New Insight into CO2 Reduction to Formate by In Situ Hydrogen Produced from Hydrothermal Reactions with Iron
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Analysis of Geometric Parameters
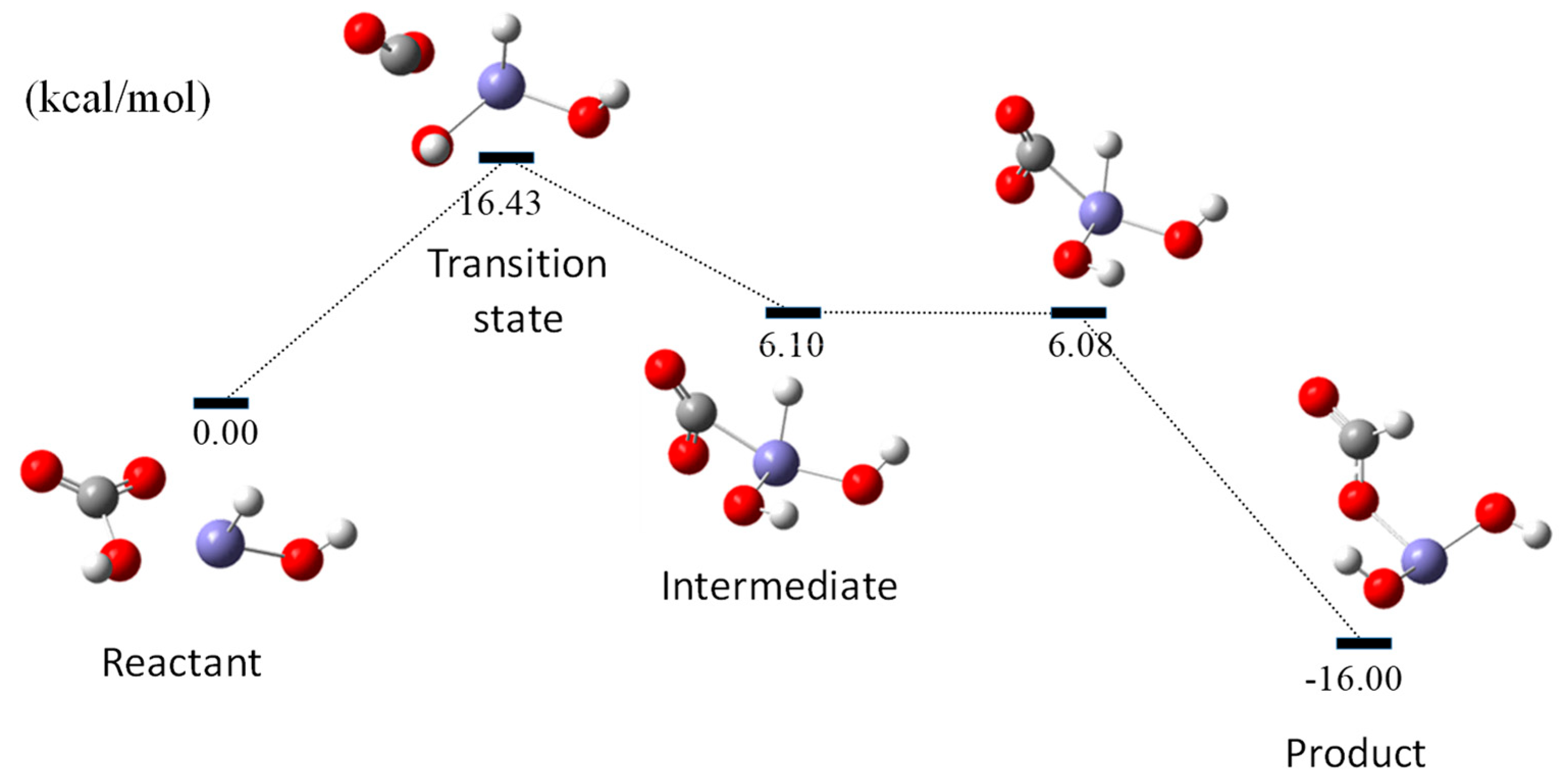
3.2. Energy Diagram for HCOO Production
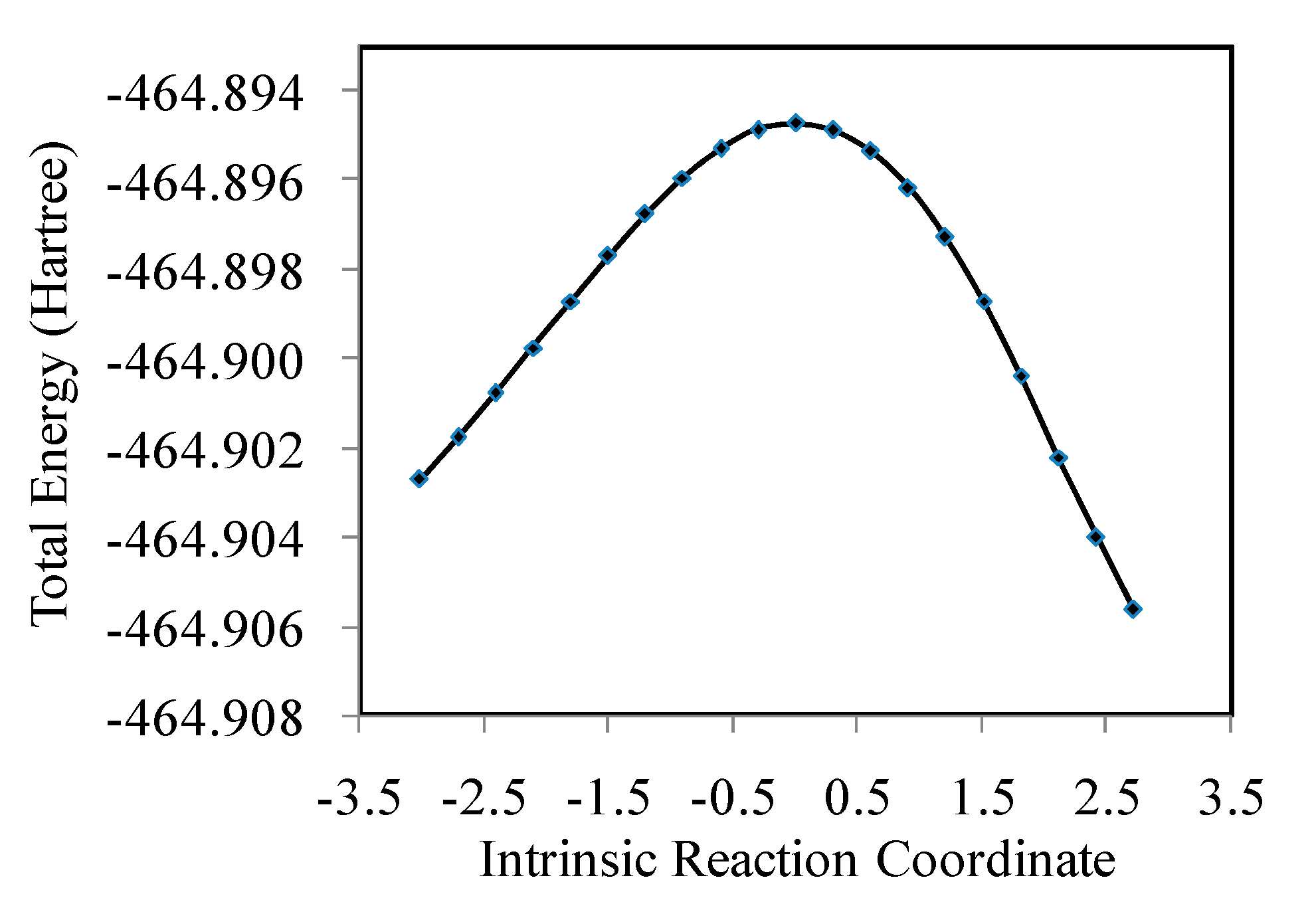
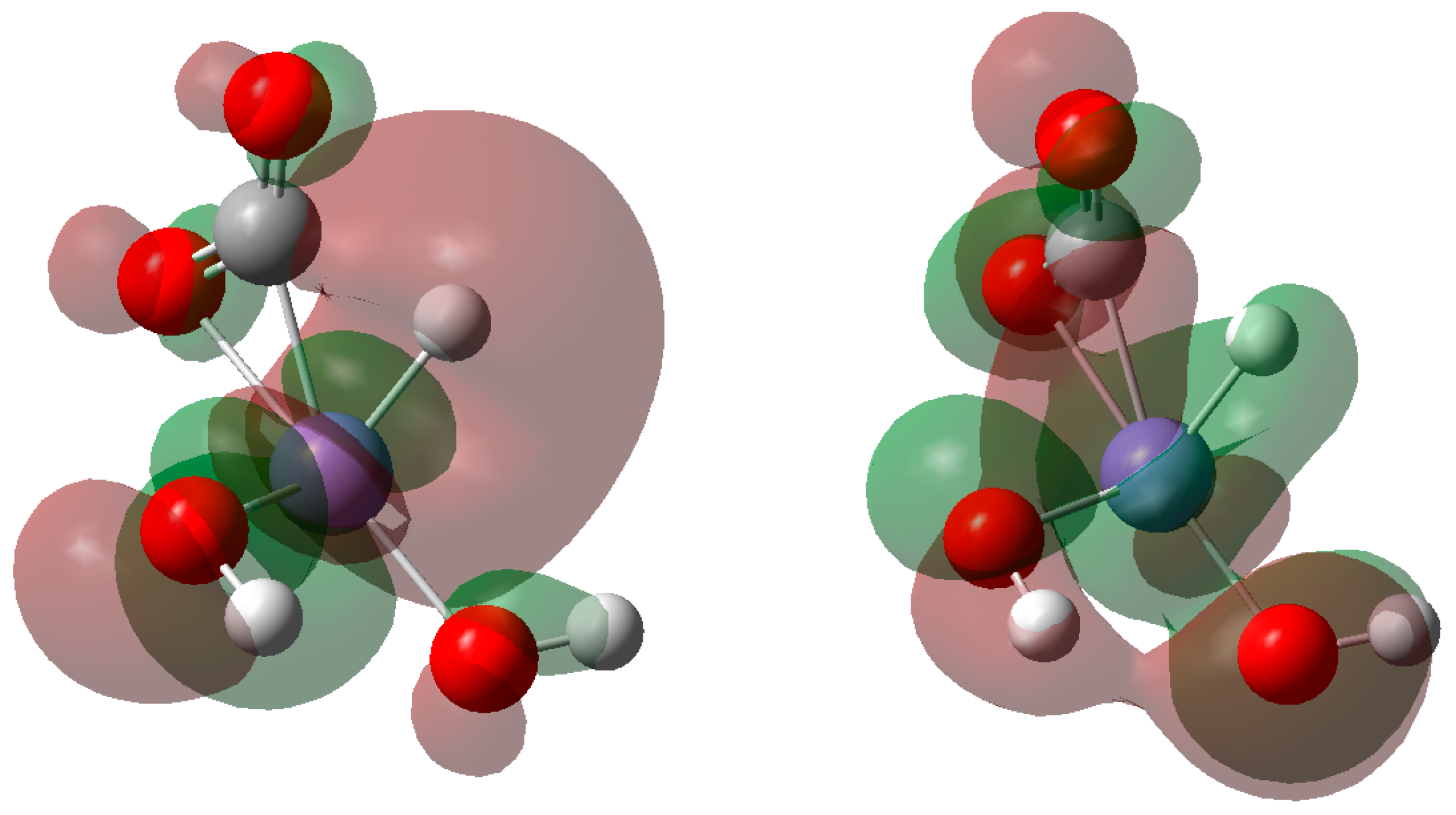
3.3. IRC and HOMO/LUMO Calculation of Transition State
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, M.; Niu, D. Modeling CO2 emissions from fossil fuel combustion using the logistic equation. Energy 2011, 36, 3355–3359. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Huff, C.A.; Sanford, M.S. Cascade catalysis for the homogeneous hydrogenation of CO2 to methanol. J. Am. Chem. Soc. 2011, 133, 18122–18125. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Koike, K.; Inoue, H.; Ishitani, O. Development of an efficient photocatalytic system for CO2 reduction using rhenium(I) complexes based on mechanistic studies. J. Am. Chem. Soc. 2008, 130, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.M.; Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: Chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
- Takahashi, H.; Kori, T.; Onoki, T.; Tohji, K.; Yamasaki, N. Hydrothermal processing of metal based compounds and carbon dioxide for the synthesis of organic compounds. J. Mater. Sci. 2008, 43, 2487–2491. [Google Scholar] [CrossRef]
- Tian, G.; He, C.; Chen, Y.; Yuan, H.M.; Liu, Z.W.; Shi, Z.; Feng, S.H. Hydrothermal Reactions from Carbon Dioxide to Phenol. ChemSusChem 2010, 3, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Gabor, L. Formic Acid as a Hydrogen Source-Recent Developments and Future Trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar]
- Galvez, M.E.; Loutzenhiser, P.G.; Hischier, I.; Steinfeld, A. CO2 splitting via two-step solar thermochemical cycles with Zn/ZnO and FeO/Fe3O4 redox reactions: Thermodynamic analysis. Energy Fuels 2008, 22, 3544–3550. [Google Scholar] [CrossRef]
- Hu, Y.H. A highly efficient photocatalyst hydrogenated black TiO2 for the photocatalytic splitting of water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hatakeyama, M.; Ogata, K.; Liu, J.K.; Wang, Y.Q.; Gao, Q.; Fujii, K.; Fujihira, M.; Jin, F.M.; Nakamura, S. New insights into highly efficient reduction of CO2 to formic acid by using zinc under mild hydrothermal conditions: A joint experimental and theoretical study. Phys. Chem. Chem. Phys. 2014, 16, 19836–19840. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Gao, Y.; Yao, G.D.; Zeng, X.; Li, Q.J.; Huo, Z.B.; Jin, F.M. Highly efficient water splitting and carbon dioxide reduction into formic acid with iron and copper powder. Chem. Eng. J. 2015, 280, 215–221. [Google Scholar] [CrossRef]
- Duo, J.; Jin, F.M.; Wang, Y.Q.; Zhong, H.; Lyu, L.Y.; Yao, G.D.; Huo, Z.B. NaHCO3-enhanced hydrogen production from water with Fe and in situ highly efficient and autocatalytic NaHCO3 reduction into formic acid. Chem. Commun. 2016, 52, 3316–3319. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.E.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Yin, G.-D.; Zhou, Y.-Y.; Zhao, J.-F. New Insight into CO2 Reduction to Formate by In Situ Hydrogen Produced from Hydrothermal Reactions with Iron. Molecules 2022, 27, 7371. https://doi.org/10.3390/molecules27217371
Zeng X, Yin G-D, Zhou Y-Y, Zhao J-F. New Insight into CO2 Reduction to Formate by In Situ Hydrogen Produced from Hydrothermal Reactions with Iron. Molecules. 2022; 27(21):7371. https://doi.org/10.3390/molecules27217371
Chicago/Turabian StyleZeng, Xu, Guo-Dong Yin, Yang-Yuan Zhou, and Jian-Fu Zhao. 2022. "New Insight into CO2 Reduction to Formate by In Situ Hydrogen Produced from Hydrothermal Reactions with Iron" Molecules 27, no. 21: 7371. https://doi.org/10.3390/molecules27217371
APA StyleZeng, X., Yin, G.-D., Zhou, Y.-Y., & Zhao, J.-F. (2022). New Insight into CO2 Reduction to Formate by In Situ Hydrogen Produced from Hydrothermal Reactions with Iron. Molecules, 27(21), 7371. https://doi.org/10.3390/molecules27217371





