Anti-Cancer and Anti-Inflammatory Activities of a Short Molecule, PS14 Derived from the Virulent Cellulose Binding Domain of Aphanomyces invadans, on Human Laryngeal Epithelial Cells and an In Vivo Zebrafish Embryo Model
Abstract
1. Introduction
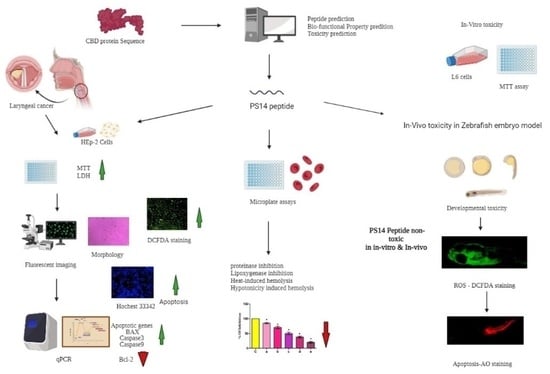
2. Results and Discussion
2.1. CBD Protein and PS14 Analysis
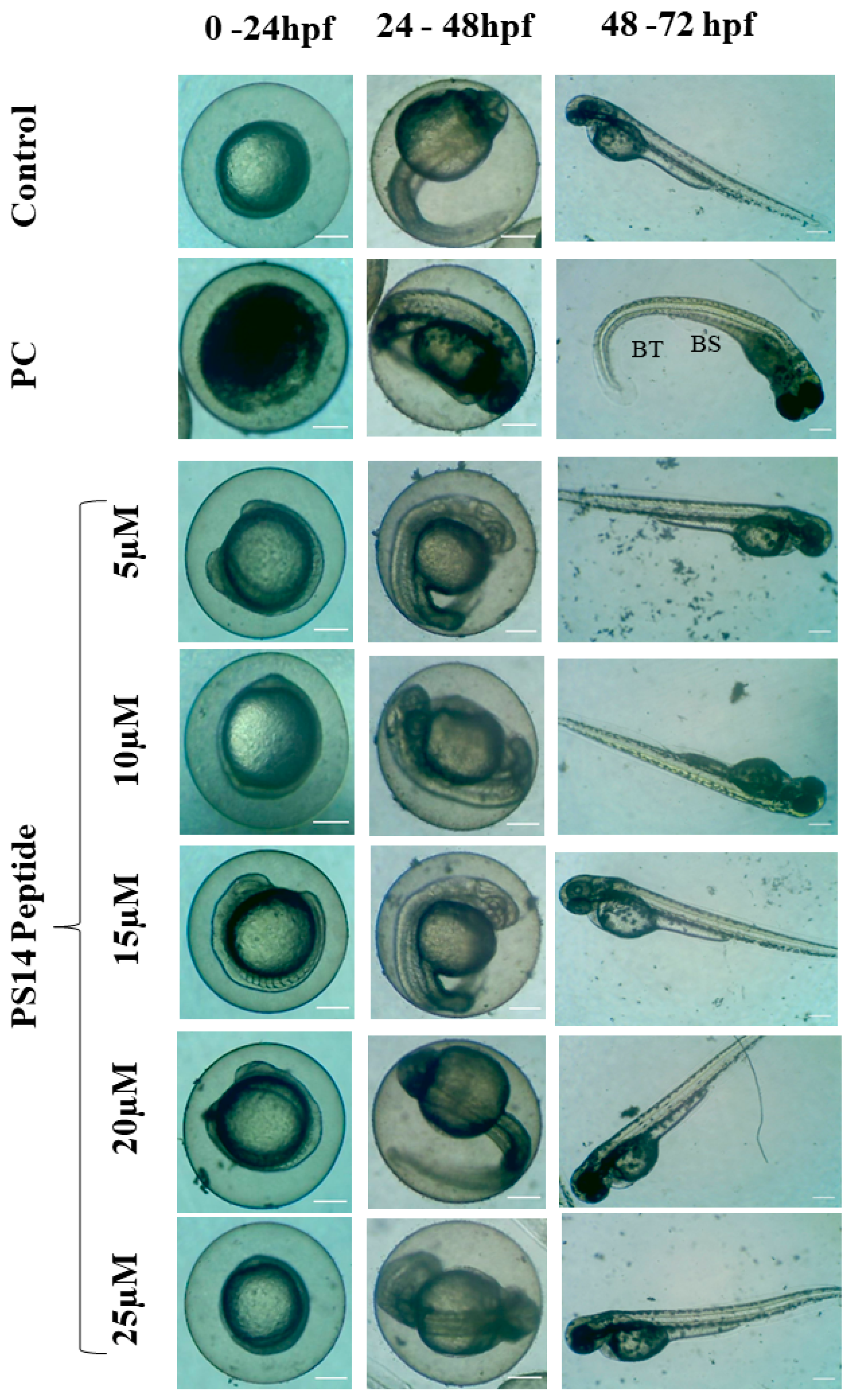
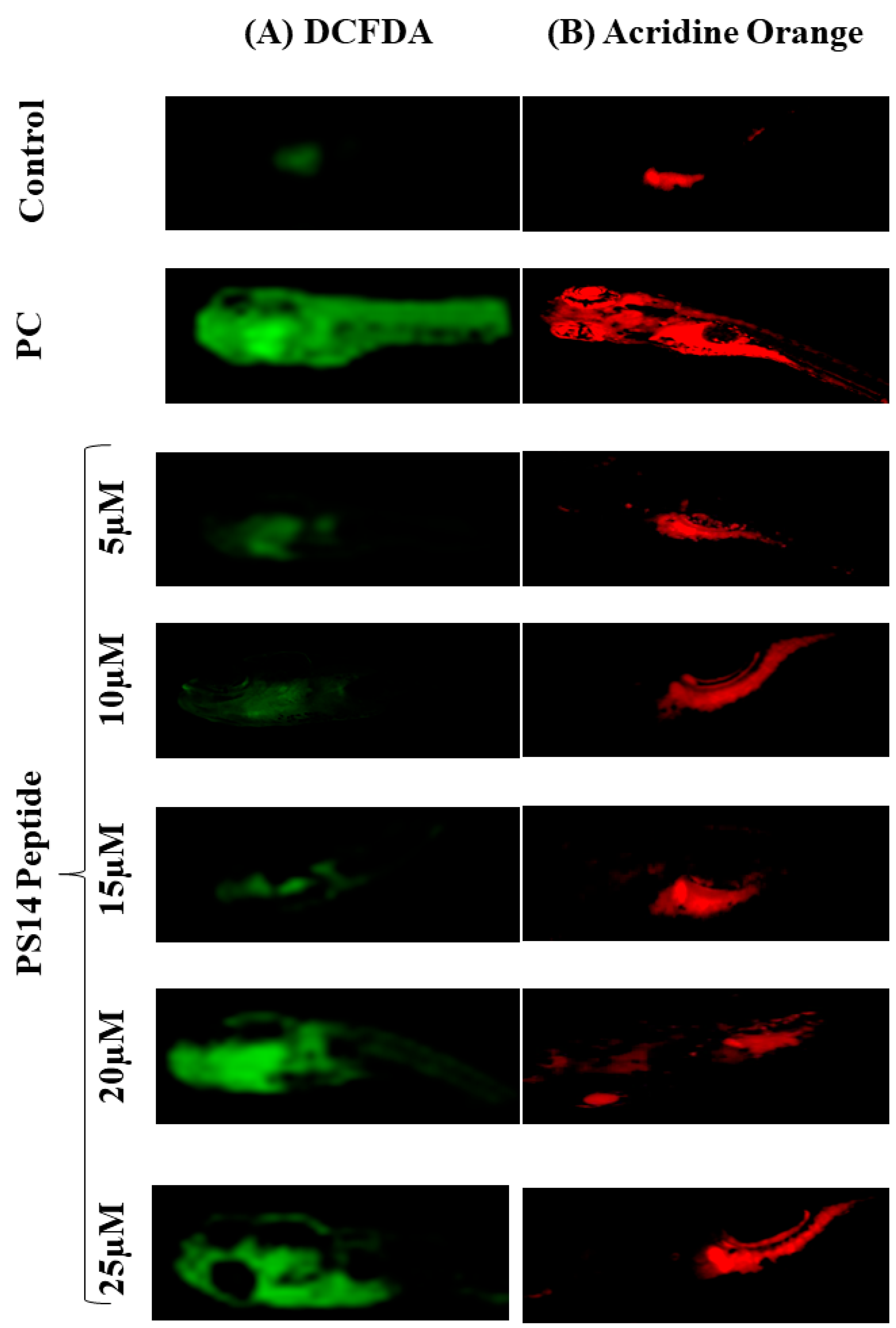
2.2. Assessment of PS14 for Toxicity
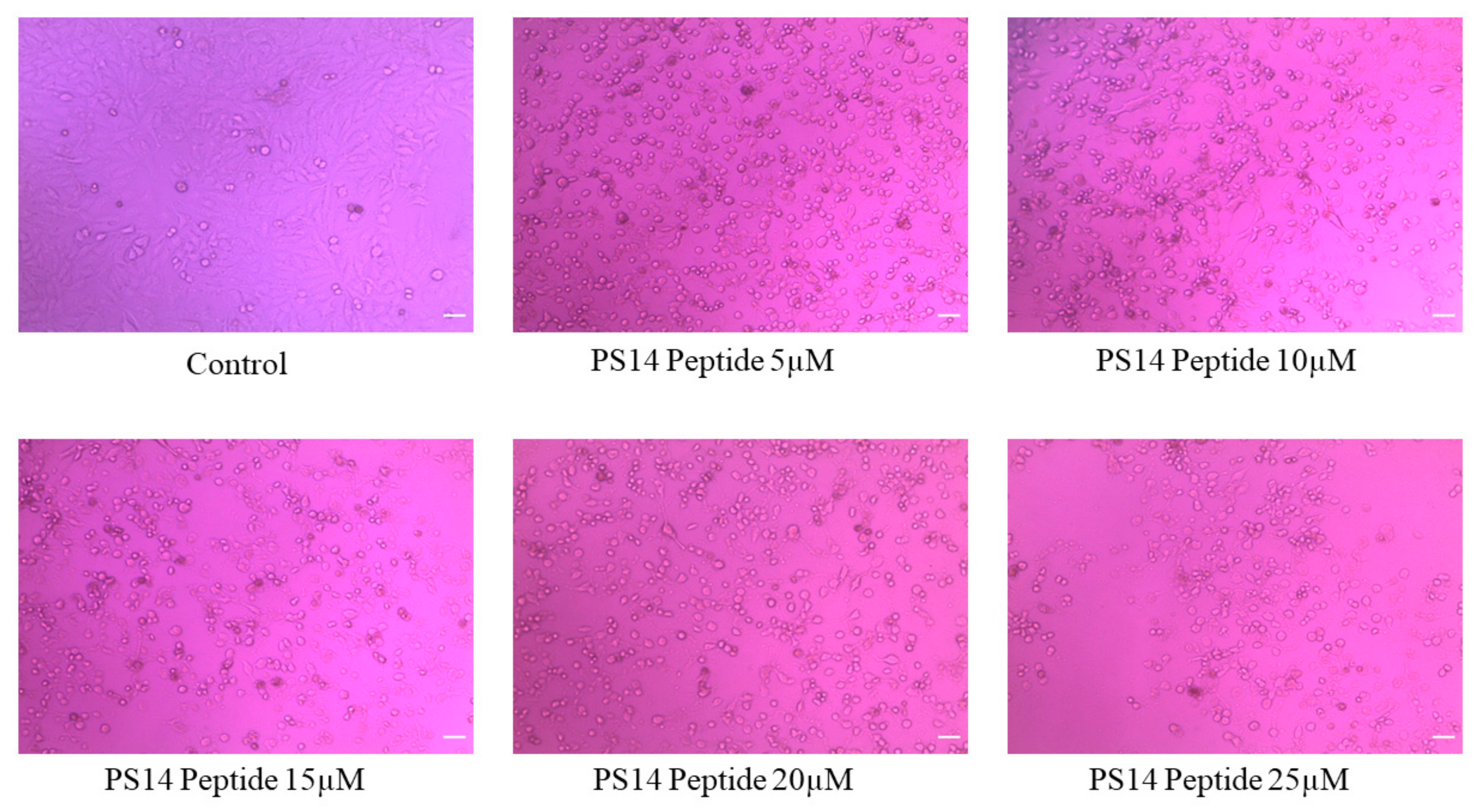
2.3. In Vitro Anti-Cancer Activity of PS14 against Hep-2 Cells
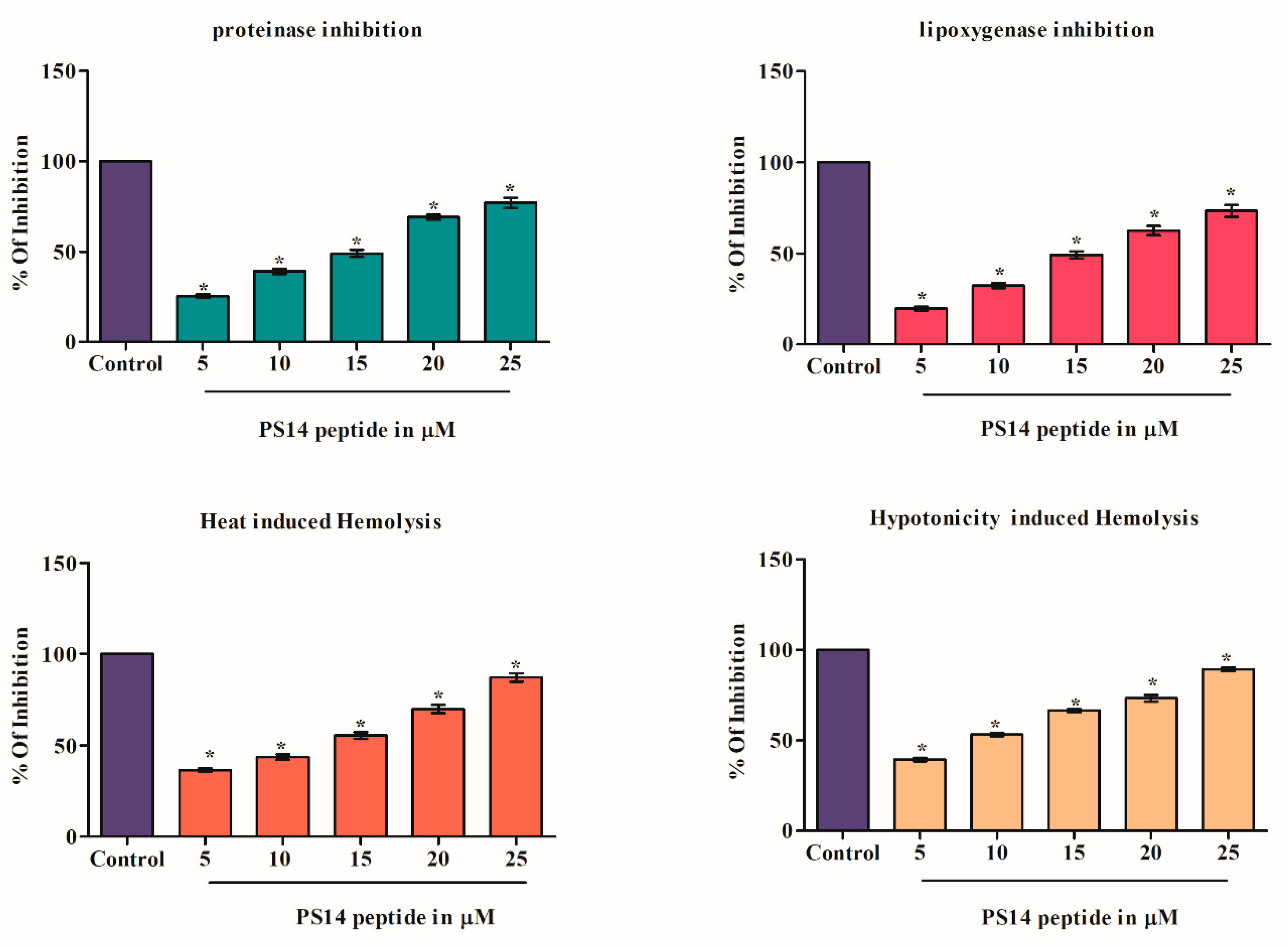
2.4. Anti-Inflammatory Activity of PS14
3. Materials and Methods
3.1. Bioinformatic Investigation and Synthesis of PS14
3.2. Assessment of PS14-Induced Toxicity In Vitro and In Vivo
3.2.1. Cell Culture and Maintenance
3.2.2. Cytotoxicity Assessment of PS14 on L6 Cells
3.2.3. In Vivo Toxicity Assessment in Zebrafish Embryo
3.2.4. Assessment of In Vivo ROS Production
3.2.5. Assessment of Apoptosis, In Vivo
3.3. In Vitro Anti-Cancer Activity of PS14
3.3.1. Assessment of Anti-Proliferative Activity
3.3.2. Trypan Blue Staining
3.3.3. LDH Assay
3.3.4. Morphological Analysis and Apoptosis Staining
3.3.5. DCFH-DA
3.3.6. RT-PCR
3.4. Assessment of In Vitro Anti-Inflammatory Activity
3.4.1. Effect of PS14 on Proteinase Inhibitory Assay
3.4.2. Effect of PS14 on Lipoxygenase Inhibition
3.4.3. Effect of PS14 on Heat-Induced Hemolysis
3.4.4. Effect of PS14 on Hypotonicity-Induced Hemolysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Casini, A.; Scozzafava, A. Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 2003, 23, 535–558. [Google Scholar] [CrossRef] [PubMed]
- Obid, R.; Redlich, M.; Tomeh, C. The Treatment of Laryngeal Cancer, Oral Maxillofac. Oral Maxillofac. Surg. 2019, 31, 1–11. [Google Scholar] [CrossRef]
- Brandstorp-Boesen, J.; Sørum Falk, R.; Folkvard Evensen, J.; Boysen, M.; Brøndbo, K. Risk of Recurrence in Laryngeal Cancer. PLoS ONE 2016, 11, e0164068. [Google Scholar] [CrossRef]
- Dutta, S.; Biswas, K.D.; Ghatak, S.; Haldar, D.; Sen, I.; Sinha, R. Postoperative hypofunctioning of the thyroid gland after total laryngectomy. Ear Nose Throat J. 2016, 95, 23–27. [Google Scholar]
- Dadar, M.; Shahali, Y.; Chakraborty, S.; Prasad, M.; Tahoori, F.; Tiwari, R.; Dhama, K. Anti-inflammatory peptides: Current knowledge and promising prospects. Inflamm. Res. 2019, 68, 125–145. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.; Li, X.; Han, W.; Su, X. Anticancer potential of bioactive peptides from animal sources. Oncol. Rep. 2017, 38, 637–651. [Google Scholar] [CrossRef]
- Velayutham, M.; Sarkar, P.; Rajakrishnan, R.; Kuppusamy, P.; Juliet, A.; Arockiaraj, J. Antiproliferation of MP12 derived from a fungus, Aphanomyces invadans virulence factor, cysteine-rich trypsin inhibitor on human laryngeal epithelial cells, and in vivo zebrafish embryo model. Toxicon 2022, 210, 100–108. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Kumaresan, V.; Pasupuleti, M.; Arasu, M.V.; Al-Dhabi, N.A.; Arshad, A.; Amin, S.M.N.; Yusoff, F.M.; Arockiaraj, J. A comparative transcriptome approach for identification of molecular changes in Aphanomyces invadans infected Channa striatus. Mol. Biol. Rep. 2018, 45, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Griffo, A.; Rooijakkers, B.J.M.; Hähl, H.; Jacobs, K.; Linder, M.B.; Laaksonen, P. Binding Forces of Cellulose Binding Modules on Cellulosic Nanomaterials. Biomacromolecules 2019, 20, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Issac, P.K.; Raju, S.V.; Elumalai, P.; Arshad, A.; Arockiaraj, J. Pathogenic bacterial toxins and virulence influences in cultivable fish. Aquac. Res. 2021, 52, 2361–2376. [Google Scholar] [CrossRef]
- Sarkar, P.; Raju, V.S.; Kuppusamy, G.; Rahman, M.A.; Elumalai, P.; Harikrishnan, R.; Arshad, A.; Arockiaraj, J. Pathogenic fungi affecting fishes through their virulence molecules. Aquaculture 2022, 548, 737553. [Google Scholar] [CrossRef]
- Carrard, G.; Koivula, A.; Söderlund, H.; Béguin, P. Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc. Natl. Acad. Sci. USA 2000, 97, 10342–10347. [Google Scholar] [CrossRef]
- Yin, W.B.; Baccile, J.A.; Bok, J.W.; Chen, Y.; Keller, N.P.; Schroeder, F.C. A nonribosomal peptide synthetase-derived iron(III) complex from the pathogenic fungus aspergillus fumigatus. J. Am. Chem. Soc. 2013, 135, 2064–2067. [Google Scholar] [CrossRef]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic anti-cancer properties of scorpion venom peptides: Rhopalurus princeps venom as an anti-cancer agent. Drug Des. Devel. Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, W.; Ding, L.; Ye, X.; Gao, H.; Tai, X.; Wu, Z.; Qian, Y.; Ruan, X.; Li, J.; et al. Bldesin, the first functionally characterized pathogenic fungus defensin with Kv1.3 channel and chymotrypsin inhibitory activities. J. Biochem. Mol. Toxicol. 2019, 33, e22244. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An insilico approach. J. Transl. Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- He, X.; Deng, Z.; Xu, W.; Li, Y.; Xu, C.; Chen, H.; Shen, J. A novel dual-response chemosensor for bioimaging of Exogenous/Endogenous hypochlorite and hydrazine in living cells, Pseudomonas aeruginosa and zebrafish. Sens. Actuators B Chem. 2020, 321, 128450. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Velayutham, M.; Guru, A.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Elumalai, P.; Harikrishnan, R.; Arshad, A.; Arockiaraj, J. GR15 peptide of S-adenosylmethionine synthase (SAMe) from Arthrospira platensis demonstrated antioxidant mechanism against H2O2 induced oxidative stress in in-vitro MDCK cells and in-vivo zebrafish larvae model. J. Biotechnol. 2021, 342, 79–91. [Google Scholar] [CrossRef]
- Flores-Guzmán, F.; Alvarado-Sansininea, J.J.; López-Muñoz, H.; Escobar, M.L.; Espinosa-Trejo, M.; Tavera-Hernandez, R.; Jiménez-Estrada, M.; Sánchez-Sánchez, L. Antiproliferative, cytotoxic and apoptotic activity of the bentonite transformation of sesquiterpene lactone glaucolide B to 5β-hydroxy-hirsutinolide on tumor cell lines. Eur. J. Pharmacol. 2019, 856, 172406. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Hong, D. Brevilaterin B from Brevibacillus laterosporus has selective antitumor activity and induces apoptosis in epidermal cancer. Res. Sq. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prabha, N.; Sannasimuthu, A.; Kumaresan, V.; Elumalai, P.; Arockiaraj, J. Intensifying the Anticancer Potential of Cationic Peptide Derived from Serine Threonine Protein Kinase of Teleost by Tagging with Oligo Tryptophan. Int. J. Pept. Res. Ther. 2020, 26, 75–83. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Z.; Ding, G.F.; Huang, F.F.; Yang, Z.S.; Yu, F.M.; Tang, Y.P.; Jia, Y.L.; Zheng, Y.Y.; Chen, R. Anti-cancer activity of Anthopleura anjunae oligopeptides in prostate cancer DU-145 cells. Mar. Drugs 2018, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Karanam, G.; Arumugam, M.K. Reactive oxygen species generation and mitochondrial dysfunction for the initiation of apoptotic cell death in human hepatocellular carcinoma HepG2 cells by a cyclic dipeptide Cyclo(-Pro-Tyr). Mol. Biol. Rep. 2020, 47, 3347–3359. [Google Scholar] [CrossRef]
- Al-Khayal, K.; Alafeefy, A.; Vaali-Mohammed, M.-A.; Mahmood, A.; Zubaidi, A.; Al-Obeed, O.; Khan, Z.; Abdulla, M.; Ahmad, R. Novel derivative of aminobenzenesulfonamide (3c) induces apoptosis in colorectal cancer cells through ROS generation and inhibits cell migration. BMC Cancer 2017, 17, 4. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Irish J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, G.; Kumaresan, V.; Bhatt, P.; Arasu, M.V.; Al-Dhabi, N.A.; Arockiaraj, J. A Cumulative Strategy to Predict and Characterize Antimicrobial Peptides (AMPs) from Protein Database. Int. J. Pept. Res. Ther. 2017, 23, 281–290. [Google Scholar] [CrossRef]
- Roy, S.; Maheshwari, N.; Chauhan, R.; Sen, N.K.; Sharma, A. Structure prediction and functional characterization of secondary metabolite proteins of Ocimum. Bioinformation 2011, 6, 315–319. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Kumaresan, V.; Anilkumar, S.; Pasupuleti, M.; Ganesh, M.-R.; Mala, K.; Paray, B.A.; Al-Sadoon, M.K.; Albeshr, M.F.; Arockiaraj, J. Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic. Biol. Med. 2019, 135, 198–209. [Google Scholar] [CrossRef]
- Wei, L.; Ye, X.; Sakurai, T.; Mu, Z.; Wei, L. ToxIBTL: Prediction of peptide toxicity based on information bottleneck and transfer learning. Bioinformatics 2022, 38, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, V.; Subramaniyam, S.; Malik, A.; Lee, G.; Manavalan, B.; Yang, D.C. MACppred: A support vector machine-based meta-predictor for identification of anti-cancer peptides. Int. J. Mol. Sci. 2019, 20, 1964. [Google Scholar] [CrossRef]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational Prediction of Anti-inflammatory Peptides by Integrating Multiple Complementary Features. Front. Genet. 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, M.; Ojha, B.; Issac, P.K.; Lite, C.; Guru, A.; Pasupuleti, M.; Arasu, M.V.; Al-Dhabi, N.A.; Arockiaraj, J. NV14 from serine O-acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biol. Int. 2021, 45, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-W.; Yeh, Y.-L.; Wang, Y.-C.; Huang, W.-J.; Ho, S.-Y.; Lin, P.; Wang, Y.-J. Combination of the novel histone deacetylase inhibitor YCW1 and radiation induces autophagic cell death through the downregulation of BNIP3 in triple-negative breast cancer cells in vitro and in an orthotopic mouse model. Mol. Cancer 2016, 15, 46. [Google Scholar] [CrossRef]
- Abdullah, A.S.H.; Mohammed, A.S.; Abdullah, R.; Mirghani, M.E.S.; Al-Qubaisi, M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract, BMC Complement. Altern. Med. 2014, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Al-Qubaisi, M.; Rozita, R.; Yeap, S.K.; Omar, A.R.; Ali, A.M.; Alitheen, N.B. Selective cytotoxicity of goniothalamin against hepatoblastoma HepG2 cells. Molecules 2011, 16, 2944–2959. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species-independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef]
- Al-Harbi, L.N.; Subash-Babu, P.; Binobead, M.A.; Alhussain, M.H.; Alsedairy, S.A.; Aloud, A.A.; Alshatwi, A.A. Potential metabolite nymphayol isolated from water lily (Nymphaea stellata) flower inhibits MCF-7 human breast cancer cell growth via upregulation of CDKN2A, PRB2, P53 and downregulation of PCNA mRNA expressions. Metabolites 2020, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, G.; Prathap, P.; Guru, A.; Rajesh, R.; Sathish, S.; Madhavan, T.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Gopinath, P.; et al. Anti-inflammatory role demonstrated both in vitro and in vivo models using nonsteroidal tetranortriterpenoid, Nimbin (N1) and its analogs (N2 and N3) that alleviate the domestication of alternative medicine. Cell Biol. Int. 2022, 46, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Singsai, K.; Charoongchit, P.; Chaikaew, W.; Boonma, N.; Fhanjaksai, P.; Chaisatan, K. Antilipoxygenase and Anti-Inflammatory Activities of Streblus asper Leaf Extract on Xylene-Induced Ear Edema in Mice. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 3176391. [Google Scholar] [CrossRef]

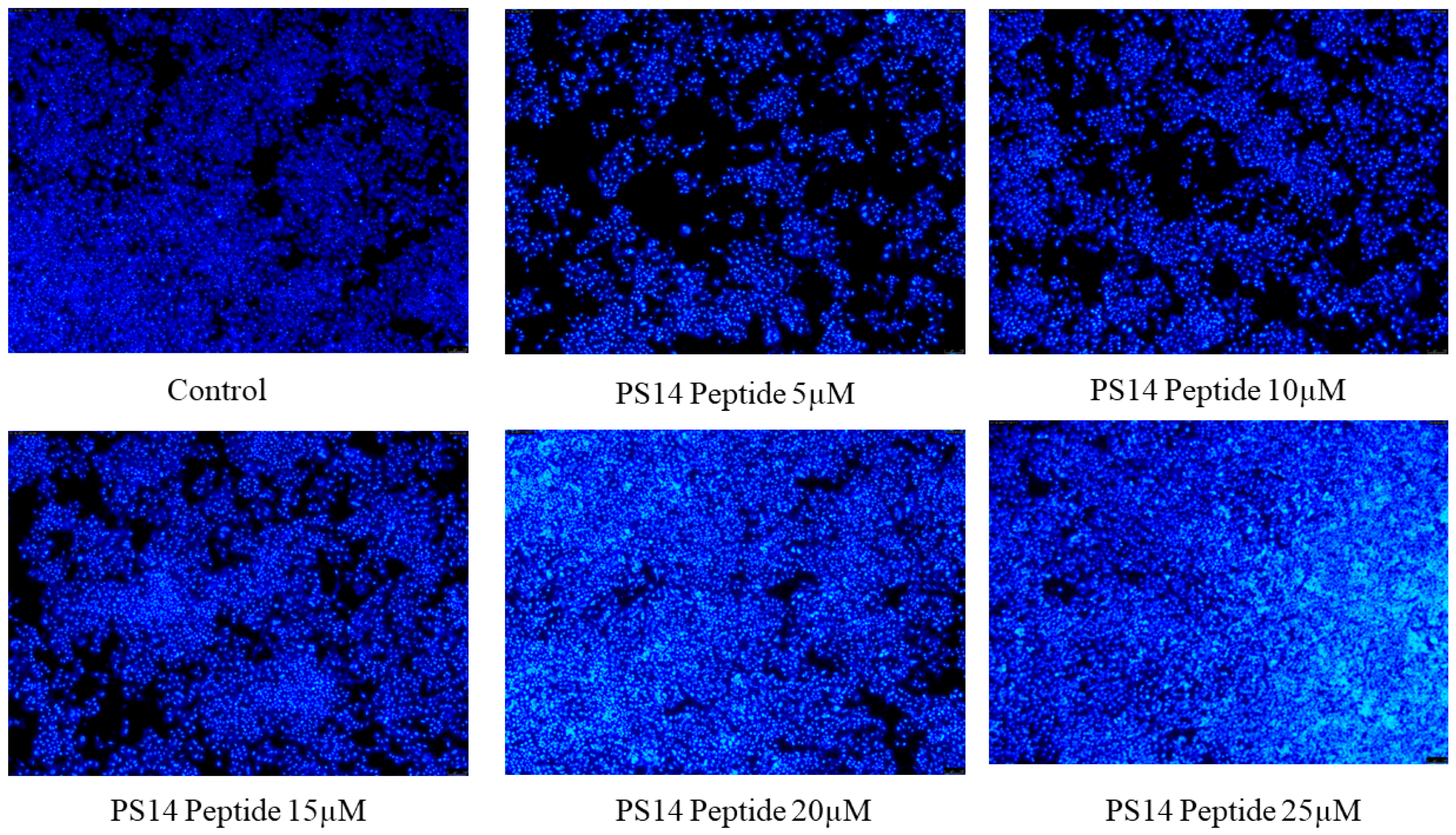
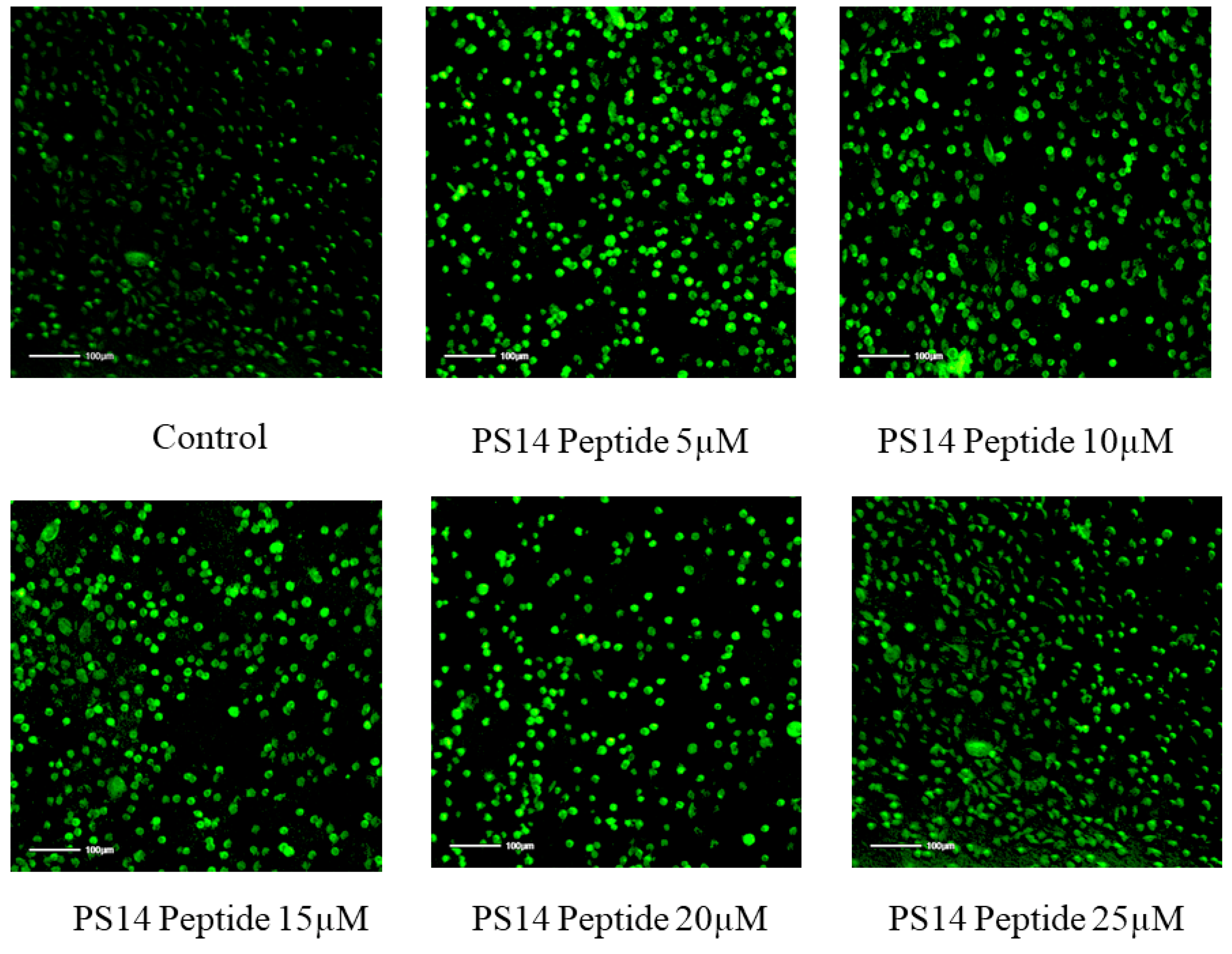

| Concentration | Percentage of Inhibition | Cell Count Percentage at 24 h | Percentage of LDH Release at 24 h | ||
|---|---|---|---|---|---|
| 24 h | 48 h | Viable | Dead | ||
| Control | 5.2 ± 0.25 | 7 ± 0.35 | 96.25 ± 1.32 | 4.28 ± 1.42 | 10.25 ± 0.25 |
| PS14 5 µM | 21.32 ± 3.2 * | 19.22 ± 1.5 * | 79.35 ± 0.94 * | 23.86 ± 0.98 * | 9.74 ± 1.54 |
| PS14 10 µM | 27.25 ± 1.89 * | 25.35 ± 0.92 * | 73.24 ± 4.25 * | 28.78 ± 2.3 * | 19.31 ± 1.09 * |
| PS14 15 µM | 34.37 ± 2.45 * | 39.93 ± 3.24 * | 61.52 ± 3.8 * | 36.48 ± 0.80 * | 34.31 ± 1.6 * |
| PS14 20 µM | 48.70 ± 2.1 * | 58.60 ± 1.7 * | 54.75 ± 4 * | 48.25 ± 1.32 * | 45.56 ± 2 * |
| PS14 25 µM | 59.75 ± 1.7 * | 57.41 ± 1.8 * | 41.25 ± 5 * | 66.32 ± 2.45 * | 69.05 ± 1.6 * |
| Gene | Primer Sequence | Reference |
|---|---|---|
| Bcl-2 | Forward: 5′-GTGGATGACTGAGTACCT-3′ Reverse: 5′-CCAGGAGAAATCAAACAGAG-3′ | [44] |
| BAX | Forward: 5′-TCAGGATGCGTCCACCAAGAAG-3′ Reverse: 5′-TGTGTCCACGGCGGCAATCATC-3′ | |
| Caspase-3 | Forward: 5′-ACATGGAAGCGAATCAATGGACTC-3′ Reverse: 5′-AAGGACTCAAATTCTGTTGCCACC-3′ | |
| Caspase-9 | Forward: 5′-GCTCTTCCTTTGTTCATC-3′ Reverse: 5′-CTCTTCCTCCACTGTTCA-3′ | |
| GAPDH (internal control) | Forward: 5′-GTCTCCTCTGACTTCAACAGCG-3′ Reverse: 5′-ACCACCCTGTTGCTGTAGCCAA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayutham, M.; Sarkar, P.; Sudhakaran, G.; Al-Ghanim, K.A.; Maboob, S.; Juliet, A.; Guru, A.; Muthupandian, S.; Arockiaraj, J. Anti-Cancer and Anti-Inflammatory Activities of a Short Molecule, PS14 Derived from the Virulent Cellulose Binding Domain of Aphanomyces invadans, on Human Laryngeal Epithelial Cells and an In Vivo Zebrafish Embryo Model. Molecules 2022, 27, 7333. https://doi.org/10.3390/molecules27217333
Velayutham M, Sarkar P, Sudhakaran G, Al-Ghanim KA, Maboob S, Juliet A, Guru A, Muthupandian S, Arockiaraj J. Anti-Cancer and Anti-Inflammatory Activities of a Short Molecule, PS14 Derived from the Virulent Cellulose Binding Domain of Aphanomyces invadans, on Human Laryngeal Epithelial Cells and an In Vivo Zebrafish Embryo Model. Molecules. 2022; 27(21):7333. https://doi.org/10.3390/molecules27217333
Chicago/Turabian StyleVelayutham, Manikandan, Purabi Sarkar, Gokul Sudhakaran, Khalid Abdullah Al-Ghanim, Shahid Maboob, Annie Juliet, Ajay Guru, Saravanan Muthupandian, and Jesu Arockiaraj. 2022. "Anti-Cancer and Anti-Inflammatory Activities of a Short Molecule, PS14 Derived from the Virulent Cellulose Binding Domain of Aphanomyces invadans, on Human Laryngeal Epithelial Cells and an In Vivo Zebrafish Embryo Model" Molecules 27, no. 21: 7333. https://doi.org/10.3390/molecules27217333
APA StyleVelayutham, M., Sarkar, P., Sudhakaran, G., Al-Ghanim, K. A., Maboob, S., Juliet, A., Guru, A., Muthupandian, S., & Arockiaraj, J. (2022). Anti-Cancer and Anti-Inflammatory Activities of a Short Molecule, PS14 Derived from the Virulent Cellulose Binding Domain of Aphanomyces invadans, on Human Laryngeal Epithelial Cells and an In Vivo Zebrafish Embryo Model. Molecules, 27(21), 7333. https://doi.org/10.3390/molecules27217333