Abstract
The new 3D coordination polymer (CP) [Mn(L)(HCOO)]n (Mn-CP) [L = 4-(pyridin-4-ylcarbamoyl)benzoate] was synthesised via a hydrothermal reaction using the pyridyl amide functionalized benzoic acid HL. It was characterized by elemental, FT-IR spectroscopy, single-crystal and powder X-ray diffraction (PXRD) analyses. Its structural features were disclosed by single-crystal X-ray diffraction analysis, which revealed a 3D structure with the monoclinic space group P21/c. Its performance as an electrocatalyst for oxygen reduction (ORR), oxygen evolution (OER), and hydrogen evolution (HER) reactions was tested in both acidic (0.5 M H2SO4) and alkaline (0.1 M KOH) media. A distinct reduction peak was observed at 0.53 V vs. RHE in 0.1 M KOH, which corresponds to the oxygen reduction, thus clearly demonstrating the material’s activity for the ORR. Tafel analysis revealed a Tafel slope of 101 mV dec−1 with mixed kinetics of 2e− and 4e− pathways indicated by the Koutecky–Levich analysis. Conversely, the ORR peak was not present in 0.5 M H2SO4 indicating no activity of Mn-CP for this reaction in acidic media. In addition, Mn-CP demonstrated a noteworthy activity toward OER and HER in acidic media, in contrast to what was observed in 0.1 M KOH.
1. Introduction
In recent years, the energy crisis and environmental pollution have driven the development of new efficient and “green” technologies for renewable energy conversion and storage, such as water splitting, metal–air batteries and fuel cells [1,2,3]. These technologies are primarily centered around three key electrocatalytic reactions: hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and oxygen reduction reaction (ORR), whose efficiency largely depends on the performance of catalysts [4,5]. Currently, efficient catalysts for the ORR and HER are considered to be Pt and its derivatives, while Ir/Ru oxides are considered to be the best catalysts for OER. However, the scarcity and high price of precious metals are critical barriers for large-scale practical applications [6,7]. Researchers around the world have addressed their studies on HER, OER and ORR catalysts, such as transition metal carbides (TMC) [8,9,10], carbon-based hybrids [11,12,13], and graphene doped with heteroatoms [14,15,16]. However, the main challenge is to find a non-precious metal-based catalyst with a high catalytic performance comparable to the already mentioned Pt and Ir/Ru oxides.
Metal–organic frameworks (MOFs), also known as coordination polymers (CPs), are materials with broad structural diversity that over the years have developed into an intriguing class of hybrid materials that have been incorporated into many applications [17,18,19,20,21]. In addition, they offer a great possibility of developing multifunctional electrocatalysts [22,23,24]. For example, Zhao et al. reported the catalytic activity of two-dimensional M3(HITP)2 (where HITP = 2,3,6,7,10,11-hexaiminotriphenylene) with M = Fe, Co, Ni, Cu and Zn for HER/OER and OER/ORR as bifunctional electrocatalysts [25]. The multifunctional catalytic activity of this catalyst is attributed to the synergistic effect of M and organic ligands and the electrocatalytic performance of M3(HITP)2 monolayers that can be modulated by changing the M atoms. Their calculations showed that a monolayer of Cu3(HITP)2 is a promising HER/OER bifunctional catalyst for water splitting with low overpotentials of only −0.02/+0.75 V, which can be compared to precious metal catalysts. On the other hand, the Cu3(HITP)2 monolayer represents an OER/ORR bifunctional catalyst with overpotentials of 0.36/0.73 V [25].
Sun and coworkers reported that MBene [where MBenes = 2D metal borides (Mo2B2)]-supported single-atom catalysts (SACs) by embedding a series of transition metal atoms in the Mo vacancy (M@Mo2B2; M = Ti, V, Cr, Mn, Fe, Co, Ni and Cu) as electrocatalysts in HER, OER and ORR [26]. Among these, Ni@Mo2B2 and Cu@Mo2B2 have shown good structural stability and excellent metal conductivity, and were tested as bifunctional electrocatalysts for HER/OER and OER/ORR. Ni@Mo2B2 as HER/OER bifunctional electrocatalyst showed a lower overpotential (0.52 V) than IrO2 (0.56 V) for OER. In addition, Cu@Mo2B2 as OER/ORR bifunctional electrocatalyst displayed a lower overpotential (0.34 V) than Pt (0.45 V) for ORR and a lower overpotential (0.31 V) than IrO2 for OER [26].
Very recently, we have reported a couple of coordination polymers derived from an amide functionalized ligand, which were found to be effective for water splitting reactions [19]. The presence of the amide backbone in combination with the metal center provided a promising material for supercapacitors and electrocatalysts for water splitting [19]. We noticed that the amide-functionalized ligands in MOFs/CPs exhibit a good stability, which is one of the most interesting prerequisites for electrocatalyst and supercapacitor properties. Recent studies have also demonstrated that MOFs/CPs equipped with amide-functionalized ligands display a good stability and exhibit relevant electrocatalyst and supercapacitor properties [19,27,28]. Thus, in line with our continued search for the development of new amide functionalized MOFs/CPs as electrocatalysts for energy storage/conversion reactions, herein we probe a novel Mn(II) CP as a trifunctional electrocatalyst for ORR, OER and HER.
2. Experimental Section
2.1. Materials and Methods
4-Aminopyridine, terepthalic acid, thionylchloride (SOCl2), the metal salt Mn(NO)3·4H2O as well as common organic solvents, were purchased from the Sigma-Aldrich Chemical Co. and used as received. FT-IR spectra were recorded on a Bruker Vertex 70 instrument in KBr pellets. 1H (300 MHz) and 13C (75.45 MHz) NMR spectra were obtained at room temperature (RT) on a Bruker Avance II + 300 (UltraShieldTMMagnet) spectrometer using tetramethylsilane [Si(CH3)4] as an internal reference. Carbon, hydrogen and nitrogen elemental analyses were carried out by the Microanalytical Service of the Instituto Superior Técnico. Single crystal X-ray diffraction data were collected using a Bruker APEX-II PHOTON 100 diffractometer with graphite monochromated Mo-Kα (λ = 0.71069) radiation. Powder X-ray diffraction (PXRD) was conducted in a D8 Advance Bruker AXS (Bragg-Brentano geometry) theta-2-theta diffractometer, with copper radiation (Cu-Kα, λ = 1.5406 Å) and a secondary monochromator, operated at 40 kV and 40 mA. A flat plate configuration was used, and the typical data collection range was between 5 and 40. Thermal properties were studied using a Perkin-Elmer Instrument system (STA6000) at a heating rate of 10 °C/min under a dinitrogen atmosphere and a flow rate of 30 mL/min. Sorption parameters were obtained from nitrogen adsorption isotherms at 77 K, using a Micromeritics ASAP 2060 SurfaceArea Analyzer with prior degassing at 130 °C for 12 h.
2.2. Synthesis and Characterization
2.2.1. Synthesis of 4-(Pyridin-4-ylcarbamoyl) Benzoic Acid (HL)
Synthesis of the pro-ligand HL was carried out in accordance with the previously reported procedure [29].
2.2.2. Synthesis of [Mn(L)(HCOO)]n (Mn-CP)
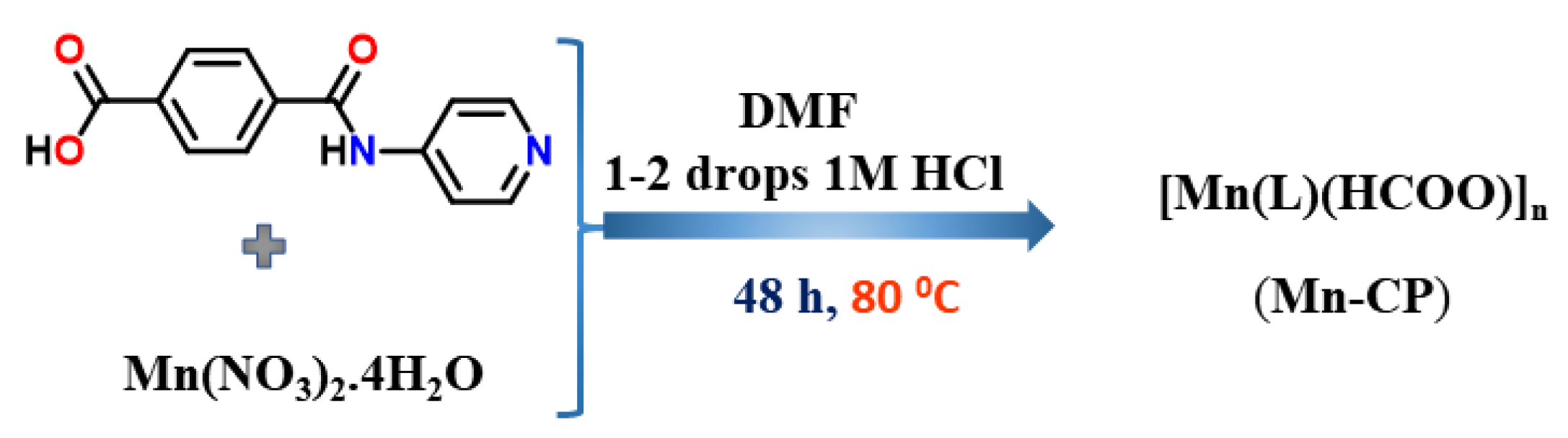
In a 5 mL glass vial, the pro-ligand HL (0.10 g, 0.41 mmol) was added to 2 mL of DMF and heated until the solution became homogeneous. With the addition of Mn(NO3)2·4H2O (0.10 g. 0.41 mmol), a few drops of 1 M HCl solution were added, and the reaction mixture was placed in a reactor for 48 h at 80 °C. We then separated the white crystals of Mn-CP formed at the bottom of the glass vial by filtration, washed them with deionized water and DMF, and then dried them in air. The white crystals of Mn-CP are insoluble in common solvents like CH2Cl2, CHCl3, MeOH, EtOH, ACN, DMF, DMSO. Isolated yield = 0.058 g (58%). Anal. Calcd for C14H10MnN2O5: C, 49.29; H, 2.95; N, 8.21; Found C, 49.36; H, 3.08; N, 8.37. FT-IR (cm−1): 1562 cm−1 νsy(OCO), 1345 cm−1 νasy(OCO).
2.2.3. Crystal Structure Determination
X-ray quality crystals of Mn-CP were immersed in cryo-oil, and a selected one was mounted in a Nylon loop and measured at 150 K. Intensity data were collected using a Bruker APEX II SMART CCD diffractometer with graphite monochromated Mo-Kα (λ = 0.71073) radiation. Cell parameters were obtained with Bruker SMART [30] software and refined with Bruker SAINT [30] on all the observed reflections. Absorption corrections were made by the multi-scan method (SADABS) [30]. Structures were solved by direct methods using the SHELXS-2014 package and refined with SHELXL-2014/7 [31]. Calculations were performed using the WinGX System-Version 2014.1 [32]. The hydrogen atoms attached to carbon and N-amide atoms were inserted at geometrically calculated positions and included in the refinement using the riding-model approximation. The least square refinements with anisotropic thermal motion parameters for all the non-hydrogen atoms were employed. Crystallographic data are summarized in Tables S1 and S2. CCDC 2,206,351 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
2.2.4. Electrochemical Measurements
The catalytic ink was made by dispersing 5 mg of the catalyst into 825 µL of a solution containing 450 µL of distilled water, 300 µL of absolute ethanol, and 75 μL of Nafion (0.5%). A small amount of Vulcan (0.4 mg Cabot Vulcan XC-72R) was added in order to increase the low conductivity of Mn-CP. The ink was ultrasonically mixed for 30 min and then 20 µL of ink was loaded onto a polished glassy carbon rotating disc electrode (RDE, 0.19625 cm2). The electrode was dried by blowing high purity N2 over it. For comparison purposes, catalytic ink was prepared with pure Mn-CP as well as with pure Vulcan.
All electrochemical measurements were performed at room temperature (25 °C) using Gamry Interface 1010 galvanostat/potentiostat with a standard three-electrode glass cell connected to a Gamry rotator (Gamry RDE710 Rotating Electrode). Counter and reference electrodes were employed with a graphite rod and saturated calomel electrode (SCE), respectively. All potentials have been converted (and presented) to the reversible hydrogen electrode (RHE) scale using the equation E(RHE) = E(SCE) + 0.242 + 0.059 pH.
The atmosphere was controlled by bubbling high-purity gases (O2 or N2, Messer, 99.9995 vol.%) into 0.1 M KOH (Sigma-Aldrich, Taufkirchen, Germany) or 0.5 M H2SO4 (Sigma-Aldrich, Taufkirchen, Germany) electrolyte solution. Electrochemical characterization and investigation of catalytic activity of the sample for ORR, HER and OER were performed using standard electrochemical methods: cyclic voltammetry (CV), linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and chronoamperometry. Voltammograms were recorded at a scan rate of 20 mV s−1 at rotation speeds of 0, 300, 600, 900, 1200, 1800, 2400, and 3600 rpm. Voltammograms were also recorded in an N2-saturated solution at different scan rates from 10 mV s−1 to 100 mV s−1. The stability and the selectivity of the catalyst were tested in O2-saturated 0.1 M KOH in chronoamperometric mode at a constant potential of 0.56 V. OER and HER polarization curves were recorded in 0.1 M KOH or 0.5 M H2SO4 at a scan rate of 20 mV s−1 at 1200 rpm. Electrochemical impedance spectroscopy (EIS) was carried out in the frequency range of 100 kHz to 0.1 Hz, with 10 mV amplitude under both OER and HER polarization conditions in alkaline and acidic media. Potentials used were −0.4 V for HER and 1.7 V for OER conditions in alkaline medium, and −0.6 V for HER and 1.6 V for OER conditions in acidic media. A stability test under OER (constant potential of 1.4 V) and HER (constant potential of −0.5 V) conditions was done in 0.5 M H2SO4 in chronoamperometric mode during 10 h.
3. Results and Discussion
3.1. Synthesis and Characterisation
The pro-ligand HL was synthesized using the procedure previously described [29]. As shown in Scheme 1, [Mn(L)(HCOO)]n (Mn-CP) was produced hydrothermally by reacting the pro-ligand HL with Mn(NO3)2·3H2O in DMF and adding a few drops of 1 M HCl acid. A combination of elemental analysis, IR and multinuclear NMR (1H and 13C) techniques was used to characterize HL, all of which agreed with the previous report [29]. On the other hand, Mn-CP has been characterized by elemental analysis, IR spectroscopy and single-crystal X-ray diffraction analysis (presented below). In the FT-IR spectrum of the pro-ligand HL, a band associated with [ν(OCO)] vibration at 1695 cm−1 is observed, while in Mn-CP strong bands due to asymmetric and symmetric stretching vibrations are observed at 1562 cm−1 and 1345 cm−1, respectively for the coordinated carboxylate groups [29]. The powder XRD analysis was also performed to verify that Mn-CP shares the same crystal features as those of the bulk material (Figure S1 Supplementary Material).
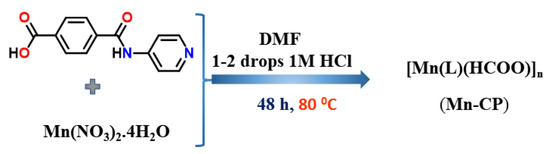
Scheme 1.
Synthesis of Mn-CP.
3.2. Crystal Structure Analysis
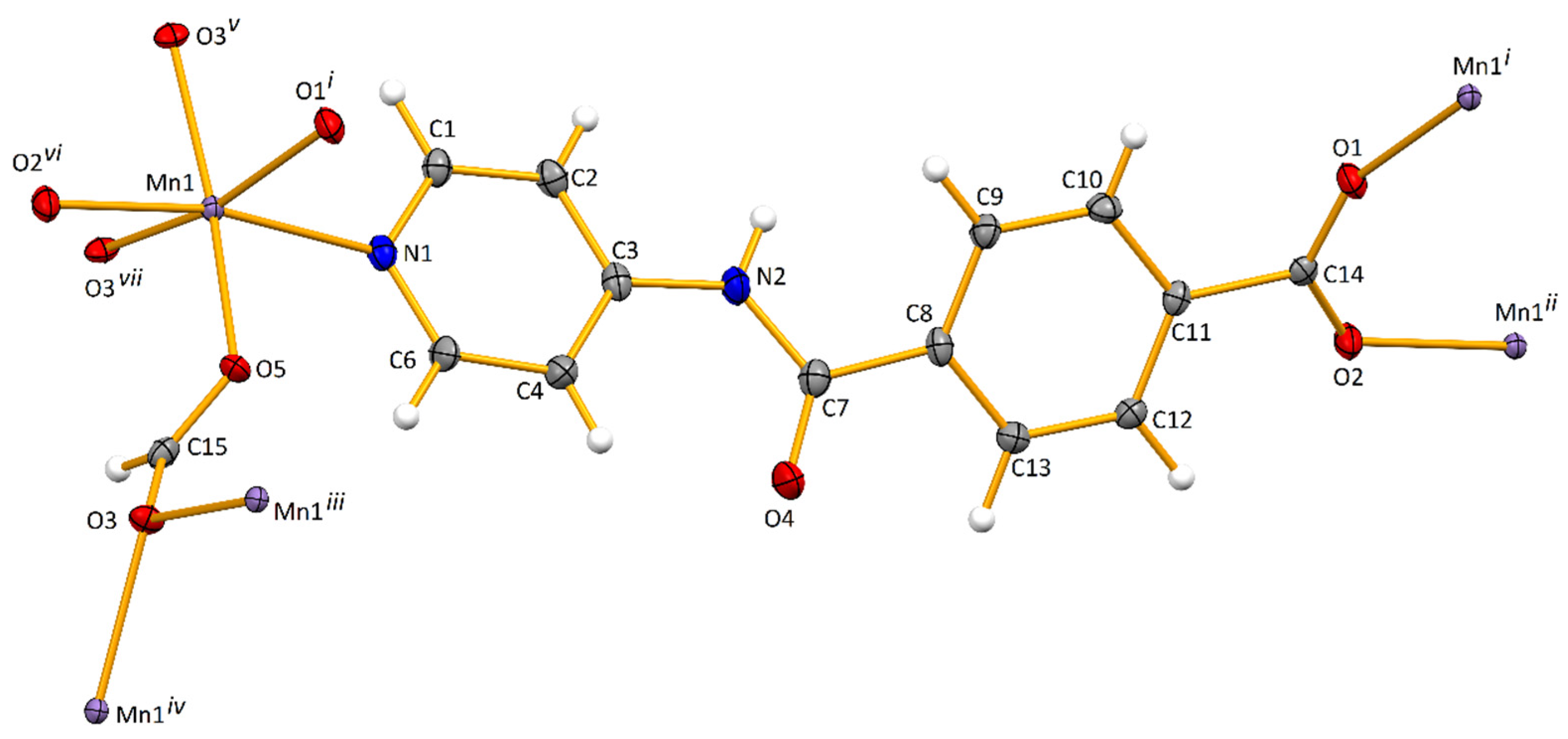
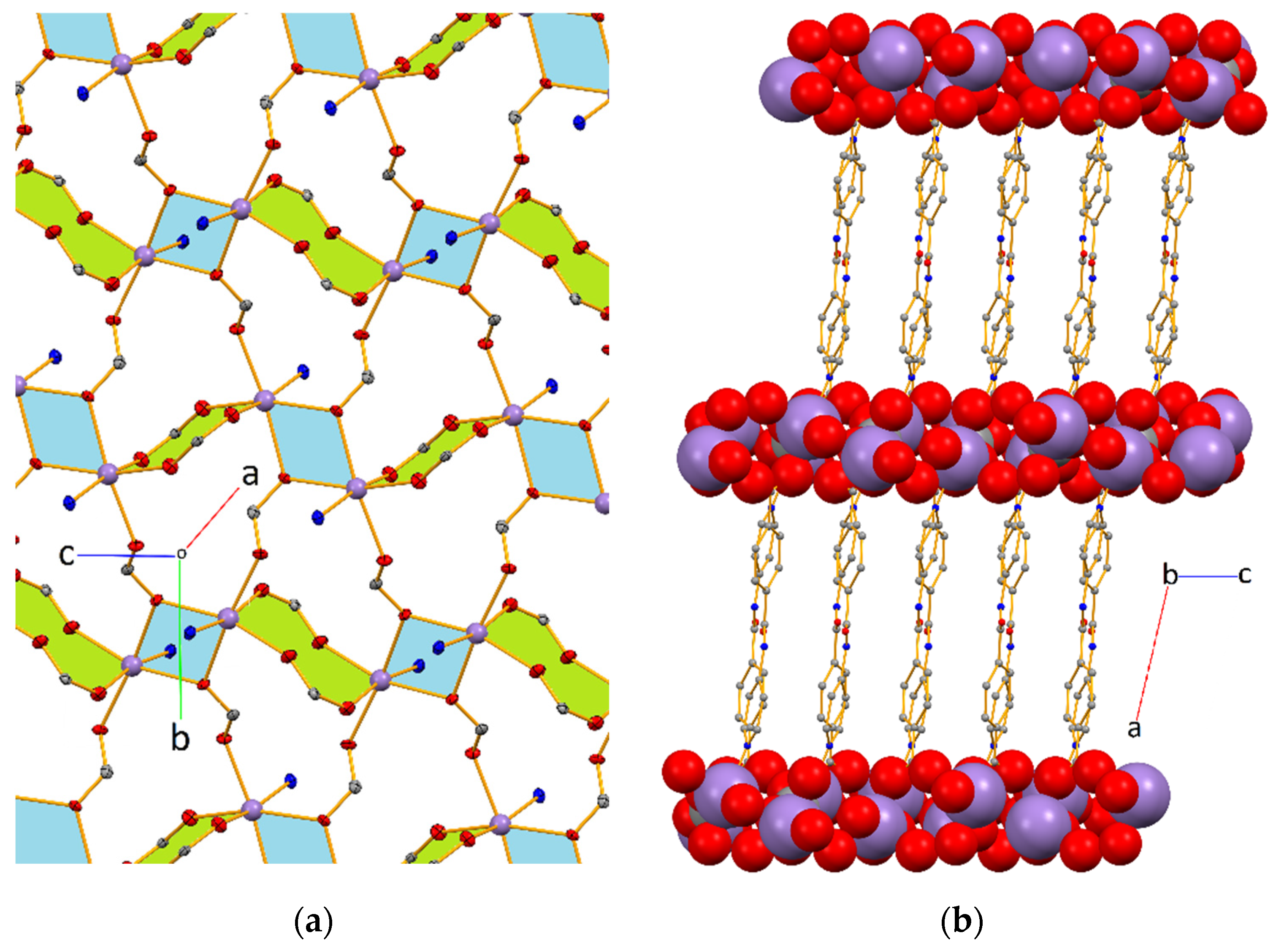
The asymmetric unit of [Mn(L)(HCOO)]n (Mn-CP; Figure 1) contains a pyridyl-based amide ligand (L–) and a formate (HCOO–) coordinated to a manganese(II) ion via the Npyridyl (N1) and one of the Oformate atoms (O5), respectively. Symmetry expansion reveals that L– acts as a bridging 1κN:2κO:3κO’ chelator and formate as an asymmetric bridging anti,syn,syn-1κO:2,3κO’chelator (Figure 1). Therefore, each Mn(II) cation presents a slightly distorted octahedral geometry defined by two Ocarboxylate and one Npyridyl atoms from three independent L– ligands, as well as three O-atoms from three independent formate groups. In this way, chains of corner-shared eight-membered Mn2O4C2 and four-membered Mn2O2 metallacycles are formed, interposed by sets of twelve-membered Mn3O6C3 (Figure 2a). The thus formed 2D layers are spread on the bc crystallographic plane, are separated by L– pillars and lead to the growth of the structure along the crystallographic a axis. The Mn⋯Mn distances in the tetra and hexa metallacycles are of 3.5961(4) and 4.7603(4) Å, respectively. The separation between metal cations bridged by a particular N-ligand assume values of 14.2651(8) and 15.7858(8) Å, as a result of the bending of L– at the level of the Camide and the twisting of the carboxylate group (see below).
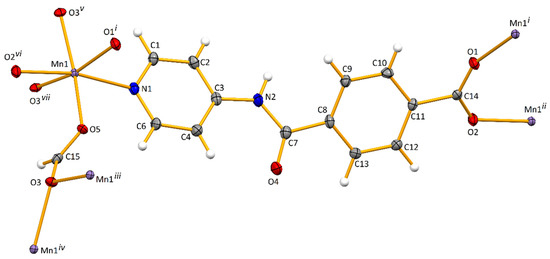
Figure 1.
Ellipsoid plot (drawn at 50% probability level) of Mn-CP with atom labelling scheme. Symmetry operations to generate equivalent atoms: (i) −x,1 − y,−z; (ii) −1 + x,y,z; (iii) x,1/2 − y,−1/2 + z; (iv) 1 − x,−1/2 + y,1/2 − z; (v) 1 − x,1/2 + y,1/2 − z; (vi) 1 + x,y,z; (vii) x,1/2 − y,1/2 + z.
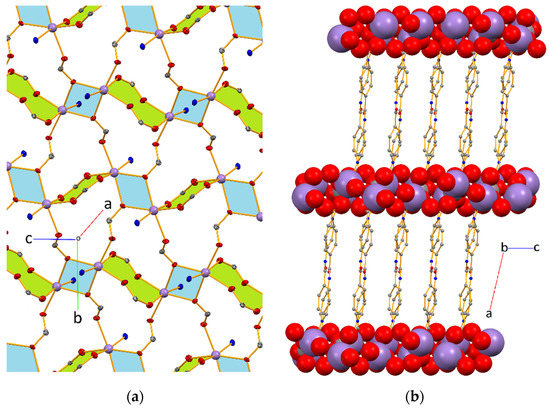
Figure 2.
(a) A fragment of the infinite 2D layer in Mn-CP revealing chains of corner-shared eight-membered Mn2O4C2 (in green) and four-membered Mn2O2 (in blue) metallacycles, intercalated with sets of twelve-membered Mn3O6C3 metallacycles (not highlighted). For clarity, the L– ligands are omitted, except for their O- and N-atoms that define the Mn coordination environments; the H-atoms of formate are also excluded. (b) A 3D packing is viewed down the crystallographic b axis; the Mn cations, the formate and the Ocarboxylate atoms of the L– ligands are drawn in spacefill model.
The Mn-O and Mn-N bond distances (Table S2) are similar, in the range of 2.1495(10)–2.2663(12) Å, and are comparable to those observed in a reported MnII derivative with an analogous N-containing moiety [33]. The L– in Mn-CP is significantly twisted, as shown by the angle of 37.32° between the least-square planes of the pyridyl and phenyl rings (Table S2). This value is higher than those found in copper(II) and zinc(II) MOFs with the same ligand [29], viz. 16.96 and 21.60° in the former, and 10.12° in the latter. The least-square plane of the carboxylate group deviates 22.65° from the one of the phenyl moieties, thus contributing to the significant non-planarity of L– and evading the possibility of efficient π⋯π stacking.
3.3. Thermogravimetric Analysis of Mn-CP
To investigate the Mn-CP thermal stability, thermogravimetric analysis (TGA) was carried out under dinitrogen between 30 and 800 °C with a heating rate of 10 °C per minute. The formate ligand was lost with a weight loss of 26.21% (calculated = 26.75%) between 183 and 240 °C. The system is thermostable in the 240–340 °C range, however above this temperature, the structure collapses and forms MnO2 (Figure S2).
3.4. Surface Area Analysis Using BET Absorption/Desorption Isotherm
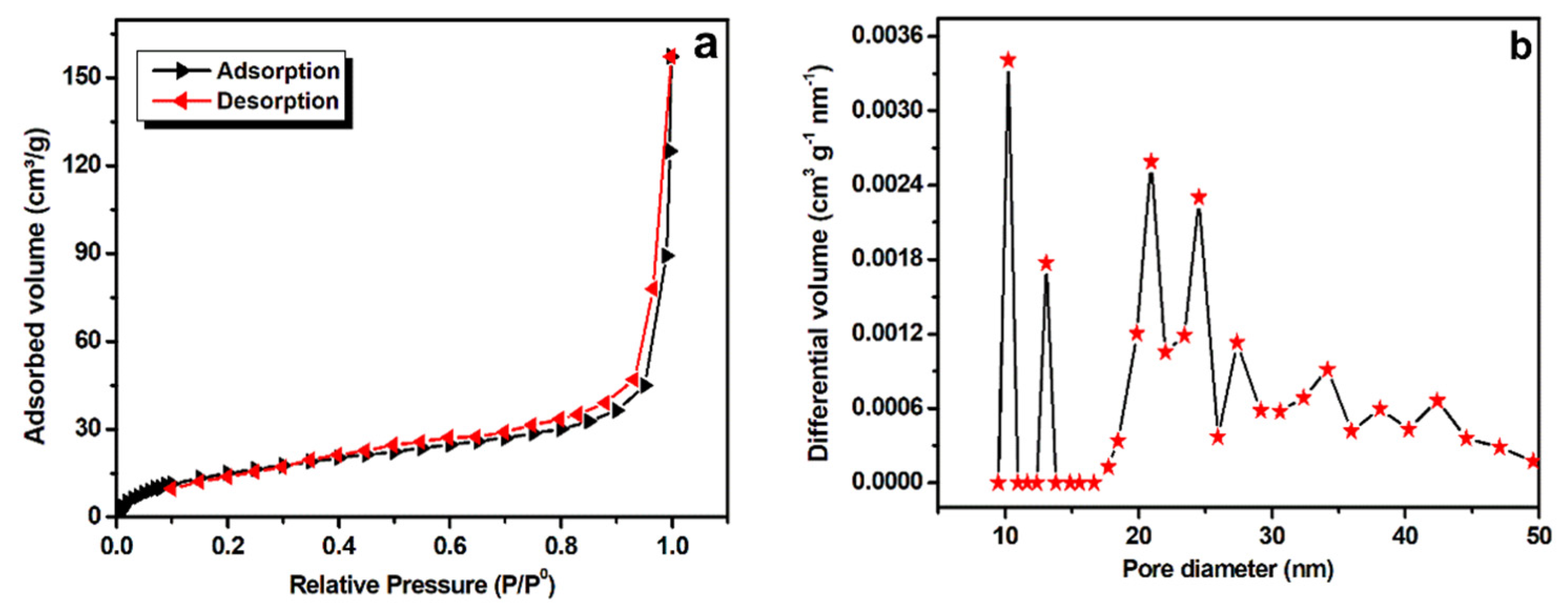
The surface area of Mn-CP was investigated using Brunauer-Emmett-Teller (BET) analysis (Figure 3). From N2-sorption isotherms, the specific surface area was calculated to be 60.2 m2g−1 (Figure 3a), comparable to those of the previously reported analogues [19] and with an average pore size distribution of about 10–50 nm (Figure 3b), suggesting it to be a mesoporous material. The Mn-CP high specific surface area, with its mesoporosity, can provide an unobstructed passage of the electrolyte ions to the redox sites, as well as oxygen/hydroxyl diffusion during the catalysis of oxygen reduction and oxygen/hydrogen evolution (see below).
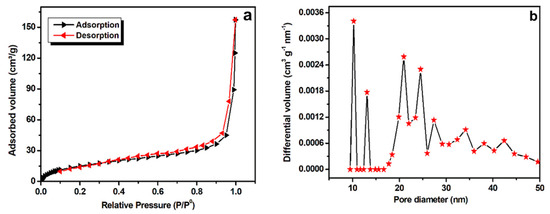
Figure 3.
(a) BET analysis of Mn-CP and (b) average pore distribution calculated from BET analysis data.
3.5. Electrochemical Analysis of Mn-CP
Electrochemical characterization was performed in order to estimate the catalyst (Mn-CP) real surface area (RSA). CVs at different polarization rates were recorded in the potential region near the open circuit potential, Figure S3. By plotting Δj = f(ν), where Δj = ja − jc and ν is scan rate in mV s−1, the double-layer capacity (Cdl) was determined to be ca. 0.11 mF cm−2. This value is higher than that reported for thermally reduced mesoporous manganese MOF@reduced graphene oxide (rGO) nanocomposites [34], and falls between values previously reported for different Cu complexes (0.29 and 0.065 mF cm−2) [35]. The double-layer capacitance is directly proportional to the real surface area (RSE = Cdl/Cref with Cref being the reference value of capacity per the unit area) and the number of active sites on the electrode surface.
3.6. Catalyst Activity of Mn-CP toward ORR, OER and HER in Alkaline Media
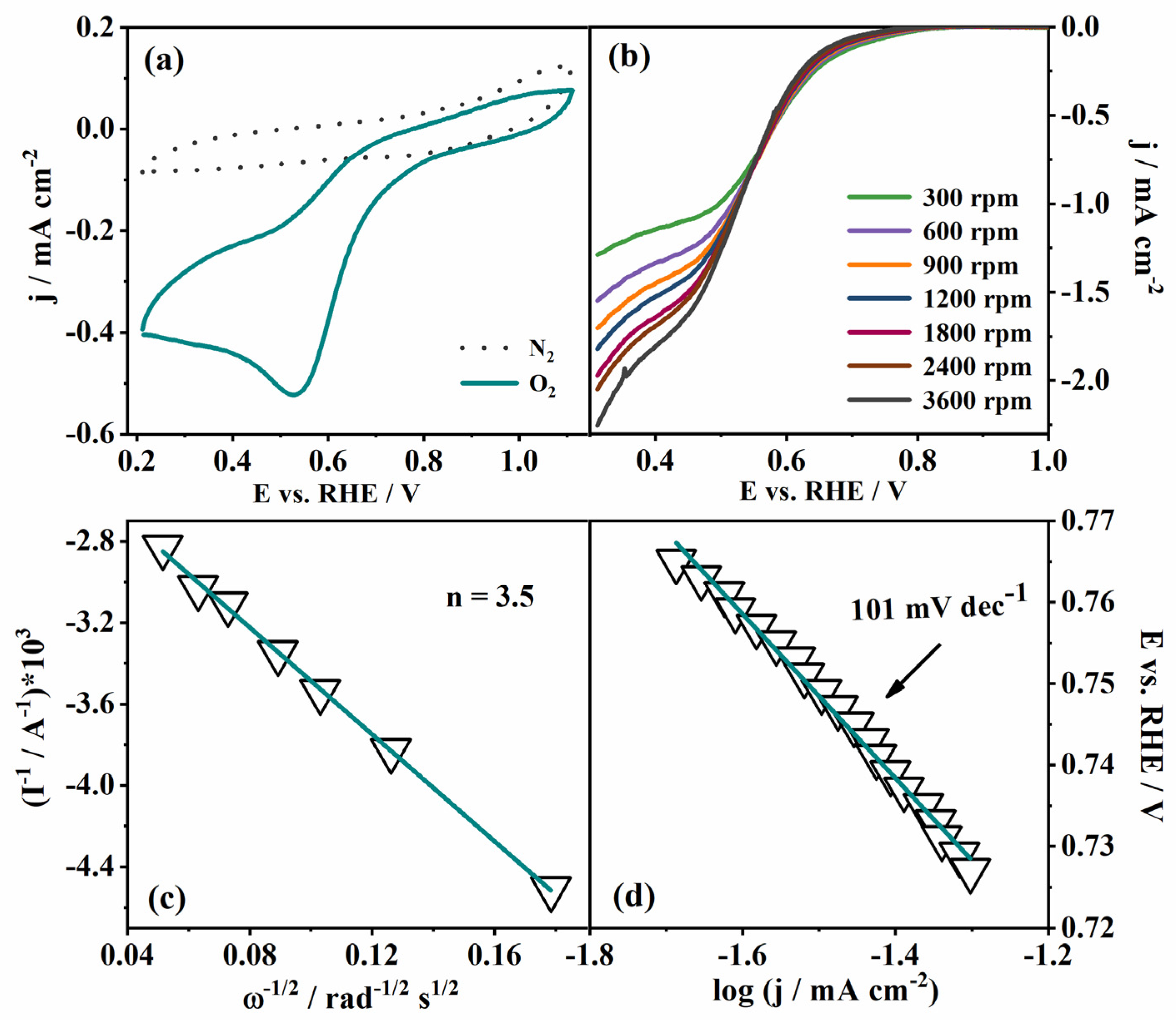
The electrocatalytic activity towards ORR, OER and HER was first investigated in 0.1 M KOH. CVs in ORR potential region in N2- and O2-saturated KOH recorded at 0 rpm (Figure 4a) revealed a distinct peak at ca. 0.53 V corresponding to O2 reduction. This peak potential value shifted toward a less positive potential by approximately 330 mV when compared to the case of the commercial Pt/C (40 wt.% of Pt) electrocatalyst tested in our previous work [2]. Namely, the oxygen reduction peak appears at ca. 0.86 V in case of commercial Pt/C [2]. Furthermore, Wahab et al. reported that peak potential corresponding to O2 reduction of a Mn-BTC MOF (BTC = benzene tricarboxyate) on reduced graphene oxide (MnBTC@rGO) shifts from 0.61 V to 0.78 V, with an increase of rGO percentage from 0 to 75%, ref. [36] while Gonen et al. reported a peak potential of 0.72 V for Mn-BTC MOF at activated carbon (Mn-BTC@AC) [37]. Thus, a comparison of pristine Mn-CP tested herein with those combined with highly conductive, high-surface-area carbon material (rGO, activated carbon) reveals a more positive O2 reduction peak potential in cases of carbon material being present. The addition of a rather small amount of Vulcan within this study indeed illustrated its contribution to the increase of Mn-CP conductivity, resulting in somewhat higher current densities of Mn-CP (with Mn-CP:Vulcan ration 12.5:1) (Figure 4b) compared to pure Mn-CP, Figure S4. Still, we want to address the activity of the pristine CP that remains a challenge, whereas there are reports on the use of CPs in the form of composites or as precursors for electrocatalysts [34].
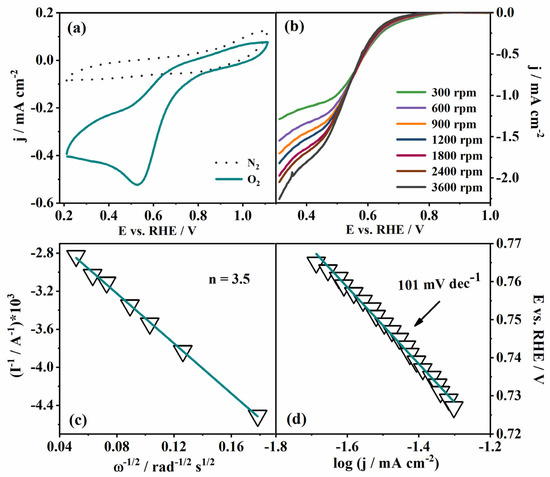
Figure 4.
(a) CVs of Mn-CP in N2- and O2-saturated 0.1 M KOH, and (b) polarization curves at different rotation rates in O2-saturated 0.1 M KOH. (c) Koutecky–Levich analysis and (d) Tafel analysis of the ORR of the Mn-CP material.
LSVs at different rotation rates were recorded in the same potential region (Figure 4b), and the Koutecky–Levich analysis was performed in order to calculate the number of exchanged electrons, n, in the elementary step of oxygen reduction, Figure 4c. The constructed K-L plot is a straight line of good linearity with an n value of 3.5 electrons, indicating a mixed oxygen reduction mechanism with both 2e− and 4e− reduction proceeding simultaneously on the catalyst surface. Additionally, the good linearity of the constructed plot suggests a first-order reaction in the electrolyte solution with respect to oxygen concentration [38]. The determined number of electrons exchanged during ORR at Mn-CP is similar to that previously reported for ORR at Mn-BTC@AC (n = 3.65) [37]. Although the n value is somewhat smaller than that of the commercial Pt/C catalyst (n = 3.96 was reported in our previous work) [2], this is understandable given that Pt is the best-known electrocatalyst for O2-reduction catalysis, but of a notably higher price.
Furthermore, Tafel analysis was performed with 1800 rpm LSV data (Figure 4d) in order to determine the Tafel slope, b, as one of the key ORR kinetics parameters. The Tafel slope value was found to be 101 mV dec−1, which is comparable with other related CP catalysts (Table 1). For example, Wahab and coworkers reported the ORR activity of a Mn-BDC (benzene 1,4-dicarboxylate) compound and its GO (graphene oxide) mixed nanocomposite labelled as MnBDC@rGO [36]. The Tafel slope values of Mn-BDC (138 mV dec−1, Table 1) and MnBDC@rGO (93.5 mV dec−1, Table 1) are comparable to that of our Mn-CP (101 mV dec−1). In another case, Chao et al. reported the ORR activity of the MnII MOF [(Tdc)(4,4′-Bpy)]n (Tdc = thiophene-2,5-dicarboxylate; 4,4′-Bpy = 4,4′-bipyridine), and the Tafel slope value (95 mV dec−1, Table 1) [34] also comparable to that of our Mn-CP. In addition, our Mn-CP displays an even better or comparable ORR activity than some of the Co and Ni-based CPs [39]. The half-wave potential (E1/2) was also determined from the LSV study and found to be 0.58 V, which is comparable to the reported ones [34,35,38,40].

Table 1.
Comparison of key parameters of ORR at Mn-CP in 0.1 M KOH with literature data a.
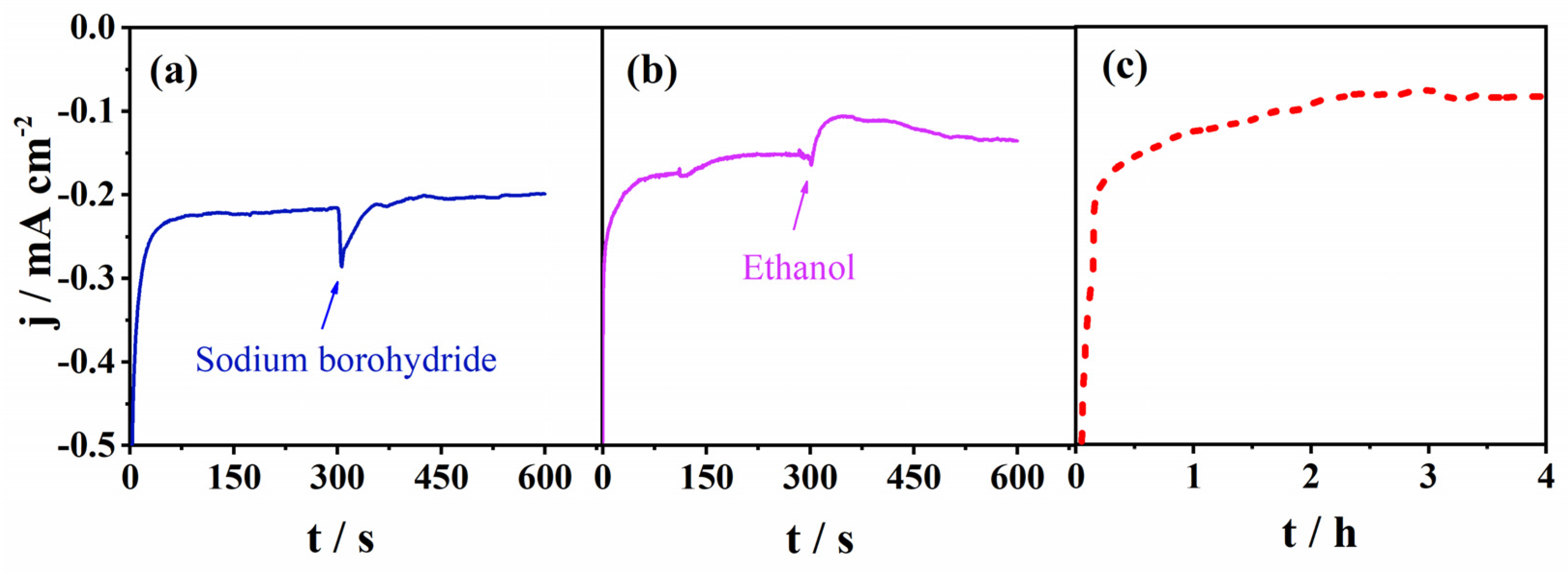
Furthermore, in order to determine its potential use in fuel cells, we investigated the selectivity of the Mn-CP catalyst towards ORR. The Mn-CP catalyst was examined in conditions typical for direct borohydride fuel cells (DBFC) and direct ethanol fuel cells (DEFC). Therefore, sodium borohydride and ethanol were added to 0.1 M KOH saturated with O2 to simulate fuel crossover and to study their effect on ORR kinetics. As shown in Figure 5a, the original cathodic current of Mn-CP showed a negligible cliff-like drop upon the addition of sodium borohydride to the solution and almost instantaneously regained its value. A somewhat more pronounced drop was observed upon the addition of ethanol, Figure 5b. These results clearly demonstrate that Mn-CP possesses a good selectivity toward ORR that is essential for a practical application.
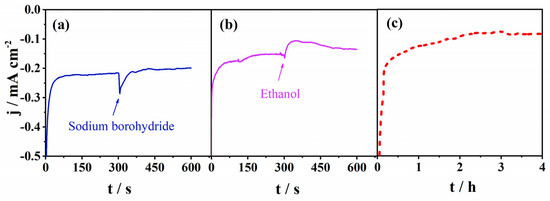
Figure 5.
Chronoamperometric responses of Mn-CP in O2-saturated 0.1 M KOH at a potential of 0.56 V. (a) With the addition of 5 mg of sodium borohydride; (b) with the addition of 3 M ethanol in the 300th s; (c) stability test during 4 h.
Next, the stability was investigated in O2-saturated 0.1 M KOH in the chronoamperometry mode at a constant potential of 0.56 V. The current density during 4 h showed an initial decay and then stabilized, indicating a relatively good stability under ORR polarization conditions, Figure 5c. The initial decay of the current density was higher than expected due to the capacitance current decrease. We believe that this decrease is not solely due to the electrocatalyst’s instability under the specific polarization conditions, but is partially due to the film instability on the conductive support. This could be improved by optimizing the ink composition, which will be presented in a future communication. Moreover, the modular architecture of CPs enables further improvement of the stability of the Mn-CP electrocatalyst by varying the chelating ligand (e.g., the donor group), and thus changing the metal–ligand binding energy.
To investigate the multifunctional abilities of the synthesized electrocatalyst, OER and HER studies were also performed in 0.1 M KOH. However, no catalytic activity of the studied catalyst towards HER and OER was observed, as evidenced by low current densities, Figure S5, and high values of the Tafel slope (798 mV dec−1 for HER and 545 mV dec−1 for OER). An electrochemical impedance spectroscopy (EIS) investigation was performed in order to determine the electrolyte resistance, Rs, and the charge-transfer resistance at the electrocatalyst/electrolyte surface during the electrocatalytic reaction, Rct, in both OER and HER potential regions (Figure S6a). Rs values within OER and HER studies were very close (34 Ω in the case of HER and 41 Ω in the case of OER), indicating a minor change in cell geometry and electrode distance during all performed experiments. Rct was found to be as high as ≈7191 Ω for HER and ≈4089 Ω for OER, illuminating a low OER and HER activity of the studied material in 0.1 M KOH.
3.7. Catalytic Activity of Mn-CP toward ORR, OER and HER in Acidic Media
The catalytic activity toward ORR in acidic media (0.5 M H2SO4) was also investigated by performing cyclic voltammetry experiments in N2- and O2-saturated electrolytes at 0 rpm (Figure S7a), followed by LSV experiments at different rotation speeds (Figure S7b). No measurable difference is observed between current densities in N2- and O2-saturated 0.5 M H2SO4, as well as between current densities at 900 rpm and 1200 rpm, indicating that no oxygen reduction occurred in acidic media on the studied catalyst.
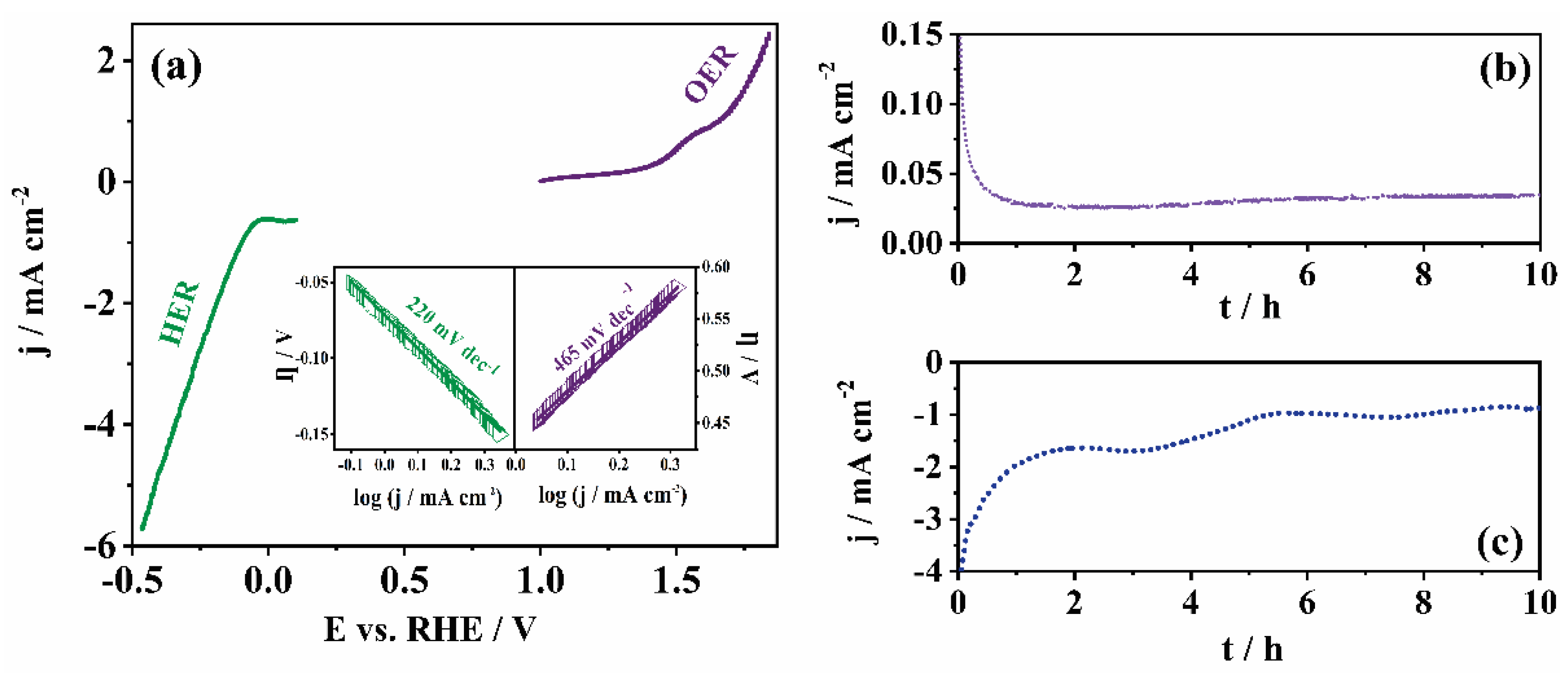
LSV experiments performed in OER and HER potential regions in 0.5 M H2SO4 at 1200 rpm are shown in Figure 6, along with Tafel plots for both reactions in the inset. A higher electrocatalytic activity toward both investigated reactions is observed in acidic media compared to the results in alkaline media (see above), with a notably higher current density recorded.
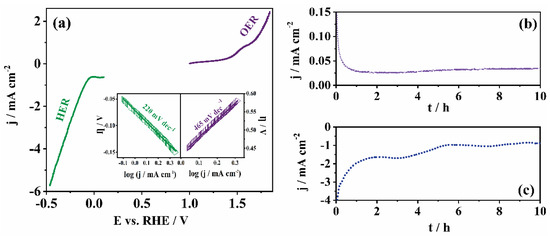
Figure 6.
HER and OER polarization curves in 0.5 M H2SO4 with the corresponding Tafel plots in the inset for Mn-CP (a). Stability tests under (b) OER (constant potential of 1.4 V) and (c) HER (constant potential of −0.5 V) conditions in 0.5 M H2SO4.
An earlier onset of both reactions is also observed in acidic media, and is presented as the onset potential in Table 2 together with other key parameters being compared with literature data. It should be noted that literature data regarding OER experiments with Mn-based MOFs in acidic media are very rare, so we opted for comparing it also with similar materials that were tested in alkaline media. Only recently, Singh et al. demonstrated OER catalysis by a pristine Mn MOF (MnTPA) (TPA = terephthalate) [41]. However, they found that MnTPA alone has a very poor OER activity. This is due to the poor electrical conductivity of Mn and the electrically insulating property of the TPA ligand. With the addition of carbon black (CB) and optimizing the MnTPA to CB ratio, they obtained a MnTPA/CB composite (MnTPA:CB ratio as high as 1:2) which achieved a current density of 10 mA cm−2 at an overpotential of 539 mV. Additionally, a higher loading of this composite material on the working electrode was used. Thus, although Table 2 might not show a better performance of the herein tested electrocatalyst compared to some reported in the literature, it serves to point out possible directions to take in order to improve the tested electrocatalyst’s performance (making a composite with carbon material certainly being one direction to follow).

Table 2.
Comparison of HER and OER parameters of the herein studied catalyst with (Mn-CP) literature data a.
Tafel analysis revealed a significantly lower value of Tafel slope for HER in acidic than that in alkaline media (220 mV dec−1 vs. 798 mV dec−1, respectively), as well as a slightly lower value for OER (465 mV dec−1 vs. 545 mV dec−1 in acidic and alkaline medium, respectively). However, both values are significantly higher than that of commercial Pt/C (68 mV dec−1 for HER and 198 mV dec−1 for OER) [43]. This is again expected, because Pt is well known for its ability to catalyze the hydrogen evolution reaction.
Figure S6b presents Nyquist plots in acidic media at both HER and OER potential regions. Rct in OER conditions was observed to be high (ca. 1944 Ω), while under HER conditions the value was significantly lower (ca. 175 Ω). This difference in the charge transfer resistance under OER and HER polarization conditions might account for the higher activity, i.e., higher current densities recorded under HER conditions.
Thus, promising features of the Mn-CP electrocatalyst may concern its high specific surface area and high porosity, providing a high number of active sites and a large contact area between those sites and electrolytes, with porosity further enabling easier diffusion of reactants/products. The presence of Mn that can exist in different oxidations states further assists the electrocatalysis as, for instance, sites in higher oxidations states are more active for OER. Still, the low conductivity impedes reaching higher current densities.
Further, stability experiments were performed under both OER and HER conditions in chronoamperometry mode for 10 h. A good stability of the tested catalyst under OER conditions was observed (Figure 6b), as well as a relatively good stability under HER conditions (Figure 6c). This indicates that Mn-CP is suitable for applications under harsh conditions in electrolysers. Namely, the current density showed a decrease during the first hour, but then a slight increase was observed during the other 9 h. The HER current densities showed a somewhat more pronounced decrease.
Though Mn-CP showed a lower HER and OER electrocatalytic activity compared to the commercial Pt (40 wt.% Pt) catalyst, i.e., lower current densities (Figure S8a,b, respectively), the promising results and its significantly lower cost make it worthy of further investigation and further studies on the improvement of its catalytic performance.
4. Conclusions
The successful design and synthesis of a novel coordination polymer of Mn(II), Mn-CP, with an amide-appended ligand are presented. The single-crystal X-ray diffraction analysis revealed that it possesses a 3D structure.
The electrocatalytic activity of Mn-CP was tested for ORR, OER and HER in both alkaline and acidic media for the first time. It showed a good performance for ORR in alkaline media, as evidenced by the low Tafel slope and the number of exchanged electrons of 3.5. In addition to the key parameters, the catalyst also showed relatively good stability for 10 h in 0.1 M KOH medium. However, the synthesized material was not active for ORR in an acidic environment.
On the other hand, for HER and OER, the same catalyst showed a better performance (in the form of higher current densities and lower Tafel slopes) in acidic than in alkaline medium; this may be a specially promising finding, as earth-abundant counterparts to noble metal electrocatalys in acidic media are still scarce.
The promising performance of Mn-CP may originate from its high specific surface area and high porosity, providing a high number of active sites and easy access of reactants to those sites. The existence of different oxidation states of Mn may enable the materials applications for electrocatalysis of different reactions. At this moment, its low electric conductivity impedes the achievement of higher current densities.
Despite having a lower electrocatalytic activity than a commercial Pt (40 wt.%) catalyst, the lower cost and good redox activity, along with good stability of Mn-CP, make this CP an interesting avenue for future investigation and improvement. Further research is already underway in this area.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217323/s1, Figures S1–S8 containing PXRD, TGA, CVs, polarization curves and Nyquist plots. Crystallographic data and refinement parameters are presented in Table S1 and selected bond distances and angles in Table S2.
Author Contributions
Conceptualization, A.P. and B.Š.; Formal analysis, A.P., K.R., S.H. and R.A.K.; Investigation, A.P., K.R. and D.M.; Supervision, A.P. and B.Š.; Visualization, A.P., K.R. and D.M.; Writing—original draft, A.P., K.R., S.H. and D.M.; Writing—review & editing, B.Š., M.F.C.G.d.S. and A.J.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
A.P., S.H. and B.Š. is grateful to the FCT and IST, Portugal, for financial support through “DL/57/2017” (Contract no. IST-ID/197/2019, IST-ID/103/2018 and IST-ID/156/2018, respectively). This work has been partially supported by the Fundação para a Ciência e a Tecnologia (FCT), Portugal, through projects UIDB/00100/2020 of Centro de Química Estrutural and PTDC/QUI-QIN/29778/2017. R.A.K. acknowledges King Saud University, Riyadh, Saudi Arabia for financial assistance through the researchers supporting project (RSP-2021/400). This publication has been prepared with the support of the RUDN University Strategic Academic Leadership Program (recipient: A.J.L.P., preparation). The authors would also like to thank the Ministry of Education, Science and Technological Development of Republic of Serbia (contract number: 451-03-68/2022-14/200146).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request to the corresponding author.
Acknowledgments
The authors acknowledge the Portuguese NMR Network (IST-UL Centre) for access to the NMR facility.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compound [Mn(L)(HCOO)]n is available from the authors.
References
- Zdolšek, N.; Rocha, R.P.; Krstić, J.; Trtić-Petrović, T.; Šljukić, B.; Figueiredo, J.L.; Vujković, M.J. Electrochemical Investigation of Ionic Liquid-Derived Porous Carbon Materials for Supercapacitors: Pseudocapacitance versus Electrical Double Layer. Electrochim. Acta 2019, 298, 541–551. [Google Scholar] [CrossRef]
- Mladenović, D.; Santos, D.M.F.; Bozkurt, G.; Soylu, G.S.P.; Yurtcan, A.B.; Miljanić, Š.; Šljukić, B. Tailoring Metal-Oxide-Supported PtNi as Bifunctional Catalysts of Superior Activity and Stability for Unitised Regenerative Fuel Cell Applications. Electrochem. Commun. 2021, 124, 106963. [Google Scholar] [CrossRef]
- Gao, R.; Dai, Q.; Du, F.; Yan, D.; Dai, L. C60-Adsorbed Single-Walled Carbon Nanotubes as Metal-Free, PH-Universal, and Multifunctional Catalysts for Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. J. Am. Chem. Soc. 2019, 141, 11658–11666. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Chen, Y.; Tang, Z.; Liu, Z.; Wang, X.; Tian, Y.; Gao, W. Hollow Nanocages of NixCo1−xSe for Efficient Zinc–Air Batteries and Overall Water Splitting. Nano-Micro Lett. 2019, 11, 28. [Google Scholar] [CrossRef]
- Milikić, J.; Balčiūnaitė, A.; Sukackienė, Z.; Mladenović, D.; Santos, D.M.F.; Tamašauskaitė-Tamašiūnaitė, L.; Šljukić, B. Bimetallic Co-Based (Com, m = Mo, Fe, Mn) Coatings for High-Efficiency Water Splitting. Materials 2021, 14, 92. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Qi, S.; Zhao, M. Stable Multifunctional Single-Atom Catalysts Resulting from the Synergistic Effect of Anchored Transition-Metal Atoms and Host Covalent-Organic Frameworks. J. Phys. Chem. C 2020, 124, 17675–17683. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.H.; Shi, F.; Zhan, J.Y.; Tu, J.P.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2015, 3, 1500286. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Ding, J.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Recent Progresses of Micro-Nanostructured Transition Metal Compound-Based Electrocatalysts for Energy Conversion Technologies. Sci. China Mater. 2021, 64, 1–26. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-Based Electrocatalysts for Sustainable Energy Applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Liu, D.; Dai, L.; Lin, X.; Chen, J.F.; Zhang, J.; Feng, X.; Müllen, K.; Zhu, X.; Dai, S. Chemical Approaches to Carbon-Based Metal-Free Catalysts. Adv. Mater. 2019, 31, 1804863. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gadipelli, S.; Yang, Y.; Li, Z.; Lu, Y.; Brett, D.J.L.; Guo, Z. An Efficient Carbon-Based ORR Catalyst from Low-Temperature Etching of ZIF-67 with Ultra-Small Cobalt Nanoparticles and High Yield. J. Mater. Chem. A 2019, 7, 3544–3551. [Google Scholar] [CrossRef]
- Kasibhatta, K.R.D.; Madakannu, I.; Prasanthi, I. Hetero Atom Doped Graphene Nanoarchitectonics as Electrocatalysts Towards the Oxygen Reduction and Evolution Reactions in Acidic Medium. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1859–1876. [Google Scholar] [CrossRef]
- Hu, C.; Liu, D.; Xiao, Y.; Dai, L. Functionalization of Graphene Materials by Heteroatom-Doping for Energy Conversion and Storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 121–132. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.Z. Activity Origin and Catalyst Design Principles for Electrocatalytic Hydrogen Evolution on Heteroatom-Doped Graphene. Nat. Energy 2016, 1, 16130. [Google Scholar] [CrossRef]
- Qiu, T.; Gao, S.; Liang, Z.; Wang, D.G.; Tabassum, H.; Zhong, R.; Zou, R. Pristine Hollow Metal–Organic Frameworks: Design, Synthesis and Application. Angew. Chem.-Int. Ed. 2021, 60, 17314–17336. [Google Scholar] [CrossRef]
- Paul, A.; Das, K.; Karmakar, A.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. A Mechanistic Insight into the Rapid and Selective Removal of Congo Red by an Amide Functionalised Zn(Ii) Coordination Polymer. Dalton Trans. 2020, 49, 12970–12984. [Google Scholar] [CrossRef]
- Paul, A.; Upadhyay, K.K.; Backović, G.; Karmakar, A.; Vieira Ferreira, L.F.; Šljukić, B.; Montemor, M.F.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Versatility of Amide-Functionalized Co(II) and Ni(II) Coordination Polymers: From Thermochromic-Triggered Structural Transformations to Supercapacitors and Electrocatalysts for Water Splitting. Inorg. Chem. 2020, 59, 16301–16318. [Google Scholar] [CrossRef]
- Paul, A.; Martins, L.M.D.R.S.; Karmakar, A.; Kuznetsov, M.L.; Novikov, A.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Environmentally Benign Benzyl Alcohol Oxidation and C-C Coupling Catalysed by Amide Functionalized 3D Co(II) and Zn(II) Metal Organic Frameworks. J. Catal. 2020, 385, 324–337. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, W.; Sun, D. Flexible Metal–Organic Frameworks for Gas Storage and Separation. Dalton Trans. 2022, 51, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Feng, X.; Dong, R. Conductive 2D Conjugated Metal–Organic Framework Thin Films: Synthesis and Functions for (Opto-)Electronics. Small Struct. 2022, 3, 2100210. [Google Scholar] [CrossRef]
- Jia, Y.; Xue, Z.; Li, Y.; Li, G. Recent Progress of Metal Organic Frameworks-Based Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reaction. Energy Environ. Mater. 2022, 1–19. [Google Scholar] [CrossRef]
- Imran, M.; Ikram, M.; Dilpazir, S.; Naseem, B.; Lin, Y.; Pan, J. Functionality and Design of Co-MOFs: Unique Opportunities in Electrocatalysts for Oxygen Reduction Reaction. Catal. Sci. Technol. 2022, 12, 17230–17240. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Qi, S.; Li, W.; Zhao, M. Bifunctional HER/OER or OER/ORR Catalytic Activity of Two-Dimensional TM3(HITP)2 with TM = Fe-Zn. J. Phys. Chem. C 2020, 124, 9350–9359. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.; Peng, Q.; Zhou, J.; Sun, Z. Mo2B2MBene-Supported Single-Atom Catalysts as Bifunctional HER/OER and OER/ORR Electrocatalysts. J. Mater. Chem. A 2021, 9, 433–441. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A.; Slawin, A.M.Z.; Carpenter-Warren, C.L.; Chen, W.; Zheng, A. Ultrafast Post-Synthetic Modification of a Pillared Cobalt(Ii)-Based Metal-Organic Framework: Via Sulfurization of Its Pores for High-Performance Supercapacitors. J. Mater. Chem. A 2019, 7, 11953–11966. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Jiang, P.G.; Li, Q.F.; Lin, J.H. Novel Metal(Ii) Coordination Polymers Based on N,N′-Bis-(4-Pyridyl) Phthalamide as Supercapacitor Electrode Materials in an Aqueous Electrolyte. Dalton Trans. 2013, 42, 1603–1611. [Google Scholar] [CrossRef]
- Paul, A.; Karmakar, A.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Amide Functionalized Metal Organic Frameworks for Diastereoselective Nitroaldol (Henry) Reaction in Aqueous Medium. RSC Adv. 2015, 5, 87400–87410. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, Face-, Point-, Schläfli-, and Delaney-Symbols in Nets, Polyhedra and Tilings: Recommended Terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Study to Develop Platinum( Iv ) Complex Chemistry for Peptide Disulfide Bond Formation. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Chen, M.S.; Hua, Q.; Bai, Z.S.; Okamura, T.A.; Su, Z.; Sun, W.Y.; Ueyama, N. Syntheses and Characterization of Inorganic-Organic Hybrids with 4-(Isonicotinamido)Phthalate and Some Divalent Metal Centers. Polyhedron 2010, 29, 2454–2461. [Google Scholar] [CrossRef]
- Chao, S.; Xia, Q.; Wang, Y.; Li, W.; Chen, W. Pristine S,N-Containing Mn-Based Metal Organic Framework Nanorods Enable Efficient Oxygen Reduction Electrocatalysis. Dalton Trans. 2020, 49, 4336–4342. [Google Scholar] [CrossRef]
- Paul, A.; Silva, T.A.R.; Soliman, M.M.A.; Karačić, J.; Šljukić, B.; Alegria, E.C.B.A.; Khan, R.A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Benzimidazole Schiff Base Copper(II) Complexes as Catalysts for Environmental and Energy Applications: VOC Oxidation, Oxygen Reduction and Water Splitting Reactions. Int. J. Hydrogen Energy 2022, 47, 23175–23190. [Google Scholar] [CrossRef]
- Wahab, A.; Iqbal, N.; Noor, T.; Ashraf, S.; Raza, M.A.; Ahmad, A.; Khan, U.A. Thermally Reduced Mesoporous Manganese MOF @reduced Graphene Oxide Nanocomposite as Bifunctional Electrocatalyst for Oxygen Reduction and Evolution. RSC Adv. 2020, 10, 27728–27742. [Google Scholar] [CrossRef]
- Gonen, S.; Lori, O.; Cohen-Taguri, G.; Elbaz, L. Metal Organic Frameworks as a Catalyst for Oxygen Reduction: An Unexpected Outcome of a Highly Active Mn-MOF-Based Catalyst Incorporated in Activated Carbon. Nanoscale 2018, 10, 9634–9641. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-Doped Graphene-Supported Transition-Metals Carbide Electrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2015, 5, 10389. [Google Scholar] [CrossRef]
- Lian, Y.; Yang, W.; Zhang, C.; Sun, H.; Deng, Z.; Xu, W.; Song, L.; Ouyang, Z.; Wang, Z.; Guo, J.; et al. Unpaired 3d Electrons on Atomically Dispersed Cobalt Centres in Coordination Polymers Regulate Both Oxygen Reduction Reaction (ORR) Activity and Selectivity for Use in Zinc–Air Batteries. Angew. Chem.-Int. Ed. 2020, 59, 286–294. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.H.; Jung, J.Y.; Wagh, N.K.; Kim, S.H.; Kim, D.H.; Lin, C.; Lee, S.U.; Lee, J.H. Unveiling Dual-Linkage 3D Hexaiminobenzene Metal-Organic Frameworks towards Long-Lasting Advanced Reversible Zn-Air Batteries. Energy Environ. Sci. 2019, 12, 727–738. [Google Scholar] [CrossRef]
- Singh, K.; Guillen Campos, J.D.J.; Dinic, F.; Hao, Z.; Yuan, T.; Voznyy, O. Manganese MOF Enables Efficient Oxygen Evolution in Acid. ACS Mater. Lett. 2020, 2, 798–800. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, L.; Shen, X.; Zhu, W.; Li, L. Mechanistic Insight on Porphyrin Based Porous Titanium Coordination Polymer as Efficient Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reactions. Dyes Pigment. 2020, 181, 108568. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Liu, J.L.; Qin, L.; Hu, Q.; Zheng, Y.; Yang, X.; Zhang, M.D. Hydrogen Evolution Reaction of One 2D Cobalt Coordination Polymer with Coordinated Sulfate Ion. J. Solid State Chem. 2021, 299, 122191. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).