Thermal and Structural Characterization of Two Crystalline Polymorphs of Tafamidis Free Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. IR Spectroscopy
2.2.2. Thermal Analyses
2.2.3. Structural X-ray Powder Diffraction Analysis
2.2.4. Variable-Temperature X-ray Powder Diffractometry
3. Results
3.1. Comparative Crystal Chemistry
3.2. High-Temperature Diffraction Studies
3.3. Thermal Analyses
3.4. IR Fingerprinting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| ATR | Attenuated Total Reflection |
| DSC | Differential Scanning Calorimetry |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FTIR | Fourier-transformed Infrared spectroscopy |
| TGA | Thermogravimetric Analysis |
| VTXRD | Variable-Temperature X-ray Diffraction |
| XRPD | X-ray Powder Diffraction |
References
- Cruz-Cabeza, A.J.; Feeder, N.; Davey, R.J. Open questions in organic crystal polymorphism. Commun. Chem. 2020, 3, 142. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism of dyes and pigments, Ch. 8. In Polymorphism of Molecular Crystals; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- van der Poll, T.S.; Zhugayevych, A.; Chertkov, E.; Bakus, R.C.; Coughlin, J.E.; Teat, S.J.; Bazan, G.C.; Tretiak, S. Polymorphism of Crystalline Molecular Donors for Solution-Processed Organic Photovoltaics. J. Phys. Chem. Lett. 2014, 5, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, P.H. Polymorphism of Active Pharmaceutical Ingredients. Chem. Eng. Technol. 2006, 29, 233–237. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18760–18776. [Google Scholar] [CrossRef]
- Brittain, H.G. Polymorphism in Pharmaceutical Solids; Informa Healthcare USA, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Lee, A.Y.; Erdemir, D.; Myerson, A.S. Crystal Polymorphism in Chemical Process Development. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 259–280. [Google Scholar] [CrossRef]
- Blandizzi, C.; Viscomi, G.C.; Scarpignato, C. Impact of crystal polymorphism on the systemic bioavailability of rifaximin, an antibiotic acting locally in the gastrointestinal tract, in health volunteers. Drug Des. Dev. Ther. 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An Extraordinary Example of Conformational Polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Chemburkar, S.R.; Bauer, J.; Deming, K.; Spiwek, H.; Patel, K.; Morris, J.; Henry, R.; Spanton, S.; Dziki, W.; Porter, W. Dealing with the Impact of Ritonavir Polymorphs on the Late Stages of Bulk Drug Process Development. Org. Process Res. Dev. 2000, 4, 413–417. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, P.; Ratan, S.; Malik, R.; Arora, S. Polymorphism: The Phenomenon Affecting the Performance of Drugs. SOJ Pharm. Pharm. Sci. 2014, 1, 10–19. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villier, M.M. Solid State Concerns During Drug Discovery and Development: Thermodynamic and Kinetic Aspects of Crystal Polymorphism and the Special Cases of Concomitant Polymorphs, Co-Crystals and Glasses. Curr. Drug Disc. Technol. 2017, 14, 72–105. [Google Scholar] [CrossRef]
- Shankland, K.; Spillman, M.J.; Kabova, E.A.; Edgeley, D.S.; Shankland, N. The principles underlying the use of powder diffraction data in solving pharmaceutical crystal structures. Acta Cryst. 2013, C69, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Abbinante, V.M.; Zampieri, M.; Barreca, G.; Masciocchi, N. Preparation and Solid-State Characterization of Eltrombopag Crystal Phases. Molecules 2021, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Girard, K.P.; Jensen, A.J.; Jones, K.N. Crystalline Solid Forms of 6-Carboxy-2-(3,5-dichlorophenyl)-benzoxazole. Patent WO 2016/038500 Al, 17 March 2016. [Google Scholar]
- Musanic, S.M.; Travancic, V.; Pavlicic, D. Solid State Forms of Tafamidis and Salts Thereof. Patent WO 2020/232325 Al, 19 November 2020. [Google Scholar]
- Le Pevelen, D.D. FT-IR and Raman Spectroscopies, Polymorphism Applications. In Encyclopedia of Spectroscopy and Spectrometry; Lindon, J., Tranter, G.E., Koppenaal, D., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 750–761. [Google Scholar] [CrossRef]
- Harris, K.D. Powder diffraction crystallography of molecular solids. Top. Curr. Chem. 2012, 315, 133–177. [Google Scholar] [CrossRef]
- Harding, M.M. Recording diffraction data for structure determination for very small crystals. J. Synchr. Radiat. 1996, 3, 250–259. [Google Scholar] [CrossRef]
- Clegg, W. The development and exploitation of synchrotron single-crystal diffraction for chemistry and materials. Phil. Trans. 2019, A377, 20180239. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Nia, S.S.; Rodriguez, J.A. Electron Diffraction of 3D Molecular Crystals. Chem. Rev. 2022, 122, 13883–13914. [Google Scholar] [CrossRef]
- Fawcett, T.; Gates-Rector, S.; Gindhart, A.; Rost, M.; Kabekkodu, S.; Blanton, J.; Blanton, T. A practical guide to pharmaceutical analyses using X-ray powder diffraction. Powder Diffr. 2019, 34, 164–183. [Google Scholar] [CrossRef]
- Iyengar, S.; Phadnis, N.; Suryanarayanan, R. Quantitative analyses of complex pharmaceutical mixtures by the Rietveld method. Powder Diffr. 2001, 16, 20–24. [Google Scholar] [CrossRef]
- Kotrlý, M. Using X-ray diffraction in forensic science. Zeit. Krist.–Cryst. Mater. 2007, 222, 193–198. [Google Scholar] [CrossRef]
- Ivanesevic, I.; McClurg, M.B.; Schields, P.J. Uses of X-ray Powder Diffraction In the Pharmaceutical Industry. In Pharmaceutical Sciences Encyclopedia (Drug Discovery, Development, and Manufacturing); Gad, S.C., Ed.; Wiley: New York, NY, USA, 2010; pp. 1–42. [Google Scholar] [CrossRef]
- TOPAS-R, V3.0; Bruker AXS: Karlsruhe, Germany, 2005.
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March model. J. Appl. Cryst. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- Ohashi, Y. A program to calculate the strain tensor from two sets of unit-cell parameters. In Comparative Crystal Chemistry; Hazen, R.M., Finger, L.W., Eds.; Wiley: Chichester, UK, 1982; pp. 92–102. [Google Scholar]
- Available online: https://www.cryst.ehu.es/cryst/strain.html (accessed on 30 September 2022).
- Kaminsky, W. Wintensor, Ein WIN95/98/NT Programm zum Darstellen tensorieller Eigenschaften. Z. Kristallogr. Suppl. 2000, 17, 51. [Google Scholar]
- Le Bail, A. Whole powder pattern decomposition methods and applications: A retrospection. Powder Diffr. 2012, 20, 316–326. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. Organic Chemical Crystallography; Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
- Burger, A.; Ramberger, A.I. On the polymorphism of pharmaceuticals and other molecular crystals. I. Mikroch. Acta 1979, II, 259–271. [Google Scholar] [CrossRef]
- Perlovich, G.; Surov, A. Polymorphism of monotropic forms: Relationships between thermochemical and structural characteristics. Acta Cryst. 2020, B76, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Nelyubina, Y.V.; Glukhov, I.V.; Antipin, M.Y.; Lyssenko, K.A. “Higher density does not mean higher stability” mystery of paracetamol finally unraveled. Chem. Commun. 2010, 46, 3469–3471. [Google Scholar] [CrossRef]
- Salari, A.; Young, R.E. Application of attenuated total reflectance FTIR spectroscopy to the analysis of mixtures of pharmaceutical polymorphs. Int. J. Pharm. 1998, 163, 157–166. [Google Scholar] [CrossRef]
- Larkin, P.J.; Dabros, M.; Sarsfield, B.; Chan, E.; Carriere, J.T.; Smith, B.C. Polymorph Characterization of Active Pharmaceutical Ingredients (APIs) Using Low-Frequency Raman Spectroscopy. Appl. Spectrosc. 2014, 68, 758–776. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Gatta, G.D.; Arletti, R.; Artioli, G.; Ballirano, P.; Cruciani, G.; Guagliardi, A.; Malferrari, D.; Masciocchi, N.; Scardi, P. Quantitative phase analysis using the Rietveld method: Towards a procedure for checking the reliability and quality of the results. Period. Miner. 2019, 8, 147–151. [Google Scholar] [CrossRef]
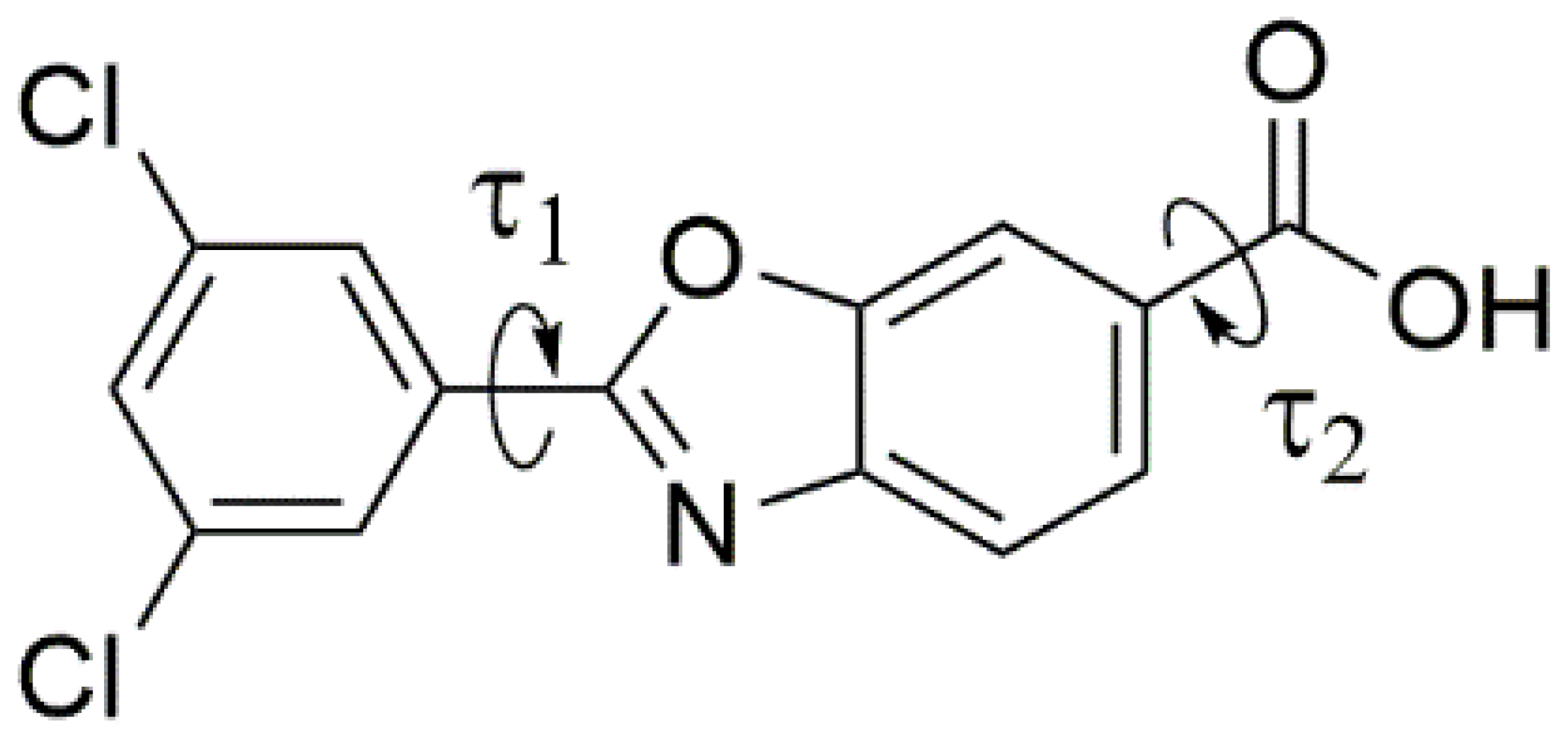
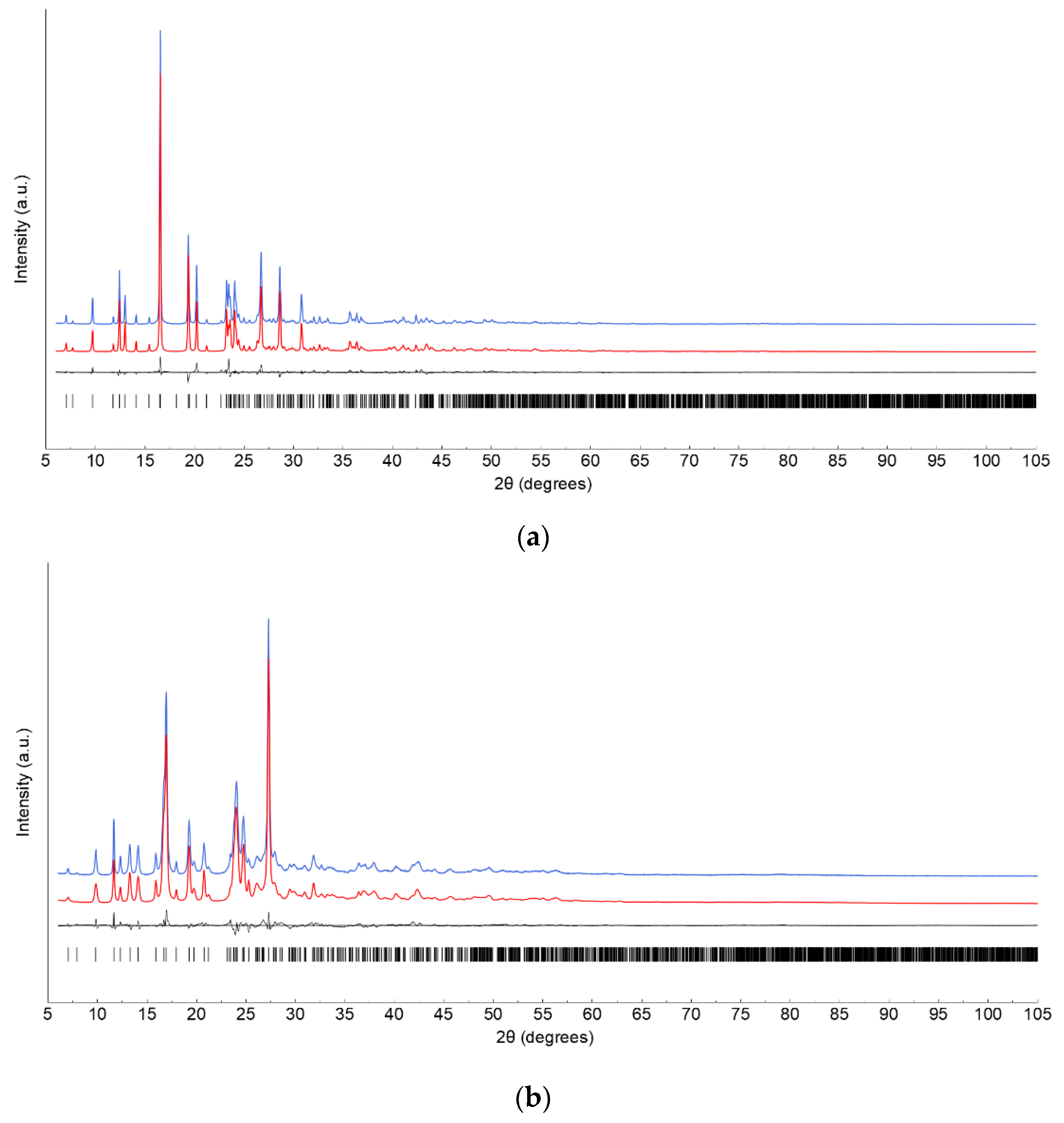
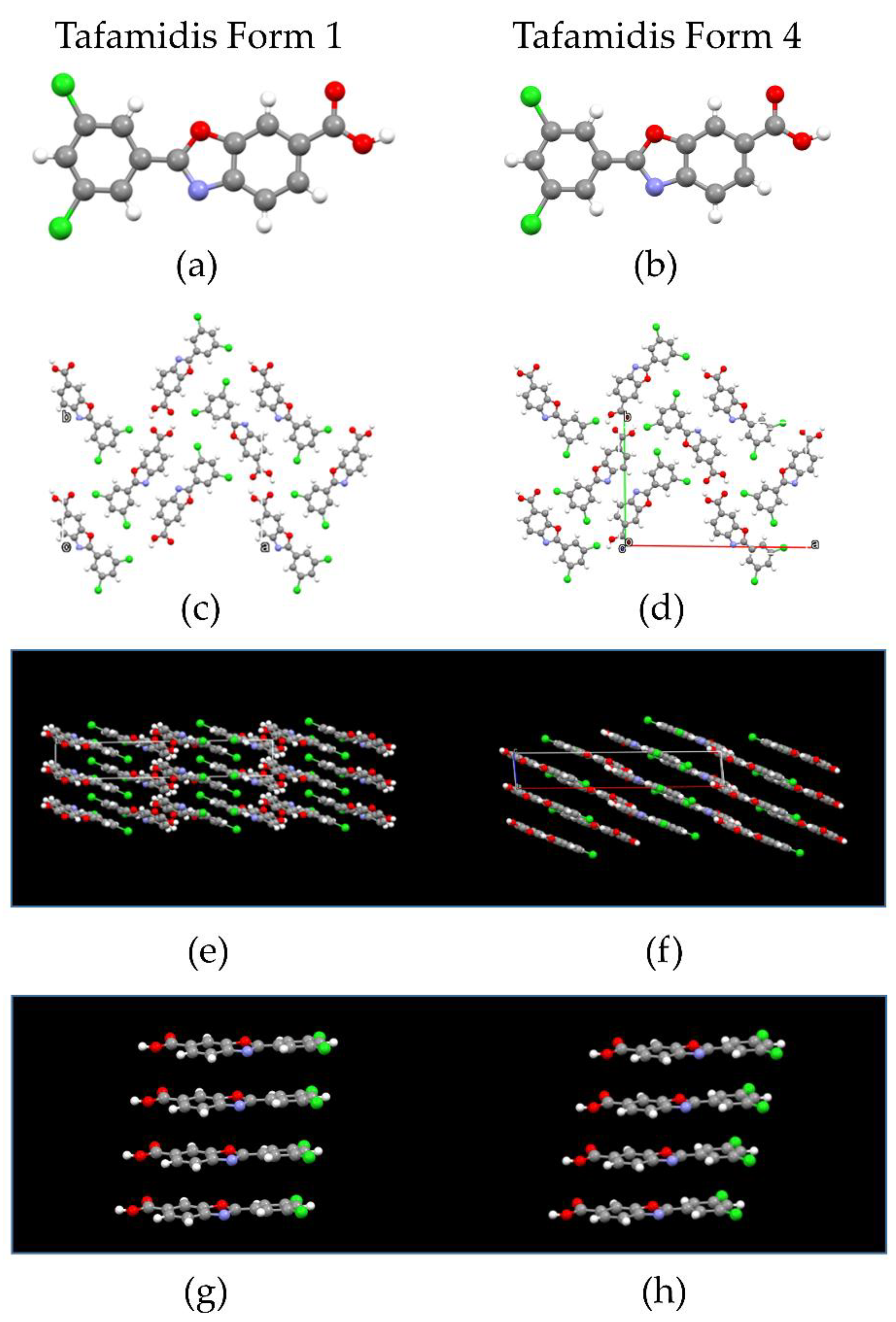
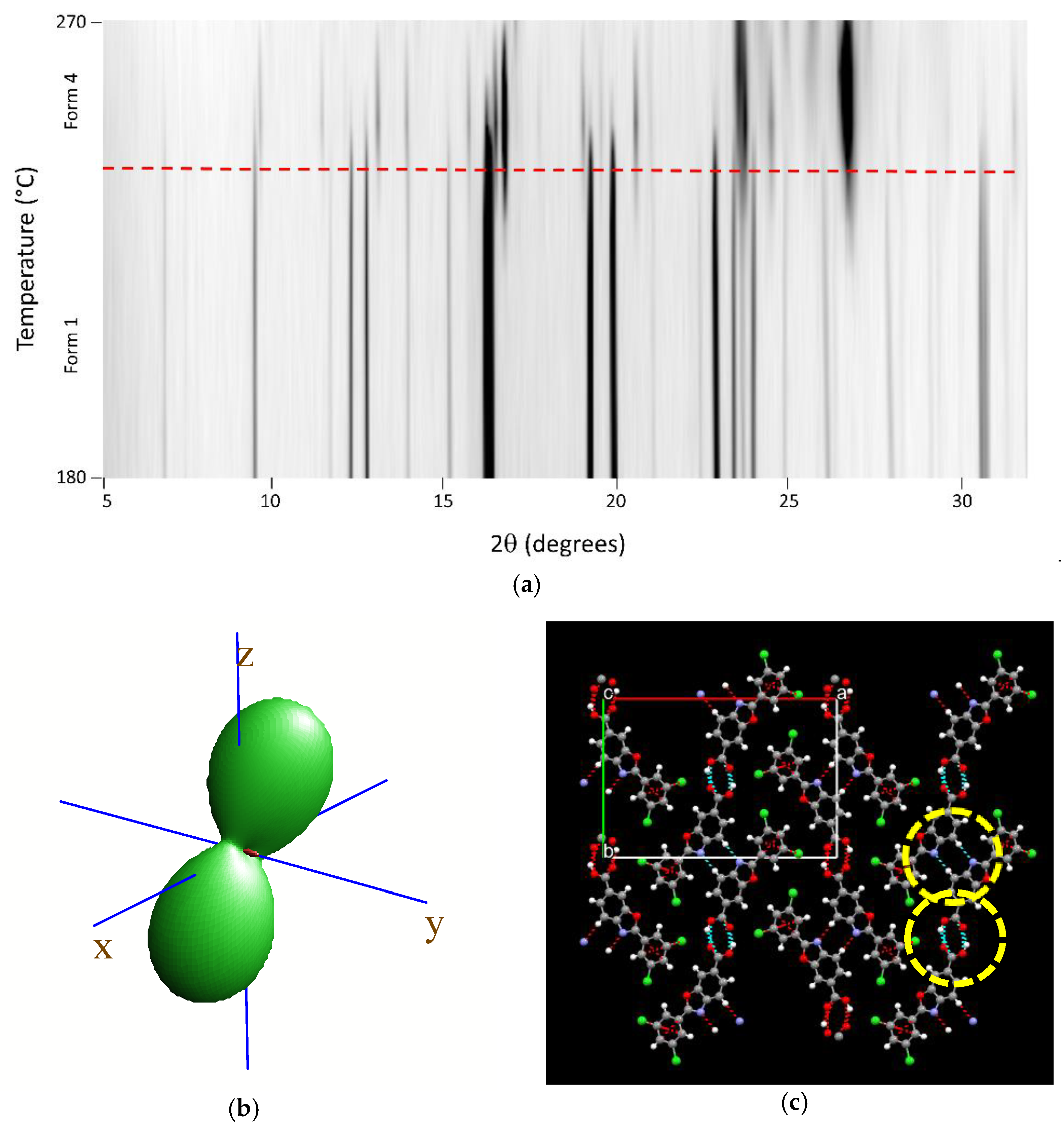
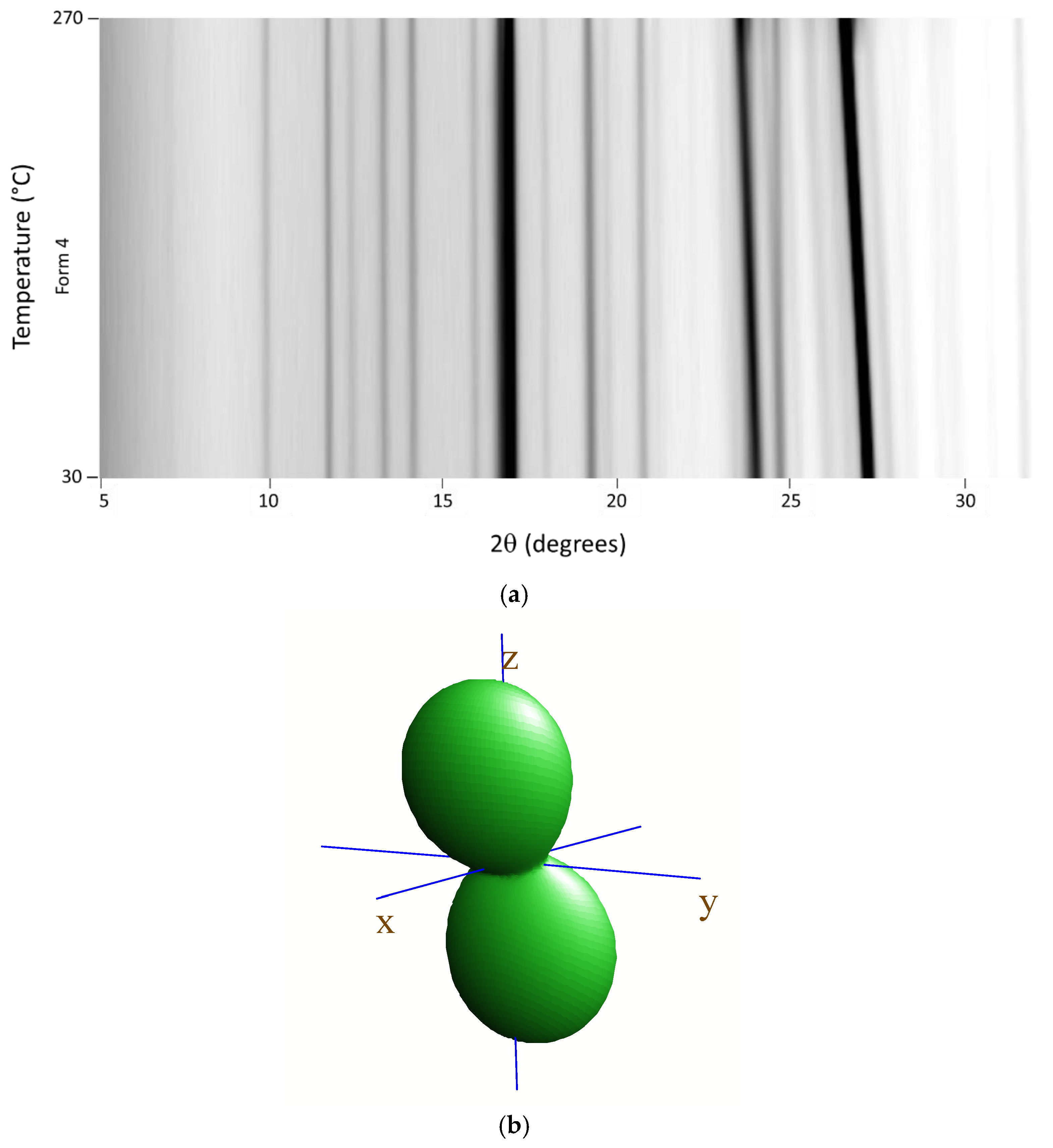
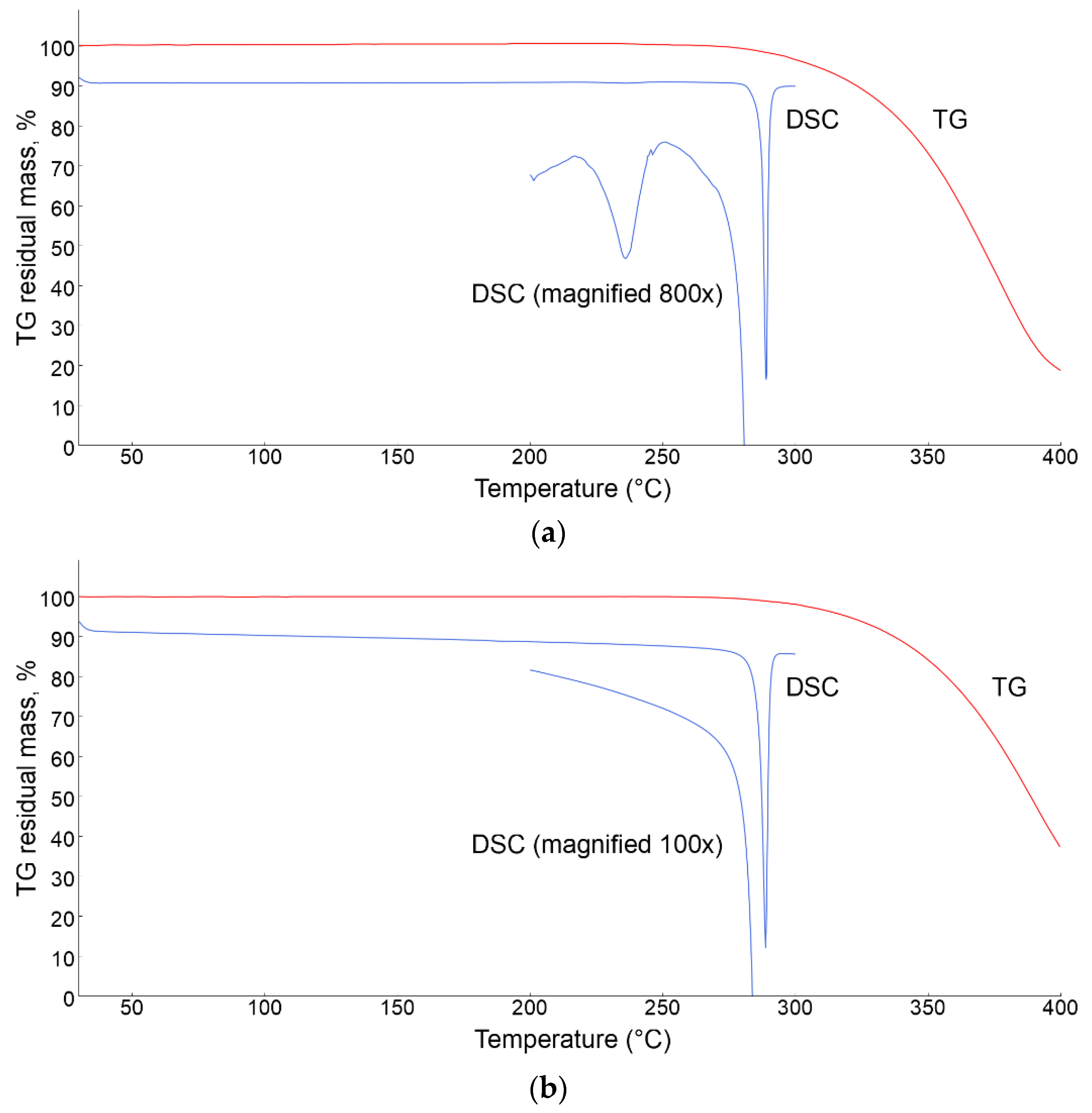
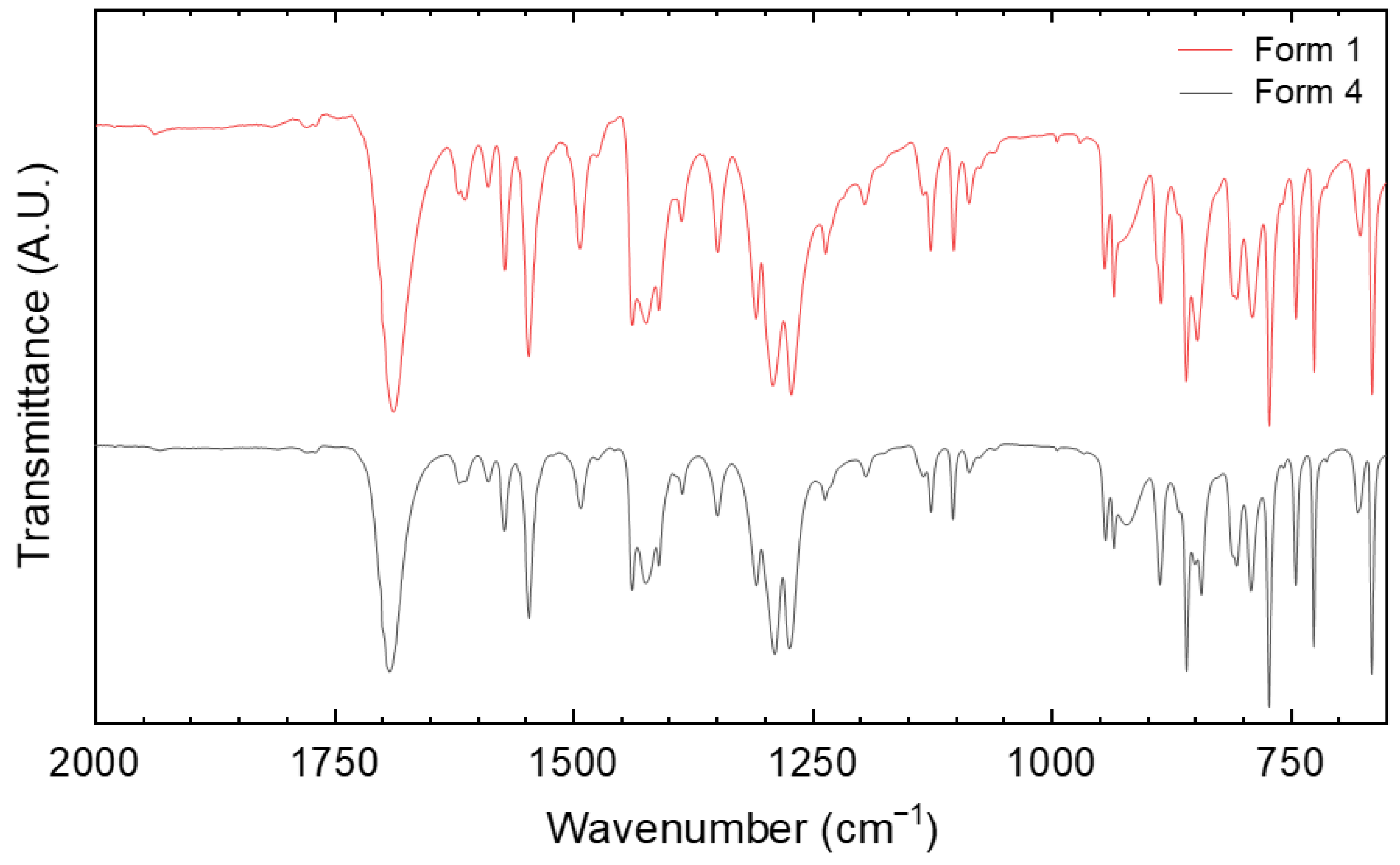
| Parameter | Form 1 | Form 4 |
|---|---|---|
| Formula | C14H7Cl2NO3 | C14H7Cl2NO3 |
| fw, g mol−1 | 308.12 | 308.12 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/a (No. 14) | P21/n (No. 14) |
| a, Å | 22.976 (1) | 22.364 (3) |
| b, Å | 14.993 (1) | 15.174 (2) |
| c, Å | 3.794 (1) | 3.819 (1) |
| β, ° | 90.938 (3) | 95.265 (5) |
| V, Å3 | 1306.9 (1) | 1290.7 (3) |
| Z | 4 | 4 |
| V/Z, Å3 | 326.7 | 322.27 |
| ρcalc, g cm−3 | 1.566 | 1.586 |
| μ (CuKα), cm−1 | 45.5 | 46.1 |
| F (000) | 624 | 624 |
| λavg, Å | 1.5418 | 1.5418 |
| T, K | 295 | 295 |
| 2θ range, ° | 6–105 | 6–105 |
| Rp, Rwp | 0.079, 0.103 | 0.062, 0.083 |
| χ2 | 4.13 | 5.16 |
| RBragg | 0.064 | 0.039 |
| Form 1 | Form 4 | A Sketch of the SV, χ and ψ Parameters | |
|---|---|---|---|
| τ1 torsional angle, ° | 9.4 (0.2) | 1.5 (0.3) |  |
| τ2 torsional angle, ° | 0.0 (0.5) | 6.0 (0.5) | |
| O–H···O, Å | 2.62 | 2.64 | |
| Stacking Vector (SV), Å | 3.794 | 3.819 | |
| χ angle, ° | 78.8 | 70.9 | |
| ψ angle, ° | 80.9 | 71.8 | |
| Interplanar Distance, Å | 3.51 | 3.41 |
| Form 1 | Form 4 | |
|---|---|---|
| κa, 106 K−1 | 50 | 20 |
| κb, 106 K−1 | −14 | 22 |
| κc, 106 K−1 | 74 | 93 |
| κβ, 106 K−1 | −48 | −29 |
| κV, 106 K−1 | 108 | 141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masciocchi, N.; Abbinante, V.M.; Zambra, M.; Barreca, G.; Zampieri, M. Thermal and Structural Characterization of Two Crystalline Polymorphs of Tafamidis Free Acid. Molecules 2022, 27, 7411. https://doi.org/10.3390/molecules27217411
Masciocchi N, Abbinante VM, Zambra M, Barreca G, Zampieri M. Thermal and Structural Characterization of Two Crystalline Polymorphs of Tafamidis Free Acid. Molecules. 2022; 27(21):7411. https://doi.org/10.3390/molecules27217411
Chicago/Turabian StyleMasciocchi, Norberto, Vincenzo Mirco Abbinante, Marco Zambra, Giuseppe Barreca, and Massimo Zampieri. 2022. "Thermal and Structural Characterization of Two Crystalline Polymorphs of Tafamidis Free Acid" Molecules 27, no. 21: 7411. https://doi.org/10.3390/molecules27217411
APA StyleMasciocchi, N., Abbinante, V. M., Zambra, M., Barreca, G., & Zampieri, M. (2022). Thermal and Structural Characterization of Two Crystalline Polymorphs of Tafamidis Free Acid. Molecules, 27(21), 7411. https://doi.org/10.3390/molecules27217411







