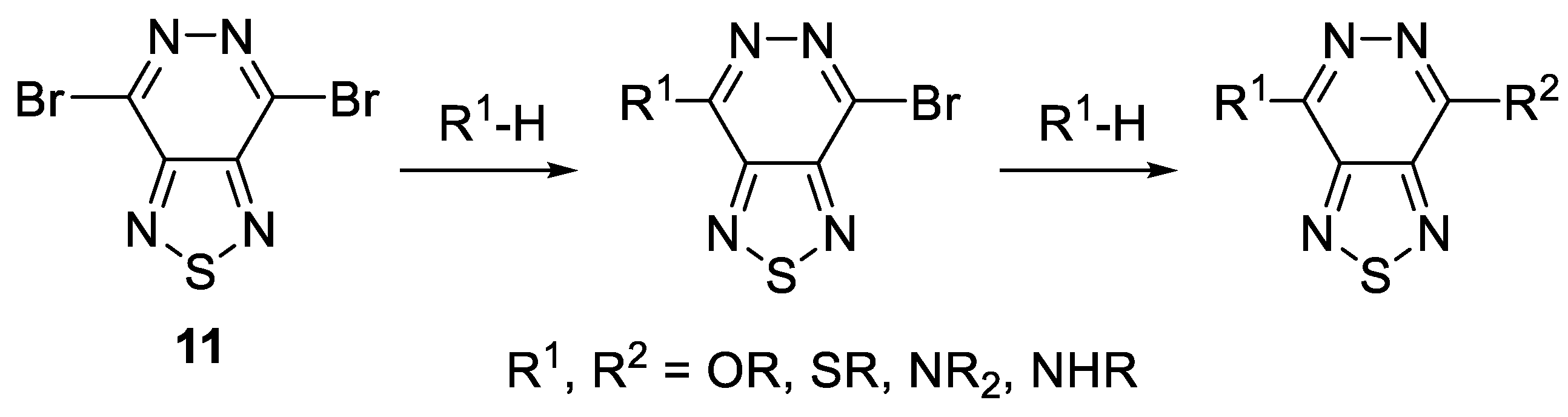
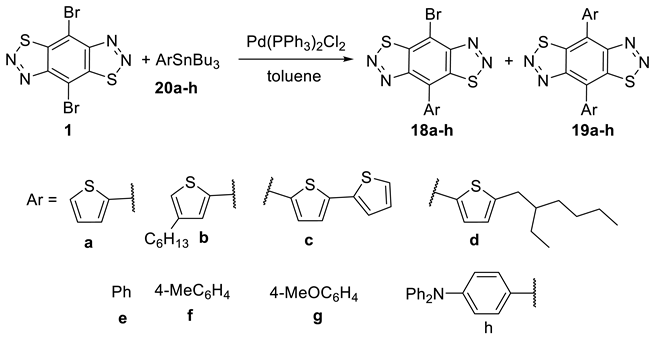
3.12. General Procedure for the Preparation of Bis-Substituted Products 19 under Stille Coupling Conditions (Procedure D)
PdCl2(PPh3)2 (14 mg, 15% mmol) and stannane 20a–h (0.28 mmol) were added to a solution of 4,8-dibromobenzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) 1 (50 mg, 0.14 mmol) in anhydrous toluene (4 mL) The resulting cloudy yellow mixture was stirred and degassed by argon in a sealed vial. The resulting yellow mixture was then stirred at 110 °C for the desired time. On completion (monitored by TLC), the mixture was washed with water and the organic layer was extracted with CH2Cl2 (3 × 35 mL), dried over MgSO4 and then concentrated in vacuo. The products were isolated by column chromatography.
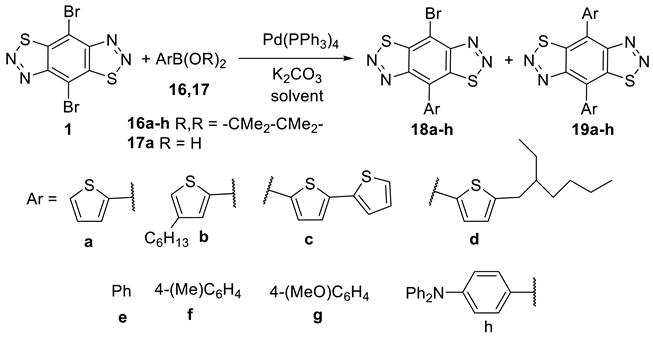
3.12.1. 4-Bromo-8-(thiophen-2-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18a)
Yellow solid, 35 mg (72%, procedure A) or 34 mg (70%, procedure C), eluent—CH2Cl2:hexane, 1:1 (v/v).Rf = 0.4 (CH2Cl2). Mp = 198–200 °C. IR νmax (KBr, cm–1): 1738, 1641, 1494, 1464, 1413, 1262, 1186, 1081, 1023, 809, 701, 544. 1H NMR (300 MHz, CDCl3): δ 8.20 (d, J = 3.9, 1H), 7.76 (d, J = 5.2, 1H), 7.36 (t, J = 4.5, 1H). 13C NMR (75 MHz, CDCl3): δ 156.1, 152.2, 139.32, 139.1, 137.0, 131.7, 130.8, 128.8, 122.4, 104.4. HRMS (ESI-TOF), m/z: calcd for C10H479BrN4S3 [M + H]+, 354.8776, found, 354.8772. MS (EI, 70 eV), m/z (I, %): 356 ([M + 2]+, 2), 355 ([M + 1]+, 31), 354 ([M]+, 35), 353 ([M − 1]+, 6), 328 (100), 298 (10), 247 (40), 219 (35), 175 (42), 151 (75), 117 (13), 93 (32), 69 (20), 45 (22).
3.12.2. 4-Bromo-8-(4-hexylthiophen-2-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18b)
Yellow solid, 43 mg (70%, procedure A) or 44 mg (73%, procedure C), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.6 (CH2Cl2:hexane, 1:1). Mp = 67–69 °C. IR νmax (KBr, cm–1): 2954, 2924, 2852, 1642, 1449, 1397, 1371, 1327, 1306, 1263, 1237, 1109, 1089, 892, 812, 727, 581, 537. 1H NMR (300 MHz, CDCl3): δ 8.09 (s, 1H), 7.35 (s, 1H), 2.76 (t, J = 7.7, 2H), 1.73 (p, J = 7.2, 2H), 1.42–1.31 (m, 6H), 0.91 (t, J = 6.9, 3H). 13C NMR (75 MHz, CDCl3): δ 155.9, 152.0, 145.4, 144.7, 138.8, 136.4, 133.3, 125.6, 122.5, 103.8, 31.6, 30.5, 30.4, 28.9, 22.6, 14.1. HRMS (ESI-TOF), m/z: calcd for C16H1679BrN4S3 [M + H]+, 438.9715, found, 438.9730. MS (EI, 70 eV), m/z (I, %): 440 ([M + 1]+, 3), 439 ([M]+, 2), 412 (3), 355 (2), 312 (2), 235 (7), 164 (8), 125 (11), 111 (15), 97 (50), 83 (52), 69 (60), 57 (98), 43 (100), 29 (75).
3.12.3. 4-([2,2′-Bithiophen]-5-yl)-8-bromobenzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18c)
Red solid, 39 mg (65%, procedure A) or 41 mg (67%, procedure C), eluent—CH2Cl2:hexane, 1:1 (v/v). Rf = 0.4 (CH2Cl2:hexane, 1:1 (v/v)). Mp = 130–132 °C. IR νmax (KBr, cm–1): 1639, 1445, 1423, 1375, 1308, 1268, 1123, 892, 812, 689, 537. 1H NMR (300 MHz, CDCl3): δ 8.09 (d, J = 4.0, 1H), 7.40–7.34 (m, 3H), 7.14–7.07 (m, 1H). 13C NMR (75 MHz, CDCl3): δ 156.3, 151.9, 144.9, 143.5, 138.4, 136.2, 135.2, 132.6, 128.4, 126.3, 125.4, 125.0, 122.0, 103.9. HRMS (ESI-TOF), m/z: calcd for C14H679BrN4S4 [M + H]+, 436.8653, found, 436.8642. MS (EI, 70 eV), m/z (I, %): 439 ([M + 2]+, 5), 438 ([M]+, 43), 437 ([M]+, 4), 436 ([M − 1], 39), 410 (37), 382 (10), 329 (52), 301 (20), 257 (62), 233 (71), 225 (20), 149 (60), 127 (55), 117 (53), 93 (70), 69 (100), 45 (95).
3.12.4. 4-Bromo-8-(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18d)
Yellow solid, 44 mg (68%, procedure A) or 45 mg (69%, procedure C), eluent—CH2Cl2:hexane, 1:1 (v/v). Rf = 0.6 (CH2Cl2). Mp = 57–60 °C. IR νmax (KBr, cm–1): 2955, 2922, 2853, 1639, 1455, 1421, 1376, 1307, 1266, 1213, 1104, 892, 813, 544. 1H NMR (300 MHz, CDCl3): δ 8.06 (d, J = 3.8, 1H), 7.01 (d, J = 3.8, 1H), 2.90 (d, J = 6.8, 2H), 1.77–1.69 (m, 1H), 1.45–1.31 (m, 8H), 0.97–0.89 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 156.0, 151.8, 151.6, 144.7, 138.3, 134.4, 131.8, 126.9, 122.7, 103.1, 41.5, 34.4, 32.4, 29.7, 25.6, 23.0, 14.1, 10.8. HRMS (ESI-TOF), m/z: calcd for C18H2079BrN4S3 [M + H]+, 467.0028, found, 467.0019. MS (EI, 70 eV), m/z (I, %): 470 ([M + 2]+, 1), 469 ([M + 1]+, 2), 468 ([M]+, 11), 467 ([M − 1]+, 2), 466 ([M − 2]+, 9), 440 (6), 369 (3), 341 (5), 326 (4), 232 (5), 188 (10), 105 (30), 83 (35), 71 (60), 57 (100), 43 (95), 29 (15).
3.12.5. 4-Bromo-8-phenylbenzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18e)
Yellow solid, 34 mg (70%, procedure A) or 32 mg (67%, procedure C), eluent—CH2Cl2:hexane, 1:1 (v/v). Rf = 0.4 (CH2Cl2). Mp = 163–165 °C. IR νmax (KBr, cm–1): 1725, 1445, 1398, 1371, 1343, 1316, 1275, 1211, 1188, 1155, 1078, 1025, 924, 898, 823, 766, 698, 538. 1H NMR (300 MHz, CDCl3): δ 7.97–7.93 (m, 2H), 7.68–7.61 (m, 3H). 13C NMR (75 MHz, CDCl3): δ 155.8, 153.9, 144.6, 141.6, 136.3, 130.5, 129.5, 129.4, 128.9, 105.3. HRMS (ESI-TOF), m/z: calcd for C12H679BrN4S2 [M + H]+, 348.9212, found, 348.9222. MS (EI, 70 eV), m/z (I, %): 350 ([M+]+, 2), 349 ([M]+, 1), 322 (20), 241 (50), 213 (18), 169 (96), 145 (100), 137 (15), 117 (18), 93 (35), 69 (45), 43 (30).
3.12.6. 4-Bromo-8-(p-tolyl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18f)
Yellow solid, 34 mg (67%, procedure A) or 35 mg (68%, procedure C), eluent—CH2Cl2:hexane, 1:1 (v/v). Rf = 0.5 (CH2Cl2). Mp = 135–137 °C. IR νmax (KBr, cm–1): 2958, 2924, 2854, 1729, 1493, 1461, 1401, 1385, 1283, 1187, 1081, 1023, 969, 894, 812, 723, 491. 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 7.9, 2H), 7.46 (d, J = 7.9, 2H), 2.52 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 155.8, 153.8, 147.2, 144.6, 141.0, 133.4, 130.2, 129.4, 128.1, 104.8, 21.6. HRMS (ESI-TOF), m/z: calcd for C13H879BrN4S2 [M + H]+, 362.9368, found, 362.9370. MS (EI, 70 eV), m/z (I, %): 363 ([M]+, 3), 362 ([M − 1]+, 1), 361 ([M − 2]+, 5), 360 ([M − 3]+, 5), 359 ([M − 4]+, 2), 318 (98), 303 (15), 285 (16), 159 (98), 115 (97), 93 (50), 69 (85), 57 (100), 43 (96).
3.12.7. 4-Bromo-8-(4-methoxyphenyl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (18g)
Yellow solid, 32 mg (60%, procedure A) or 26 mg (50%, procedure C), eluent—CH2Cl2:hexane, 1:1 (v/v). Rf = 0.2 (CH2Cl2). Mp = 125–127 °C. IR νmax (KBr, cm–1): 2957, 2925, 2854, 1641, 1609, 1502, 1460, 1429, 1376, 1301, 1264, 1179, 1081, 1029, 967, 885, 829, 815, 702, 614, 534. 1H NMR (300 MHz, CDCl3): δ 7.92 (d, J = 8.7, 2H), 7.16 (d, J = 8.7, 2H), 3.94 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 161.3, 155.8, 153.8, 144.6, 141.3, 131.1, 128.9, 128.5, 114.9, 104.3, 55.6. HRMS (ESI-TOF), m/z: calcd for C13H879BrN4OS2 [M + H]+, 378.9317, found, 378.9314. MS (EI, 70 eV), m/z (I, %): 380 ([M + 1]+, 7), 379 ([M]+, 5), 350 (20), 271 (80), 199 (90), 175 (100), 132 (55), 28 (9).
3.12.8. 4-(8-Bromobenzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole)-4-yl)-N,N-diphenylaniline (18h)
Red solid, 52 mg (72%, procedure A) or 46 mg (64%, procedure C), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.5 (CH2Cl2:hexane, 1:1 (v/v)). Mp = 165–168 °C. IR νmax (KBr, cm–1): 1588, 1468, 1432, 1331, 1315, 1274, 1193, 1075, 1026, 814, 753, 695, 618, 512. 1H NMR (300 MHz, CDCl3): δ 7.83 (d, J = 8.7, 2H), 7.35 (t, J = 7.7, 4H), 7.24–7.08 (m, 8H). 13C NMR (75 MHz, CDCl3): δ 155.9, 153.7, 150.0, 146.8, 144.7, 141.0, 130.6, 129.7, 129.1, 128.4, 125.9, 124.5, 121.4, 103.8. HRMS (ESI-TOF), m/z: calcd for C24H1479BrN5S2 [M]+, 514.9869, found, 514.9874. MS (EI, 70 eV), m/z (I, %): 519 ([M + 2]+, 5), 518 ([M + 1]+, 10), 517 ([M]+, 75), 516 ([M − 1]+, 11), 515 ([M − 2]+, 70), 489 (8), 459 (6), 408 (50), 380 (35), 336 (100), 312 (65), 167 (12), 149 (15), 77 (20), 51 (11).
3.12.9. 4,8-Di(thiophen-2-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19a)
Red solid, 32 mg (65%, procedure B) or 33 mg (67%, procedure D), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.5 (CH2Cl2:hexane, 1:1 (v/v)). Mp > 250 °C. IR νmax (KBr, cm–1): 1637, 1513, 1442, 1412, 1376, 1341, 1315, 1267, 1064, 819, 797, 700, 549. 1H NMR (300 MHz, CDCl3): δ 8.20 (dd, J = 3.8, 1.2, 2H), 7.74 (dd, J = 5.1, 1.2, 2H), 7.37 (dd, J = 5.1, 3.8, 2H). 13C NMR (75 MHz, CDCl3): δ 154.1, 139.6, 137.7, 131.1, 130.2, 128.4, 120.2. HRMS (ESI-TOF), m/z: calcd for C14H7N4S4 [M + H]+, 358.9548, found, 358.9544. MS (EI, 70 eV), m/z (I, %): 360 ([M + 2]+, 21), 359 ([M + 1]+, 22), 358 ([M]+, 96), 330 (45), 302 (98), 270 (30), 226 (28), 151 (50), 69 (48), 57 (85), 43 (100), 29 (99), 17 (98).
3.12.10. 4,8-Bis(4-hexylthiophen-2-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19b)
Red solid, 46 mg (63%, procedure B) or 47 mg (57%, procedure D), eluent—CH2Cl2:hexane, 1:3 (v/v). Rf = 0.7 (CH2Cl2:hexane, 1:1 (v/v)). Mp = 134–136 °C. IR νmax (KBr, cm–1): 2961, 2928, 2851, 1645, 1461, 1180, 1071, 925, 761, 555, 459. 1H NMR (300 MHz, CDCl3): δ 8.09 (d, J = 1.3, 2H), 7.32 (d, J = 1.3, 2H), 2.77 (t, J = 7.7, 4H), 1.75 (p, J = 7.6, 4H), 1.45–1.33 (m, 12H), 0.94–0.89 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 154.0, 145.0, 139.4, 137.4, 132.7, 125.0, 120.8, 31.6, 30.5, 30.4, 29.0, 22.6, 14.1. HRMS (ESI-TOF), m/z: calcd for C26H31N4S4 [M + H]+, 527.1426, found, 527.1407. MS (EI, 70 eV), m/z (I, %): 528 ([M + 2]+, 37), 527 ([M + 1]+, 50), 526 ([M]+, 98), 479 (11), 455 (40), 441 (93), 427 (30), 413 (42), 329 (25), 235 (40), 165 (98), 120 (70), 105 (99), 69 (96), 55 (99), 29 (100).
3.12.11. 4,8-Di([2,2′-bithiophen]-5-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19c)
Violet solid, 36 mg (50%, procedure B) or 471 mg (57%, procedure D), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.5 (CH2Cl2:hexane, 1:1 (v/v)). Mp > 250 °C. IR νmax (KBr, cm–1): 1728, 1632, 1504, 1456, 1373, 1319, 1276, 1225, 1165, 1122, 1093, 1052, 824, 783, 701, 643, 548, 485. 1H NMR (300 MHz, CDCl3): δ 8.07 (d, J = 4.1 Hz, 2H), 7.39 (d, J = 4.1 Hz, 3H), 7.37 (d, J = 5.1 Hz, 2H), 7.12–7.09 (m, 3H). 13C NMR (75 MHz, CDCl3): δ 153.9, 142.7, 138.9, 136.4, 136.2, 131.9, 128.1, 125.8, 125.0, 124.7, 120.3. HRMS (ESI-TOF), m/z: calcd for C22H11N4S6 [M]+, 522.9302, found, 522.9299. MS (EI, 70 eV), m/z (I, %): 524 ([M + 2]+, 26), 523 ([M + 1]+, 25), 522 ([M]+, 100), 466 (90), 434 (35), 421 (32), 389 (30), 233 (80), 201 (50), 177 (45), 127 (30), 18 (60).
3.12.12. 4,8-Bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19d)
Red solid, 55 mg (68%, procedure B) or 58 mg (72%, procedure D), eluent—CH2Cl2:hexane, 1:4 (v/v). Rf = 0.7 (CH2Cl2:hexane). Mp = 78–80 °C. IR νmax (KBr, cm–1): 2957, 2924, 2855, 1664, 1376, 1313, 1272, 1245, 1139, 1088, 1013, 871, 816, 782, 703, 642, 545, 508. 1H NMR (300 MHz, CDCl3): δ 8.01 (d, J = 3.8, 2H), 7.00 (d, J = 3.8, 2H), 2.90 (d, J = 6.8, 4H), 1.73 (p, J = 5.9, 2H), 1.46–1.30 (m, 16H), 0.94–0.90 (m, 12H). 13C NMR (75 MHz, CDCl3): δ 153.7, 150.5, 138.6, 135.3, 131.1, 126.8, 120.3, 41.5, 34.4, 32.5, 28.9, 25.7, 23.0, 14.2, 10.9. HRMS (ESI-TOF), m/z: calcd for C30H39N4S4 [M + H]+, 583.2052, found, 583.2045. MS (EI, 70 eV), m/z (I, %): 584 ([M + 2]+, 4), 583 ([M + 1]+, 6), 582 ([M]+, 27), 525 (2), 497 (3), 483 (6), 427 (8), 343 (6), 328 (7), 252 (4), 177 (12), 164 (8), 121 (6), 57 (100), 41 (97), 29 (60).
3.12.13. 4,8-Diphenylbenzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19e)
Yellow solid, 24 mg (50%, procedure B) or 31 mg (65%, procedure D), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.5 (CH2Cl2:hexane, 1:1 (v/v)). Mp > 250 °C. IR νmax (KBr, cm–1): 1572, 1491, 1445, 1374, 1317, 1275, 1210, 1186, 1153, 1077, 1024, 978, 923, 898, 823, 766, 743, 696, 643, 539, 476. 1H NMR (300 MHz, CDCl3): δ 7 8.01 (d, J = 7.0, 4H), 7.69–7.58 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 155.3, 141.7, 137.0, 130.0, 129.5, 129.2, 127.9. HRMS (ESI-TOF), m/z: calcd for C18H11N4S2 [M + H]+, 347.0420, found, 347.0421. MS (EI, 70 eV), m/z (I, %): 348 ([M + 2]+, 1), 347 ([M + 1]+, 2), 346 ([M]+, 7), 317 (3), 290 (100), 258 (10), 245 (4), 145 (30), 51 (3).
3.12.14. 4,8-Di-p-tolylbenzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19f)
Yellow solid, 28 mg (55%, procedure B) or 34 mg (65%, procedure D), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.4 (CH2Cl2:hexane 1:1 (v/v)). Mp > 250 °C. IR νmax (KBr, cm–1): 1607, 1446, 1373, 1311, 1277, 1209, 1182, 1119, 1101, 1021, 940, 898, 831, 802, 716, 652, 627, 582, 530, 491. 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 7.9, 4H), 7.90 (d, J = 7.9, 4H), 2.52 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 155.5, 141.7, 140.4, 134.3, 130.0, 129.6, 127.9, 21.6. HRMS (ESI-TOF), m/z: calcd for C20H15N4S2 [M + H]+, 375.0733, found, 375.0725. MS (EI, 70 eV), m/z (I, %): 374 ([M]+, 8), 318 (70), 303 (5), 285 (3), 159 (100), 115 (97), 93 (25), 63 (14), 39 (30).
3.12.15. 4,8-Bis(4-methoxyphenyl)benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole) (19g)
Orange solid, 32 mg (60%, procedure B) or 26 mg (50%, procedure D), eluent—CH2Cl2:hexane, 1:1 (v/v). Rf = 0.2 (CH2Cl2:hexane, 1:1 (v/v)). Mp > 250 °C. IR νmax (KBr, cm–1): 1605, 1519, 1450, 1371, 1316, 1302, 1282, 1256, 1178, 1026, 816, 718, 546, 515. 1H NMR (300 MHz, CDCl3): δ 7.97 (d, J = 8.3, 4H), 7.17 (d, J = 8.3, 4H), 3.95 (s, 6H). HRMS (ESI-TOF), m/z: calcd for C20H15N4O2S2 [M + H]+, 407.0631, found, 407.0626. MS (EI, 70 eV), m/z (I, %): 407 ([M + 1]+, 1), 406 ([M]+, 10), 335 (100), 307 (25), 292 (12), 264 (8), 175 (20), 160 (21), 132 (98), 93 (6), 15 (70).
3.12.16. 4,4′-(Benzo[1,2-d:4,5-d’]bis([1,2,3]thiadiazole)-4,8-diyl)bis(N,N-diphenylaniline) (19h)
Red solid, 63 mg (67%, procedure B) or 65 mg (69%, procedure D), eluent—CH2Cl2:hexane, 1:2 (v/v). Rf = 0.5 (CH2Cl2:hexane, 1:1 (v/v)). Mp > 250 °C. IR νmax (KBr, cm–1): 1587, 1514, 1489, 1444, 1329, 1315, 1277, 1192, 1176, 841, 817, 753, 734, 695, 653, 620, 509. 1H NMR (300 MHz, CDCl3): δ 7.85 (d, J = 8.2, 4H), 7.35–7.30 (m, 8H), 7.24–7.08 (m, 16H). 13C NMR (75 MHz, CDCl3): δ 155.4, 149.4, 147.0, 141.2, 130.5, 129.6, 129.5, 127.0, 125.6, 124.0, 121.6. HRMS (ESI-TOF), m/z: calcd for C42H29N6S2 [M + H]+, 681.1890, found, 681.1901.