRef]
- Strecker, T.E.; Odutola, S.O.; Lopez, R.; Cooper, M.S.; Tidmore, J.K.; Charlton-Sevcik, A.K.; Li, L.; MacDonough, M.T.; Hadimani, M.B.; Ghatak, A.; et al. The Vascular Disrupting Activity of OXi8006 in Endothelial Cells and its Phosphate Prodrug OXi8007 in Breast Tumor Xenografts. Cancer Lett. 2015, 369, 229–241. [Google Scholar] [CrossRef]
- Barresi, E.; Bruno, A.; Taliani, S.; Cosconati, S.; Da Pozzo, E.; Salerno, S.; Simorini, F.; Daniele, S.; Giacomelli, C.; Marini, A.M.; et al. Deepening the Topology of the Translocator Protein Binding Site by Novel N,N-Dialkyl-2-arylindol-3-ylglyoxylamides. J. Med. Chem. 2015, 58, 6081–6092. [Google Scholar] [CrossRef]
- Kathirvelan, D.; Haribabu, J.; Reddy, B.S.R.; Balachandran, C.; Duraipandiyan, V. Facile and Diastereoselective Synthesis of 3,2′-Spiropyrrolidine-oxindoles Derivatives, their Molecular Docking and Antiproliferative Activities. Bioorg. Med. Chem. Lett. 2015, 25, 389–399. [Google Scholar] [CrossRef]
- Horbert, R.; Pinchuk, B.; Johannes, E.; Schlosser, J.; Schmidt, D.; Cappel, D.; Totzke, F.; Schaechtele, C.; Peifer, C. Optimization of Potent DFG-in Inhibitors of Platelet Derived Growth Factor Receptorβ (PDGF-Rβ) Guided by Water Thermodynamics. J. Med. Chem. 2015, 58, 170–182. [Google Scholar] [CrossRef]
- Singh, P.; Prasher, P.; Dhillon, P.; Bhatti, R. Indole Based Peptidomimetics as Anti-inflammatory and Anti-hyperalgesic Agents: Dual Inhibition of 5-LOX and COX-2 Enzymes. Eur. J. Med. Chem. 2015, 97, 104–123. [Google Scholar] [CrossRef]
- Mielczarek, M.; Thomas, R.V.; Ma, C.; Kandemir, H.; Yang, X.; Bhadbhade, M.; Black, D.S.C.; Griffith, R.; Lewis, P.J.; Kumar, N. Synthesis and Biological Activity of Novel Mono-indole and Mono-benzofuran Inhibitors of Bacterial Transcription Initiation Complex Formation. Bioorg. Med. Chem. 2015, 23, 1763–1775. [Google Scholar] [CrossRef]
- Khan, A.H.; Chen, J.S. Synthesis of Breitfussin B by Late-Stage Bromination. Org. Lett. 2015, 17, 3718–3721. [Google Scholar] [CrossRef]
- Ye, Q.; Mao, W.L.; Zhou, Y.B.; Xu, L.; Li, Q.; Gao, Y.X.; Wang, J.; Li, C.H.; Xu, Y.Z.; Xu, Y. Synthesis and Biological Evaluation of 3-([1,2,4]triazolo[4,3-a]pyridin-3-yl)-4-(indol-3-yl)-maleimides as Potent, Selective GSK-3β Inhibitors and Neuroprotective Agents. Bioorg. Med. Chem. 2015, 23, 1179–1188. [Google Scholar] [CrossRef]
- Yoo, E.; Salunke, D.B.; Sil, D.; Guo, X.Q.; Salyer, A.C.D.; Hermanson, A.R.; Kumar, M.; Malladi, S.S.; Balakrishna, R.; Thompson, W.H.; et al. Determinants of Activity at Human Toll-like Receptors 7 and 8: Quantitative Structure–Activity Relationship (QSAR) of Diverse Heterocyclic Scaffolds. J. Med. Chem. 2014, 57, 7955–7970. [Google Scholar] [CrossRef]
- Kamalraja, J.; Sowndarya, R.; Perumal, P.T. A Greener Approach for the Regioselective Synthesis of Multifunctionalized Indolylpyrrole and Indolyltriazolylpyrrole Hybrids via Michael Addition of α-Azido Ketones. Synlett 2014, 25, 2208–2212. [Google Scholar] [CrossRef]
- Muscia, G.C.; Hautmann, S.; Buldain, G.Y.; Asis, S.E.; Gutschow, M. Synthesis and Evaluation of 2-(1H-indol-3-yl)-4-Phenylquinolines as Inhibitors of Cholesterol Esterase. Bioorg. Med. Chem. Lett. 2014, 24, 1545–1549. [Google Scholar] [CrossRef]
- Kumar, D.; Maruthi Kumar, N.; Tantak, M.P.; Ogura, M.; Kusaka, E.; Ito, T. Synthesis and Identification of α-Cyano Bis(indolyl)chalcones as Novel Anticancer Agents. Bioorg. Med. Chem. Lett. 2014, 24, 5170–5174. [Google Scholar] [CrossRef]
- Wu, W.L.; Su, W.P. Mild and Selective Ru-Catalyzed Formylation and Fe-Catalyzed Acylation of Free (N–H) Indoles Using Anilines as the Carbonyl Source. J. Am. Chem. Soc. 2011, 133, 11924–11927. [Google Scholar] [CrossRef]
- Gu, L.J.; Liu, J.Y.; Zhang, L.Z.; Xiong, Y.; Wang, R. Synthesis of 3-Acylindoles via Decarboxylative Cross-coupling Reaction of Free (N-H) Indoles with α-Oxocarboxylic Acids. Chin. Chem. Lett. 2014, 25, 90–92. [Google Scholar] [CrossRef]
- Xia, X.F.; Zhang, L.L.; Song, X.R.; Niu, Y.N.; Liu, X.Y.; Liang, Y.M. Palladium–copper-cocatalyzed Intramolecular Oxidative Coupling: An Efficient and Atom-economical Strategy for the Synthesis of 3-Acylindoles. Chem. Commun. 2013, 49, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, P.H.; Wang, L. Copper-promoted Decarboxylative Direct C3-acylation of N-substituted Indoles with α-Oxocarboxylic Acids. Chem. Commun. 2013, 49, 2368–2370. [Google Scholar] [CrossRef]
- Ma, Y.H.; You, J.S.; Song, F.J. Facile Access to 3-Acylindoles through Palladium-Catalyzed Addition of Indoles to Nitriles: The One-Pot Synthesis of Indenoindolones. Chem. Eur. J. 2013, 19, 1189–1193. [Google Scholar] [CrossRef]
- Jiang, T.S.; Wang, G.W. Synthesis of 3-Acylindoles by Palladium-Catalyzed Acylation of Free (N–H) Indoles with Nitriles. Org. Lett. 2013, 15, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.Y.; Guo, X.K.; Xiang, J.N.; Li, J.H. Palladium-Catalyzed Synthesis of 3-Acylated Indoles Involving Oxidative Cross-Coupling of Indoles with α-Amino Carbonyl Compounds. J. Org. Chem. 2013, 78, 11163–11171. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Farkas, M.E.; Qiu, Z.L.; Yang, Z. Friedel–Crafts Acylation of Indoles in Acidic Imidazolium Chloroaluminate Ionic Liquid at Room Temperature. Tetrahedron Lett. 2002, 43, 5793–5795. [Google Scholar] [CrossRef]
- Xu, H.; Yang, W.-B.; Wang, Q. Antifungal Agents. Part 3: Synthesis and Antifungal Activities of 3-Acylindole Analogs against Phytopathogenic Fungi In Vitro. Chem. Biol. Drug. Des. 2011, 78, 864–868. [Google Scholar] [CrossRef]
- Ottoni, O.; Neder, A.D.F.; Dias, A.K.B.; Cruz, R.P.A.; Aquino, L.B. Acylation of Indole under Friedel−Crafts ConditionsAn Improved Method to Obtain 3-Acylindoles Regioselectively. Org. Lett. 2001, 3, 1005–1007. [Google Scholar] [CrossRef]
- Guchhait, S.K.; Kashyap, M.; Kamble, H. ZrCl4-Mediated Regio- and Chemoselective Friedel–Crafts Acylation of Indole. J. Org. Chem. 2011, 76, 4753–4758. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xue, H.; Li, H.L.; Kang, H.P.; Feng, J.; Lin, A.J.; Liu, S.X. Collective Synthesis of 3-Acylindoles, Indole-3-carboxylic Esters, Indole-3-sulfinic Acids, and 3-(Methylsulfonyl)indoles from Free (N–H) Indoles via Common N-Indolyl Triethylborate. Org. Lett. 2016, 18, 3918–3921. [Google Scholar] [CrossRef] [PubMed]
- Okauchi, T.; Itonaga, M.; Minami, T.; Owa, T.; Kitoh, K.; Yoshino, H. A General Method for Acylation of Indoles at the 3-Position with Acyl Chlorides in the Presence of Dialkylaluminum Chloride. Org. Lett. 2000, 2, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Suzuki, K.; Singh, S.K.; He, H.Y. Regiospecific C-Acylation of Pyrroles and Indoles Using N-Acylbenzotriazoles. J. Org. Chem. 2003, 68, 5720–5723. [Google Scholar] [CrossRef] [PubMed]
- Wynne, J.H.; Lloyd, C.T.; Jensen, S.D.; Boson, S.; Stalick, W.M. 3-Acylindoles via a One-Pot, Regioselective Friedel-Crafts Reaction. Synthesis 2004, 14, 2277–2282. [Google Scholar] [CrossRef]
- Yao, S.J.; Ren, Z.H.; Guan, Z.H. Recent Advances on the Synthesis of Acylindoles. Tetrahedron Lett. 2016, 57, 3892–3901. [Google Scholar] [CrossRef]
- Bohstroem, Z.; Holmberg, K. Friedel–Crafts Acylation of 2-methylindole with Acetic Anhydride Using Mesoporous HZSM-5. J. Mol. Catal. A-CHEM 2013, 366, 64–73. [Google Scholar] [CrossRef]
- Tran, P.H.; Nguyen, A.T.D.; Nguyen, H.T.; Le, T.N. Brønsted Acidic Ionic Liquid-Promoted Direct C3-acylation of N-unsubstituted Indoles with Acid Anhydrides under Microwave Irradiation. RSC Adv. 2017, 7, 54399–54406. [Google Scholar] [CrossRef]
- Cornel, V.; Lovely, C.J. Boron Trifluoride Etherate, e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Kuhl, N.; Hopkinson, M.N.; Glorius, F. Selective Rhodium(III)-Catalyzed Cross-Dehydrogenative Coupling of Furan and Thiophene Derivatives. Angew. Chem. Int. Ed. 2012, 51, 8230–8234. [Google Scholar] [CrossRef]
- Zou, Y.J.; Yue, G.Z.; Xu, J.W.; Zhou, J.R. General Suzuki Coupling of Heteroaryl Bromides by Using Tri-tert-butylphosphine as a Supporting Ligand. Eur. J. Org. Chem. 2014, 5901–5905. [Google Scholar] [CrossRef]
- Donald, J.R.; Taylor, R.J.K. Tandem Meinwald Rearrangement-Fischer Indolisation: A One-Pot Conversion of Epoxides into Indoles. Synlett 2009, 1, 59–62. [Google Scholar] [CrossRef]
- Shi, W.M.; Ma, X.P.; Pan, C.X.; Su, G.F.; Mo, D.L. Tandem C–O and C–N Bonds Formation Through O-Arylation and [3,3]-Rearrangement by Diaryliodonium Salts: Synthesis of N-Aryl Benzo[1,2,3]triazin-4(1H)-one Derivatives. J. Org. Chem. 2015, 80, 11175–11183. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Shi, W.M.; Mo, X.L.; Li, X.H.; Li, L.G.; Pan, C.X.; Chen, B.; Su, G.F.; Mo, D.L. Synthesis of α,β-Unsaturated N-Aryl Ketonitrones from Oximes and Diaryliodonium Salts: Observation of a Metal-Free N-Arylation Process. J. Org. Chem. 2015, 80, 10098–10107. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y.; Xu, Q.L.; Keene, C.; Kurti, L. Scalable, Transition-Metal-Free Direct Oxime O-Arylation: Rapid Access to O-Arylhydroxylamines and Substituted Benzo[b]furans. Chem. Eur. J. 2014, 20, 8883–8887. [Google Scholar] [CrossRef]
- Ito, Y.; Kobayashi, K.; Saegusa, T. Indole Syntheses with o-Tolyl Isocyanide. 3-Acylindoles and 2-substituted Indoles. J. Org. Chem. 1979, 44, 2030–2032. [Google Scholar] [CrossRef]
- Yu, J.B.; Zhang, C.; Yang, X.J.; Su, W.K. Decarboxylative Acylation of N-free Indoles Enabled by a Catalytic Amount of Copper Catalyst and liquid-assisted grinding. Org. Biomol. Chem. 2019, 17, 4446–4451. [Google Scholar] [CrossRef]
- Davies, J.R.; Kane, P.D.; Moddy, C.J.; Slawin, A.M.Z. Control of Competing N−H Insertion and Wolff Rearrangement in Dirhodium(II)-Catalyzed Reactions of 3-Indolyl Diazoketoesters. Synthesis of a Potential Precursor to the Marine 5-(3-Indolyl)oxazole Martefragin A. J. Org. Chem. 2005, 70, 5840–5851. [Google Scholar] [CrossRef]
- Ali, M.A.; Punniyamurthy, T. Domino Ligand-Free Copper-Catalyzed Synthesis of Polysubstituted Indoles. Synlett 2011, 5, 623–626. [Google Scholar] [CrossRef]
- Kamenov, L.; Yudin, L.G.; Budylin, V.A.; Kost, A.N. The Chemistry of Indole. Chem. Heterocycl. Compd. 1970, 6, 856–859. [Google Scholar] [CrossRef]
- Qu, J.; Kumar, N.; Alamgir, M.; Black, D. StC. A Versatile Dynthetic Route to 11H-indolo[3,2-c]isoquinolines. Tetrahedron Lett. 2009, 50, 5628–5630. [Google Scholar] [CrossRef]
- Buchmann, G.; Rossner, D. Beitrag zur Chemie des 2-Phenyl-indols. II. Über acylierte 2-Phenyl-indole und ihre Reaktivität. J. Fuer Prakt. Chem. 1964, 25, 117–134. [Google Scholar] [CrossRef]
- Na, Y.M.; Borgne, M.L.; Pagniez, F.; Baut, G.L.; Pape, P.L. Synthesis and Antifungal Activity of New 1-Halogenobenzyl-3-imidazolylmethylindole Derivatives. Eur. J. Med. Chem. 2003, 38, 75–87. [Google Scholar] [CrossRef]
- Barreca, M.L.; Ferro, S.; Rao, A.; De Luca, L.; Zappala, M.; Monforte, A.M.; Debyser, Z.; Witvrouw, M.; Chimirri, A. Pharmacophore-Based Design of HIV-1 Integrase Strand-Transfer Inhibitors. J. Med. Chem. 2005, 48, 7084–7088. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Palmieri, G.; Petrini, M. A New Approach to the Synthesis of 2-Substituted Indoles: Reaction of Dimetallated Ortho-trimethylsilylmethylanilides with Esters. Tetrahedron 1990, 46, 1379–1384. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.F.; Sun, L.; Ding, L.; Gu, Y.C.; Gong, P. Synthesis and Anti-tumor Activity of 2-Amino-3-Cyano-6-(1H-indol-3-yl)-4-Phenylpyridine. Eur. J. Med. Chem. 2011, 46, 3149–3157. [Google Scholar] [CrossRef]
- Giraud, F.; Alves, G.; Debiton, E.; Nauton, L.; Thery, V.; Durieu, E.; Ferandin, Y.; Lozach, O.; Meijer, L.; Anizon, F.; et al. Synthesis, Protein Kinase Inhibitory Potencies, and in Vitro Antiproliferative Activities of Meridianin Derivatives. J. Med. Chem. 2011, 54, 4474–4489. [Google Scholar] [CrossRef]
- Yurovskaya, M.A.; Druzhinina, V.V.; Budylin, V.A.; Bundel, Y.G.; Yufit, D.S.; Struchkov, Y.T. Structure of 3-acylindole oximes. Chem. Heterocycl. Compd. 1983, 19, 184–187. [Google Scholar] [CrossRef]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Filisti, E.; Sferrazza, A. 3-Aroylindoles via Copper-Catalyzed Cyclization of N-(2-Iodoaryl)enaminones. Synlett 2009, 9, 1480–1484. [Google Scholar] [CrossRef]



 | ||||||
|---|---|---|---|---|---|---|
| Entry | 2a (Eq.) | BF3·Et2O (Eq.) | Solvent | Temp. (°C) | Time (h) | Yield (%) |
| 1 | 1.0 | 1.0 | DCM | rt | 1.2 | 70 |
| 2 | 1.0 | 1.0 | DCE | rt | 1.0 | 40 |
| 3 | 1.0 | 1.0 | CHCl3 | rt | 1.0 | 41 |
| 4 | 1.0 | 1.0 | THF | rt | 8.0 | trace |
| 5 | 1.0 | 1.0 | CH3CN | rt | 3.0 | 55 |
| 6 | 1.0 | 1.0 | 1,4-dioxane | rt | 1.5 | 62 |
| 7 | 1.0 | 0 | DCM | rt | 1.0 | nr |
| 8 | 1.0 | 0.5 | DCM | rt | 1.5 | 60 |
| 9 | 1.0 | 1.2 | DCM | rt | 1.0 | 66 |
| 10 | 1.0 | 1.5 mL | ― | rt | 0.5 | 30 |
| 11 | 1.1 | 1.0 | DCM | rt | 1.0 | 78 |
| 12 | 1.2 | 1.0 | DCM | rt | 1.0 | 83 |
| 13 | 1.3 | 1.0 | DCM | rt | 1.0 | 82 |
| 14 | 1.2 | 1.0 | DCM | 0 | 2.5 | 80 |
| 15 | 1.2 | 1.0 | DCM | 50 | 0.3 | 40 |
 | ||||
|---|---|---|---|---|
| Entry | 2 | R | 3 | Yield (%) |
| 1 | 2b | Et | 3ab | 91 |
| 2 | 2c | n-Pr | 3ac | 85 |
| 3 | 2d | cyclohexyl | 3ad | 72 |
| 4 | 2e | Ph | 3ae | 76 |
| 5 | 2f | 4-CH3O-C6H4 | 3af | 80 |
| 6 | 2g | 3-CH3-C6H4 | 3ag | 71 |
| 7 | 2h | 4-Cl-C6H4 | 3ah | np |
| 8 | 2j | 4-NO2-C6H4 | 3aj | np |
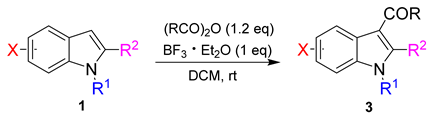 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | R1 | R2 | X | 3 | Yield (%) |
| 1 | Me | Me | H | H | 3ba | 87 |
| 2 | Et | Me | H | H | 3bb | 82 |
| 3 | n-Pr | Me | H | H | 3bc | 71 |
| 4 | Ph | Me | H | H | 3bd | 53 |
| 5 | Me | H | Me | H | 3ca | 68 |
| 6 | Et | H | Me | H | 3cb | 71 |
| 7 | n-Pr | H | Me | H | 3cc | 74 |
| 8 | Ph | H | Me | H | 3cd | 56 |
| 9 | Me | H | Ph | H | 3da | 62 |
| 10 | Et | H | Ph | H | 3db | 65 |
| 11 | n-Pr | H | Ph | H | 3dc | 70 |
| 12 | Ph | H | Ph | H | 3dd | 59 |
| 13 | Me | H | H | 5-Br | 3ea | 87 |
| 14 | Et | H | H | 5-Br | 3eb | 93 |
| 15 | n-Pr | H | H | 5-Br | 3ec | 84 |
| 16 | Ph | H | H | 5-Br | 3ed | 70 |
| 17 | Me | H | H | 5-CN | 3fa | 93 |
| 18 | Et | H | H | 5-CN | 3fb | 91 |
| 19 | n-Pr | H | H | 5-CN | 3fc | 88 |
| 20 | Ph | H | H | 5-CN | 3fd | 53 |
| 21 | Me | H | H | 5-CH3 | 3ga | 88 |
| 22 | Et | H | H | 5-CH3 | 3gb | 83 |
| 23 | n-Pr | H | H | 5-CH3 | 3gc | 76 |
| 24 | Ph | H | H | 5-CH3 | 3gd | 67 |
| 25 | Me | H | H | 5-OCH3 | 3ha | 80 |
| 26 | Et | H | H | 5-OCH3 | 3hb | 75 |
| 27 | n-Pr | H | H | 5-OCH3 | 3hc | 68 |
| 28 | Ph | H | H | 5-OCH3 | 3hd | 64 |
| 29 | Me | H | H | 6-F | 3ia | 92 |
| 30 | Et | H | H | 6-F | 3ib | 88 |
| 31 | n-Pr | H | H | 6-F | 3ic | 81 |
| 32 | Ph | H | H | 6-F | 3id | 67 |
| 33 | Me | H | H | 7-Br | 3ja | 91 |
| 34 | Et | H | H | 7-Br | 3jb | 89 |
| 35 | n-Pr | H | H | 7-Br | 3jc | 88 |
| 36 | Ph | H | H | 7-Br | 3jd | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Li, J.; Wei, K. Boron Trifluoride Etherate Promoted Regioselective 3-Acylation of Indoles with Anhydrides. Molecules 2022, 27, 8281. https://doi.org/10.3390/molecules27238281
Zheng Y, Li J, Wei K. Boron Trifluoride Etherate Promoted Regioselective 3-Acylation of Indoles with Anhydrides. Molecules. 2022; 27(23):8281. https://doi.org/10.3390/molecules27238281
Chicago/Turabian StyleZheng, Yunyun, Jiuling Li, and Kai Wei. 2022. "Boron Trifluoride Etherate Promoted Regioselective 3-Acylation of Indoles with Anhydrides" Molecules 27, no. 23: 8281. https://doi.org/10.3390/molecules27238281
APA StyleZheng, Y., Li, J., & Wei, K. (2022). Boron Trifluoride Etherate Promoted Regioselective 3-Acylation of Indoles with Anhydrides. Molecules, 27(23), 8281. https://doi.org/10.3390/molecules27238281