Abstract
The present study aimed to characterize the physical properties of nanoemulsion-based sodium alginate edible coatings containing myrtle (Myrtus communis L.) essential oil and to determine its inhibitory effects on Listeria monocytogenes in fresh Kasar cheese during the 24-day storage at 4 °C. The GC-MS analysis showed that the main components of myrtle essential oil were 1,8-cineol (38.64%), α-pinene (30.19%), d-limonene (7.51%), and α-ocimene (6.57%). Myrtle essential oil showed an inhibitory effect on all tested L. monocytogenes strains and this effect significantly increased after ultrasonication. Minimum inhibitory and minimum bactericidal concentrations of myrtle essential oil nanoemulsion were found to be 4.00–4.67 mg/mL and 5.00–7.33 mg/mL, respectively. The antibacterial activity of myrtle essential oil nanoemulsion against L. monocytogenes was confirmed by the membrane integrity and FESEM analyses. Nanoemulsion coatings containing myrtle essential oil showed antibacterial activity against L. monocytogenes with no adverse effects on the physicochemical properties of cheese samples. Nanoemulsion coatings containing 1.0% and 2.0% myrtle essential oil reduced the L. monocytogenes population in cheese during the storage by 0.42 and 0.88 log cfu/g, respectively. These results revealed that nanoemulsion-based alginate edible coatings containing myrtle essential oil have the potential to be used as a natural food preservative.
1. Introduction
Listeria monocytogenes is one of the most important foodborne pathogens that causes listeriosis in humans and animals. Soft and semi-hard cheeses are considered important risk products for foodborne listeriosis and were identified as the major food vehicle of L. monocytogenes infections. Control of L. monocytogenes in cheese processing is particularly difficult due to its high cold and osmotic stress tolerance, and its ability to form environmentally stable biofilms resistant to sanitation. L. monocytogenes contamination is associated with inadequately pasteurized milk and post-process contamination or inadequate processing [1,2]. Kasar cheese is a semi-hard cheese commercially produced in Turkey and constitutes a significant part of the annual total cheese consumption (242 thousand tons/year). Kasar cheese has an ideal environment for the growth of many microorganisms, including L. monocytogenes due to its high-water content (45%) and suitable pH (5.5–5.8) [3,4].
The microbiological safety of food is a major concern for consumers, regulatory agencies, researchers, and the food industry around the world. There is increasing interest in the use of natural or plant-based preservatives such as essential oils for maintaining food quality and safety. Essential oils and their constituents have great potential as natural antimicrobial agents to prevent the growth of pathogenic and spoilage microorganisms in foods. Essential oils are naturally aromatic and volatile liquids obtained from different parts of plants such as flowers, roots, bark, leaves, seeds, and fruits. It has been reported that essential oils exert potent antimicrobial, antiviral, antifungal, and antioxidant effects, depending on their active constituents [5,6,7]. Nevertheless, their poor solubility in water, susceptibility to oxidative degradation, high volatility, and intense aroma all limit their use in food products. These disadvantages of essential oils can be overcome by incorporating them into nanoemulsion-based delivery systems. Recently, researchers have focused on the use of bioactive compounds with natural antimicrobial properties in food preservation in nanoemulsion against pathogenic and spoilage microorganisms [8]. Ultrasonication has been considered an efficient and eco-friendly physical method for nanoemulsification. The reduction in particle size of emulsions by ultrasound treatment enhances the accessibility of bioactive agents and improves their antimicrobial properties. When the bioactive components are encapsulated with a suitable transport system, their interaction with other food components is prevented, the physical stability of the active substances is increased, and their controlled release into the food can be realized. Nanoemulsions have a key role in the development of a new generation of active food packaging. Bio-based packaging has been increasing in popularity given its beneficial impact on the environment. Sodium alginate is one of the most important natural biopolymers used in the preparation of biodegradable films and edible coatings. It has been reported that nanoemulsions containing active ingredients can be applied as edible coatings, which is a promising method to improve the quality and safety of foods [9,10,11].
Myrtle (Myrtus communis L.) belongs to the Myrtaceae family and is a characteristic plant representative of the Mediterranean flora. It has been reported that the chemical composition of myrtle essential oil consists mainly of α-pinene, limonene, 1,8-cineole, linalool, α-terpineol, linalyl acetate, α-terpineol acetate, and geranyl acetate. The antibacterial properties of myrtle essential oils against pathogenic bacteria were reported in many studies and obtained results are promising. However, there are only a few reports on the application of myrtle essential oil as an antimicrobial in foods for the control of L. monocytogenes [12,13]. The antibacterial activity of myrtle essential oil was associated with α-pinene, 1,8-cineole, and linalool [14,15,16]. The use of essential oils as natural preservatives in different types of cheese has been widely studied in recent years. On the other hand, despite the essential oils exhibiting promising antimicrobial and antioxidant activity, their use is still limited in the cheese industry due to their negative effect on the physicochemical and sensorial properties of the final products. To overcome these drawbacks, nanoemulsion-based edible coating applications in which essential oils are encapsulated could be a promising alternative approach. To the best of our knowledge, no studies on ultrasound-treated sodium alginate-based edible coating containing myrtle essential oil and the application of these coatings on food have been reported. The present study aimed to determine the antibacterial effect of sodium alginate nanoemulsion-based edible coatings containing different concentrations of myrtle essential oil produced by ultrasound treatment on L. monocytogenes in Kasar cheese. In addition, the effect was examined of the nanoemulsion coating on the physicochemical and sensory properties of cheese.
2. Results and Discussion
2.1. The Chemical Composition of Myrtle Essential Oil
Essential oils and their bioactive constituents play an important role in antimicrobial activity. Twenty-five components accounting for 97.86% of the total composition of myrtle essential oil were identified. The relative percentages of the components are presented in Table 1. The major components of myrtle essential oil were 1,8-cineol (38.64%), α-pinene (30.19%), d-limonene (7.51%), and α-ocimene (6.57%), whereas α-terpineol (3.91%), β-cis-ocimene (2.68%), isosylvestrene (2.2%), o-cymene (1.14%), and myrtenol (1.03%) were also present in relatively high amounts. Previous studies reported that the main constituents of myrtle essential oil were 1,8-cineol (5.92–32.12%), α-pinene (9.00–44.62%), d-limonene (trace–23.55%), linalool (2.07–29.08%), and α-terpineol (0.42–8.12%) [17,18,19,20]. The variability of the chemical composition of myrtle essential oil has been associated with geographic origin, variety, plant parts, season, extraction type, and storage conditions of the essential oil [16].

Table 1.
Chemical composition of myrtle essential oil.
2.2. Antibacterial Efficiency of Myrtle Essential Oil Nanoemulsion
The antibacterial effect of myrtle essential oil emulsion and nanoemulsion against L. monocytogenes strains was determined quantitatively by the microtube dilution method, and the obtained minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) values are given in Table 2. Myrtle essential oil emulsion and nanoemulsion showed an inhibitory effect on all tested L. monocytogenes strains. Monoterpene hydrocarbons and oxygenated monoterpenes, such as 1,8-cineol, α-pinene, linalool, α-terpineol, and γ-terpinene in the myrtle essential oil, are responsible for the antibacterial activity against Gram-positive and Gram-negative bacteria, mainly Listeria spp. [12,13,21,22]. Similarly, in the present study, the antimicrobial activity of myrtle essential oil was associated with the high content of 1,8-cineol (38.64%), and α-pinene (30.19%). In addition, it has been reported that antimicrobial activity was highly correlated with the synergetic effect between the major and minor compounds in myrtle essential oil, rather than a single compound [23]. The MIC and MBC values of myrtle essential oil for L. monocytogenes strains were found to be 6.00–8.67 mg/mL and 8.00–14.67 mg/mL, respectively (Table 2). The results revealed that L. monocytogenes ATCC 13932 was the most resistant strain in the group. There are limited studies in the literature examining the antimicrobial effect of myrtle essential oil on L. monocytogenes. Dhifi et al. [12] reported that the MIC and MBC values of myrtle essential oil for L. monocytogenes was 4 mg/mL and 8 mg/mL, respectively. Similarly, Akin et al. [21] and Caputo et al. [22] declared that the MIC value of myrtle essential oil was 5 mg/mL and 3 mg/mL, respectively. These differences can be explained by the chemical composition of the essential oil and the difference in the strains used. In contrast, myrtle essential oil nanoemulsion exhibited higher antimicrobial activity against all tested L. monocytogenes strains compared to that of the myrtle essential oil emulsion (p < 0.05). The MIC and MBC values of the myrtle essential oil nanoemulsion were determined to be 4.00–4.67 mg/mL and 5.00–7.33 mg/mL, respectively. The antibacterial effect significantly increased as a result of ultrasound treatment (p < 0.05). Similarly, Kazemeini et al. [24] have reported that the nanoemulsion of Trachyspermum ammi essential oil had lower MIC values against L. monocytogenes than its pure oil. Moghimi et al. [25] have reported that the antimicrobial activity of sage essential oil increased after its incorporation into the nanoemulsion. Thyme essential oil [26], thymus daenensis essential oil [27], and cinnamon essential oil [28] yielded similar results in studies on the antibacterial activity of nanoemulsions. In ultrasonic emulsification, ultrasonic waves generate cavitation forces that convert the macroemulsion into nanoemulsion. Nanoemulsions obtained by ultrasonication have higher stability and lower droplet size. The encapsulation of essential oil in nanoemulsion systems can enhance their antimicrobial activity by the reduction in the size of essential oil droplets in nanoemulsion. The increased surface area of antimicrobial compounds provides better interaction with the cell membrane leading to increasing antimicrobial activity.

Table 2.
Minimum inhibitory (MIC) and minimum bactericidal (MBC) concentrations of emulsion and nanoemulsion of myrtle essential oil against L. monocytogenes strains.
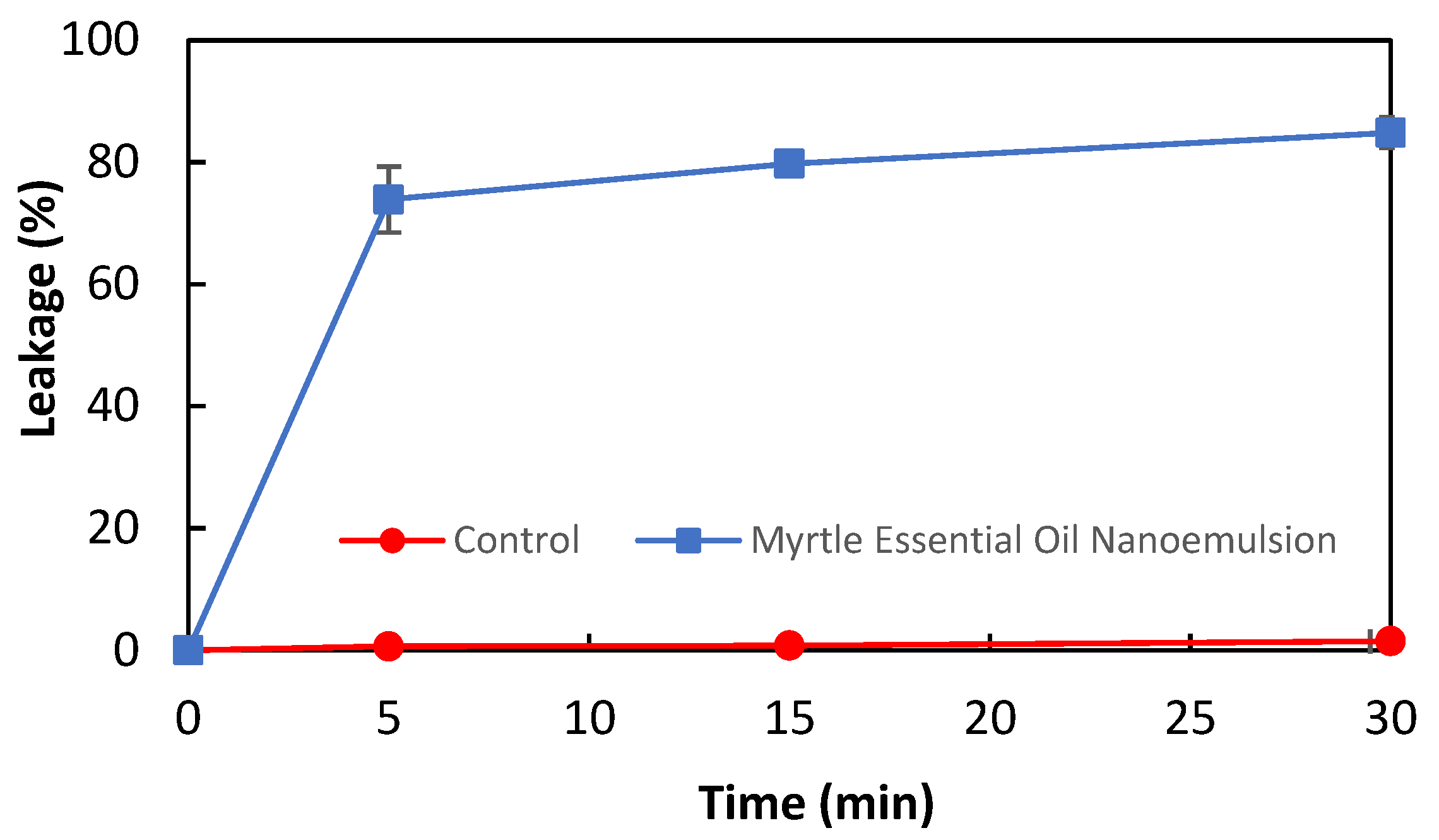
The antibacterial activity of myrtle essential oil nanoemulsion against L. monocytogenes was confirmed by the membrane integrity analysis. The release of nucleic acids from bacterial cells is considered an indicator of a decrease in cell membrane integrity. The effect of myrtle essential oil nanoemulsion on the membrane integrity of L. monocytogenes is shown in Figure 1. The nucleic acid leakage in bacterial cells treated and untreated with myrtle essential oil nanoemulsion was 73.88% and 0.64% at the 5th minute, and 84.85% and 1.5% at the 30th minute, respectively. These results revealed that the myrtle essential oil nanoemulsion damaged the cell membrane of L. monocytogenes and caused the intracellular components to leak out in a short time. It has been reported that essential oils damage the cell membrane of bacteria and then lead to the leakage of intracellular contents such as nucleic acids and protein [29,30]. Sikkema et al. [31] have reported that the cyclic terpene hydrocarbons such as α-pinene, β-pinene, γ-terpinene, and limonene affect the structure and function of the bacterial cell membranes.
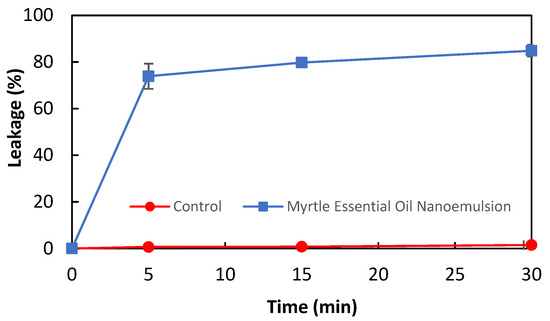
Figure 1.
The effect of myrtle essential oil nanoemulsion on the leakage of nucleic acids of L. monocytogenes cells.
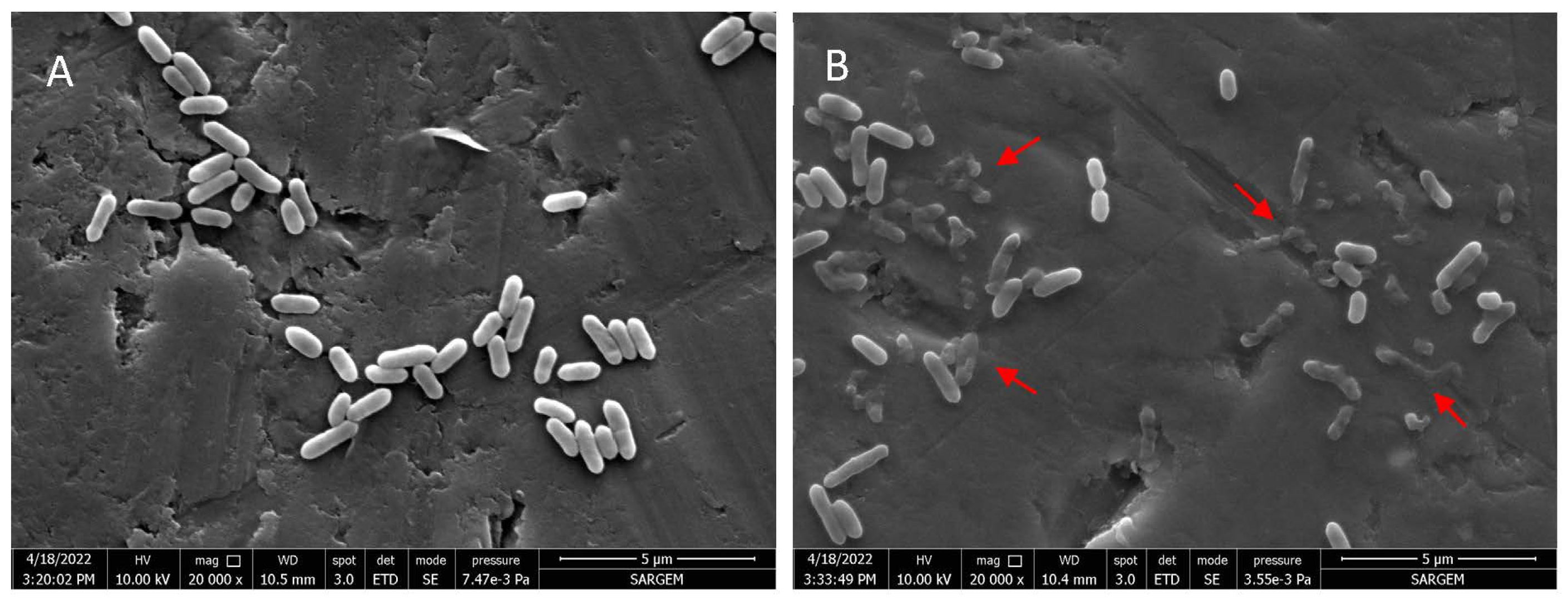
The effect of myrtle essential oil nanoemulsion on the cell morphology of L. monocytogenes was investigated by Field Emission Electron Microscopy (FESEM). The cells treated or untreated with myrtle essential oil nanoemulsion are shown in Figure 2. The untreated L. monocytogenes cells exhibited rod-shaped structures with intact cell integrity and smooth and robust surfaces, while a wide variety of structural disruptions with varying degrees of shrinkage and destruction were observed in bacterial cells treated with myrtle essential oil nanoemulsion. The FESEM images showed that the myrtle essential oil nanoemulsion caused significant damage to bacterial cell integrity.
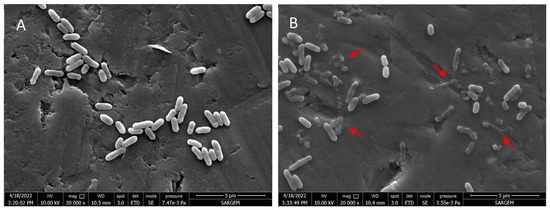
Figure 2.
Scanning electron micrographs of cell morphology of L. monocytogenes untreated (A), treated with nanoemulsion of myrtle essential oil at MBC for 15 min (B).
2.3. Characterization of Emulsions and Nanoemulsions Coating
The mean particle size, zeta potential, and whiteness index (WI) values of alginate-based emulsions and nanoemulsions containing myrtle essential oil are shown in Table 3. Nanoemulsions with smaller particle sizes were obtained by the ultrasound process compared to those of coarse emulsions. The particle sizes of nanoemulsions containing 0.5%, 1.0%, and 2.0% myrtle essential oil were 157 ± 22 nm, 172 ± 30 nm, and 122.7 ± 1.2 nm, respectively. The results were similar to those reported by Artiga-Artigas et al. [32] who studied alginate-based nanoemulsions containing different concentrations of oregano essential oil and mandarin fiber. Similar particle size results for nanoemulsions produced by ultrasound have been also reported by Rahmasari and Polat Yemiş [33], Salvia-Trujillo et al. [34], and Chu et al. [35].

Table 3.
Particle size, zeta potential and whiteness index of alginate nanoemulsion-coating solutions.
The ζ-potential is a key parameter for evaluating the stability of the colloidal system. The particle with a ζ-potential value more positive than +30 mV or more negative than −30 mV represents appropriate stability of the emulsion. All nanoemulsions obtained by ultrasound treatment showed negative ζ-potential values of lower than −30 mV and a more stable structure (−38.47 ± 2.48, −32.27 ± 2.94, and −37.37 ± 2.48 for nanoemulsions containing 0.5%, 1.0%, and 2.0% myrtle essential oil, respectively). There were no significant differences between the formulations (p > 0.05). The coarse emulsions, on the other hand, exhibited a weak surface charge with negative values of lower than −30 mV. The nanoemulsions obtained in the study showed higher stability compared to the coarse emulsions. It has been reported that several mechanical stresses such as the micro-fluidization and ultrasound process can lead to the release of free carboxyl and hydroxyl groups, which are responsible for the negative charge of the essential oil nanoemulsion [11,36].
The optical properties of nanoemulsions are an important factor in food applications. The whiteness index (WI) of emulsions containing different concentrations of myrtle essential oil decreased significantly (p < 0.05) after the ultrasound treatment. Similarly, Salvia-Trujillo et al. [37] have reported a decrease in WI values of alginate-based nanoemulsions containing essential oil after the micro-fluidization process. The whiteness index is closely related to the particle size of the nanoemulsions. It has been reported that larger particles scatter light more intensely than small particles, causing an increase in the whiteness index of emulsions [38]. However, the WI values of the obtained emulsions and nanoemulsions decreased due to the increase in essential oil concentration (p < 0.05). It has been reported that the color of the emulsion depends on the scattered light apart from the droplet size, the refractive index of the continuous and dispersed phase, and the oil concentration [39].
2.4. Application of Nanoemulsion Edible Coatings on Cheese Samples
2.4.1. Antibacterial Activity against L. monocytogenes in Cheese Samples
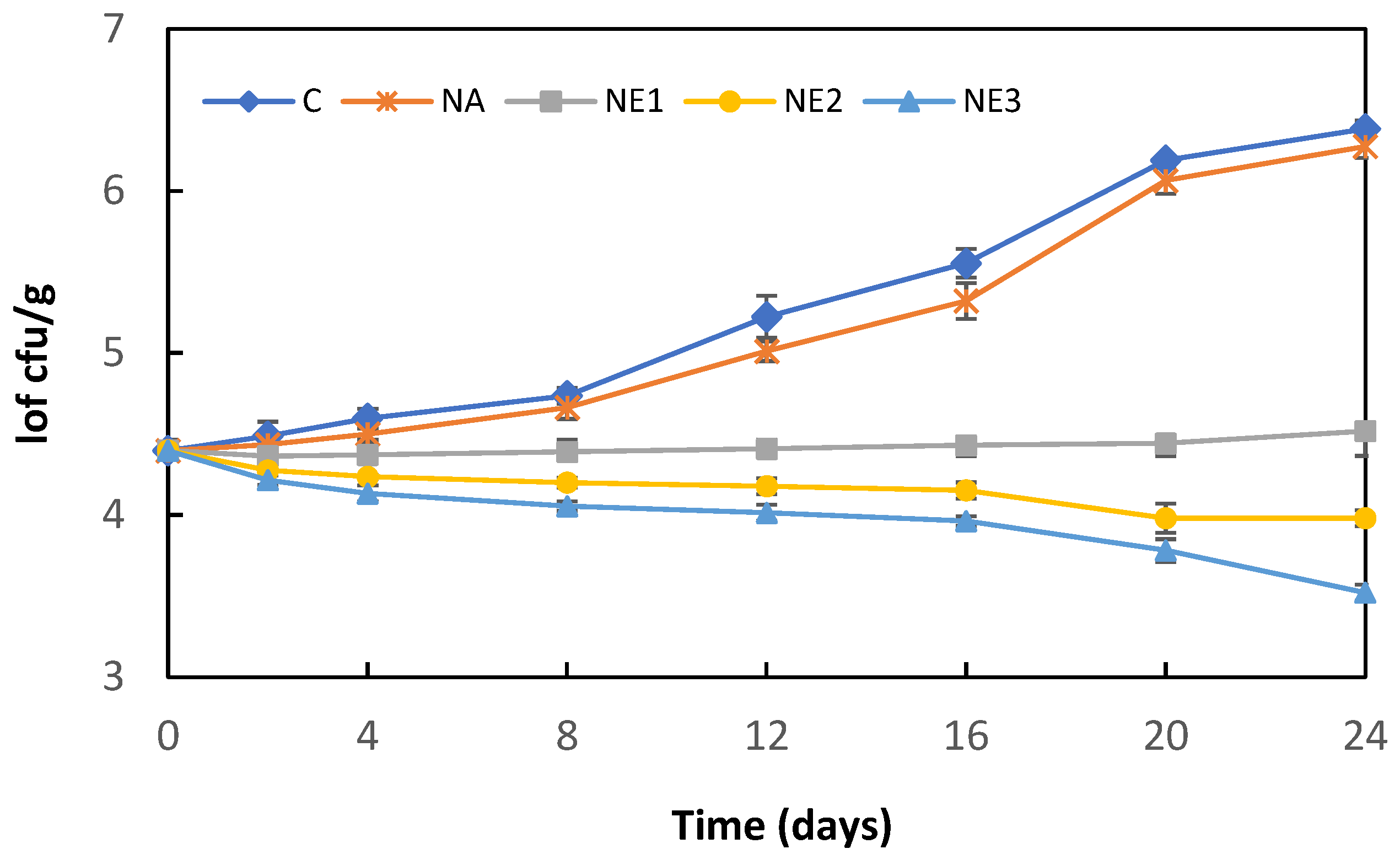
The effect of sodium alginate nanoemulsion coatings containing different concentrations of myrtle essential oil on the L. monocytogenes counts in fresh Kasar cheese during storage at 4 °C is shown in Figure 3. The initial L. monocytogenes count (4.40 log cfu/g) increased significantly after 24 days of storage in both control (C) and alginate nanoemulsion coating (NA)-samples, reaching 6.38 and 6.27 log cfu/g, respectively (p < 0.05). The L. monocytogenes counts in the C and NA samples were higher than those of the alginate nanoemulsion-coating samples containing myrtle essential oil (NA1, NA2, and NA3) during the storage period. The growth of L. monocytogenes was suppressed in the coating samples (NE1) containing 0.5% myrtle essential oil; however, no significant changes were observed in the bacterial counts during the storage (p > 0.05). Similarly, previous studies have reported myrtle essential oil has a lower anti-listerial effect in the food matrix than in vitro analyses [12,13]. Saraiva et al. [13] evaluated the efficacy of myrtle essential oil against L. monocytogenes in sheep milk cheese and reported that the addition of myrtle essential oil showed a lower L. monocytogenes (approximately 1–2 log cfu/g) count during the ripening period compared to the control samples. It has also been stated that myrtle essential oil prevented the growth of L. monocytogenes in cheeses; however, it had no effect on reducing the bacterial load. Similarly, Dhifi et al. [12] have reported that Myrtus communis flower essential oil added to ground beef at the rate of 0.4% and 0.8% had a bacteriostatic effect against L. monocytogenes and delayed the growth of bacteria during storage. In the present study, 0.42 and 0.88 log cfu/g reductions were determined in cheese samples coated with sodium alginate nanoemulsion containing 1.0% and 2.0% myrtle essential oil, respectively. Nanoencapsulation of essential oils is an effective approach to increase the physical stability of active compounds and protect them from interaction with food components. The nanoemulsion-based dispersion system can increase the antimicrobial activity of encapsulated essential oils because of its high surface-to-volume ratio and small particle size [34,40].
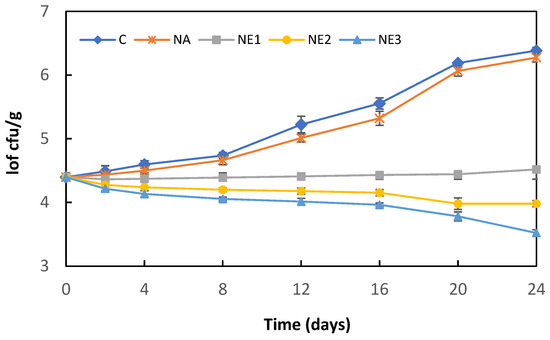
Figure 3.
Changes in L. monocytogenes counts of cheese samples during storage. C: Control, uncoated cheese; NA: samples coated ultrasound-treated sodium alginate solution without the incorporation of myrtle essential oil; NE1, NE2, NE3: samples coated ultrasound-treated sodium alginate solution with the incorporation of 0.5, 1.0, or 2.0% myrtle essential oil, respectively. Error bars represent standard deviation.
2.4.2. Change of Physicochemical Properties of Cheese Samples
The changes in the physicochemical properties of Kasar cheese samples during the 24-day storage are shown in Table 4. pH is regarded to be one of the most important factors affecting the texture and flavor of cheese, as it affects the solubility of caseins and the activity of enzymes involved in ripening [41,42]. The pH in all cheese samples ranged from 5.66 ± 0.01 to 5.69 ± 0.01, indicating no significant effect of alginate nanoemulsion coatings with or without myrtle essential oil on the pH of Kasar cheese (Table 4). Silva et al. [43] stated that the alginate coating did not have an effect on the pH of the cheese. On the other hand, the pH increase was observed in all samples on day 6 of the storage period. The slight increase in cheese pH, especially on day 6, can be associated with the release of alkaline compounds during proteolysis [42]. Water activity (aw) is the main factor affecting stability in semi-hard cheeses during ripening. The water activity of cheese samples showed relatively high aw values (0.95–0.97) at the beginning of the storage (Table 4). These values remained constant during the storage period for all tested cheese samples without significant differences (p > 0.05). At the beginning of the storage, the coated cheese samples showed lower hardness values than the uncoated samples (Table 4). The effect of the coating on the decrease in cheese hardness can be explained by the hydration of the cheese [44]. The hardness values of the cheese samples decreased with increasing essential oil concentration (p < 0.05). The hardness of all cheese samples significantly increased during storage (p < 0.05). There were no statistical differences among treatments for the hardness parameter at the end of storage (p > 0.05). This increase in hardness values could be attributed to the water loss and proteolysis during cheese maturation [32,45]. Similarly, Zhong et al. [46] measured low hardness values at the beginning of the storage in the coated cheeses, but observed that the hardness value was close to that of the control sample at the end of storage. Artiga-Artigas et al. [32] have also reported that the cheese hardness decreased with the coating application; however, this value was not affected by the increase in essential oil concentration. Nanoemulsions are often described as transparent systems due to their smaller droplet size. The transparency of coating solutions is especially important in food applications and affects the acceptability of the product for the consumer. The uncoated control cheese samples had the highest WI value (74.34 ± 0.53) and did not change significantly during storage (p > 0.05). On the other hand, the cheese samples coated with alginate nanoemulsion showed lower WI values (68.11 ± 0.82–69.88 ± 0.82) compared to the control (p < 0.05). A gradual increase in WI value was observed during storage in all coated samples. Similarly, Artiga-Artigas et al. [32] have reported an increase in WI values during storage in cheeses coated with alginate nanoemulsions containing 1.5% oregano essential oil.

Table 4.
The effect of nanoemulsion coating on the physicochemical properties of the cheese samples.
2.4.3. Sensory Evaluation
The effect of different concentrations of myrtle essential oil nanoemulsion coatings on the sensory characteristics of Kasar cheese is shown in Table 5. The coated cheese samples obtained higher sensory scores in terms of color and appearance than those of the control samples because nanoemulsion-based edible coatings offer a glossy appearance to the cheese samples. The odor, flavor, and general acceptability were significantly affected by myrtle essential oil (p < 0.05). The highest overall acceptance scores were observed in the samples coated with sodium alginate (7.50) followed by uncoated samples (7.33). The general acceptability, odor, and flavor scores decreased depending on the increase in myrtle essential oil concentration in the alginate nanoemulsion coating. It was found that the acceptability of the Kasar cheese coated with 2% myrtle essential oil was the lowest (5.11) which can be attributed to the taste and the intense smell of myrtle essential oil. However, Kasar cheese samples coated with nanoemulsion edible coating containing myrtle essential oil were considered to be moderately acceptable at all concentrations used in the present study. The results of the sensory analysis revealed that nanoemulsion edible coating incorporated with essential oil can be used without seriously negatively affecting the sensory attributes of the cheese product.

Table 5.
The effect of nanoemulsion coating on the sensory properties of the cheese samples.
3. Materials and Methods
3.1. Materials
Fresh Kasar cheese; (protein 24.99%, fat 28.59%, and moisture content 42.22%) was kindly provided by Güneşoğlu Süt A.Ş. (Sakarya, Turkey). Food grade sodium alginate, glycerol, and Tween 80 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was used for the preparation of the coating solutions. Myrtle essential oil was obtained from BIOMESI Bioagrotechnology R&D (Adana, Turkey). The wild myrtle plants were collected for essential oil analyses from Adana Province in Turkey in November 2021. The dried myrtle leaves were submitted to the hydro-distillation process by using an industrial type of Clevenger apparatus for 4 h. The recovered essential oil was dried over anhydrous sodium sulfate and stored in darkness at 4 °C [47].
3.2. Bacterial Strains and Cultural Conditions
Listeria monocytogenes ATCC 19111, L. monocytogenes ATCC 7644, and L. monocytogenes ATCC 13932 were obtained from the American Type Culture Collection ((Manassas, VA, USA). All strains were kept at −18 °C in Tryptic Soy Broth (TSB; Merck, Darmstadt, Germany) containing 15% glycerol. L. monocytogenes strains were grown in TSB supplemented with 5 g/L yeast extract (TSBYE; Merck, Darmstadt, Germany) for 24 h at 37 °C.
3.3. Analysis of the Composition of Essential Oil
The chemical composition of the myrtle essential oil was determined using gas chromatography–mass spectrometry (GC-MS, Claurus 500; Perkin-Elmer Instruments, Waltham, MA, USA) according to the method of Özoğul et al. [47]. Analysis was performed on an SGE non-polar fused silica capillary column (60 × 0.25 mm, ID-BPX5, 0.25 μm; Perkin Elmer, Shelton, CT, USA) under the following conditions: the oven temperature was programmed at 60–250 °C (4 °C/min), 250 °C (10 min); injector and detector temperature 220 °C; helium was used as carrier gas at a flow rate of 1.5 mL/min; the injection volume was 1μL in splitless mode; the electron ionization mode at 70 eV; the ion source temperature 230 °C; scan mass range 40–550 m/z, and interface line temperature 250 °C. The compounds of the myrtle essential oil were identified using the NIST-MS and Wiley libraries and compared with the mass spectral data from the literature.
3.4. Preparation and Characterization of Nanoemulsion
3.4.1. Nanoemulsion Preparation
The nanoemulsion was formulated using the procedure previously described by Yazgan et al. [48], with minor modifications. The coarse emulsion was prepared from a mixture of myrtle essential oil (10% w/w), Tween 80 (1% w/w), and ultrapure water (89% w/w) and homogenized at 10,000 rpm for 2 min (T25 digital Ultra-Turrax; IKA, Staufen, Germany). The coarse emulsions were sonicated using an ultrasonic processor (VCX 750; Sonics & Materials, Inc., Newtown, CT, USA) for 5 min at 80% power amplitudes with a 13-mm-diameter titanium probe to obtain nanoemulsion.
3.4.2. Particle Size and Zeta Potential
The particle size and zeta potential of coarse emulsions and nanoemulsions were determined by dynamic light scattering (DLS) with a Zetasizer NanoZS laser diffractometer (Malvern Instruments Ltd., Worcestershire, UK) at 25 °C. The mean particle size and ζ-potential of myrtle essential oil nanoemulsion were measured to be 144.6 ± 4.41 nm and, −37.3 ± 0.61, respectively.
3.4.3. Measurement of Minimum Inhibitory and Minimum Bactericidal Concentrations of Myrtle Essential Oil Emulsions and Nanoemulsions
Minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of myrtle essential oil emulsions and nanoemulsions were determined by a broth dilution method in ¼ strength TSBYE amended with 0.15% agar as described by Delaquis et al. [49]. L. monocytogenes ATCC 19111, L. monocytogenes ATCC 7644, and L. monocytogenes ATCC 13932 were cultured individually in TSBYE for 24 h at 37 °C. The essential oil emulsions and nanoemulsions were diluted in a 1:1 ratio with ½ strength TSBYE + 0.30% agar (50 mg/mL). The stock solutions were dispensed into the wells of microtiter plates along with sterile ¼ strength TSBYE + 0.15% agar to achieve concentrations ranging between 0.5 to 50 mg/mL. Each well was then inoculated with 10 µL of culture (final cell concentration ~5 log cfu/mL). MIC was defined as the lowest concentration that prevented visible growth. A loopful of medium from wells without evidence of growth was applied to tryptic soy agar supplemented with 5 g/L yeast extract (TSAYE) which was incubated for 24 h at 37 °C to determine MBC.
3.4.4. Membrane Integrity
The effect of myrtle essential oil nanoemulsion on the membrane integrity of L. monocytogenes was performed according to Sugumar et al. [50], with minor modifications. L. monocytogenes ATCC 7644 was cultured in TSBYE at 37 °C for 24 h. The cells were harvested by centrifugation at 6000 rpm for 10 min, washed twice, and resuspended in a sterile 0.85% NaCl (final cell concentration ~7 log cfu/mL). The cell suspensions were treated with the MBC of the nanoemulsions at 37 °C for 5, 15, and 30 min. After incubation, the mixture was filtered through 0.22 μm cellulose acetate membrane filter and the absorbance of the treated cell filtrate was read using a UV–visible spectrophotometer (UV-1240; Shimadzu, Kyoto, Japan) at 260 nm (A1). The leakage of UV absorbance was calculated as (A1/A0) × 100. Cell culture treated with Triton X-100 (A0) or without any treatment was used as positive and negative controls, respectively.
3.4.5. Field Emission Scanning Electron Microscopy (FESEM)
The mode of action of essential oil nanoemulsion on L. monocytogenes was confirmed using field emission scanning electron microscopy (FESEM, Quanta 450 FEG; FEI, Hillsboro, OR, USA) as described by Shi et al. [51], with minor modifications. L. monocytogenes ATCC 7644 cells were treated with MBC concentration of myrtle essential oil nanoemulsion, and incubated at 37 °C for 15 min. The cells were harvested by centrifugation at 6000 rpm for 10 min, and washed twice with phosphate-buffered saline solution (pH 7.0). The microorganisms were resuspended in water containing 2.5% glutaraldehyde, and then held at 4 °C for 12 h. Subsequently, the cells were gradually dehydrated in water–ethanol series (30%, 50%, 70%, 80%, 90%, and 100% ethanol) for 10 min. Finally, the samples were dried, and gold-sputter coated to prepare for analysis under a FESEM at 20,000× magnification.
3.5. Preparation and Characterization of the Nanoemulsion Coating Solution
3.5.1. Preparation of Nanoemulsion-Coating Solution
Sodium alginate (2% w/w) was dissolved in ultrapure water at 70 °C for 2 h. Coarse emulsions were prepared by mixing the alginate solution (2% w/v), myrtle essential oil (0.5, 1, and 2% w/w), glycerol (1.5% w/w), and Tween 80 (1% w/w). The mixtures were homogenized at 10,000 rpm for 2 min (T25 digital Ultra-Turrax; IKA, Germany). Then, the coarse emulsions were subjected to ultrasonication using an ultrasonic processor (VCX 750; Sonics & Materials, Inc., Newtown, CT, USA) for 5 min at 80% power amplitudes with a 13-mm-diameter titanium probe.
3.5.2. Particle Size and Zeta Potential
The particle size and zeta potential of the coarse emulsions and nanoemulsions were determined as previously mentioned in Section 3.4.2.
3.5.3. Whiteness Index (WI)
The color values of coarse emulsions and nanoemulsions were determined using a colorimeter (Minolta CM-3600d, Osaka, Japan). The parameters L* (lightness), a* (red-green scale), and b* (yellow-blue scale) were measured. The whiteness index (WI) was calculated using the following equation [32]:
WI = 100 − [(100 − L*)2 + (a*2 + b*2)]0.5
3.6. Application of Coatings Solutions on Cheese Samples
The cheese samples were aseptically cut into approximately 10 g pieces (70 mm × 45 mm × 3 mm) and placed onto sterile trays. The cheese samples were randomly divided into five groups (shown in Table 6. All of the groups were individually immersed in alginate nanoemulsion solutions for 2 min and were allowed to drain for 2 min except for the control group. The uncoated samples were immersed in ultrapure water following the same procedure. The cheese samples were placed separately in a sterile bag (Whirl-Pak; Nasco, Wisconsin, USA), and sealed. A total of 300 cheese samples (five treatments × three replicates × four samples × five days) were prepared on different days for physicochemical analyses. Each treatment was stored at 5 °C for 24 days and sampled at days 0, 6, 12, 18, and 24 for the physicochemical analyses. A total of 90 cheese samples (five treatments × three replicates × six panelists) were prepared for the sensory evaluation.

Table 6.
List of treatments in the present study.
3.6.1. Antibacterial Activity against Inoculated L. monocytogenes
The L. monocytogenes strains were cultured in TSBYE at 37 °C for 24 h. The sliced cheese pieces were irradiated with ultraviolet light in a laminar airflow cabinet for 15 min to eliminate the background microflora. The cheese samples were tested for the presence of L. monocytogenes before the experiments. Each sample was inoculated with 50 µL aliquots of the cocktail of L. monocytogenes strains to obtain a final concentration of approximately 4.5 log cfu/g. The inocula were evenly spread over the surface of the cheese samples and allowed to dry for 15 min for the bacterial attachment. The cheese samples were coated as described in Section 3.6, placed separately in a sterile bag (Whirl-Pak; Nasco, WI, USA), and sealed. A total of 120 cheese samples (five treatments × three replicates × eight days) were prepared on different days. Each treatment was stored at 5 °C for 24 days and sampled at regular time-intervals for the analysis. The sample was homogenized with sterile 0.1% (w/v) peptone water using a stomacher (BagMixer®; Interscience, France) for 1 min. Appropriate serially diluted samples were spread onto Oxford Agar plates which were incubated at 37 °C for 24 h. The population of L. monocytogenes was expressed as log cfu/g samples.
3.6.2. Physicochemical Properties of Cheese
Water Activity, pH, and Color
The water activity (aw) was measured twice for each sample using a water activity meter (Aqua Lab Series 3TE; Decagon Devices Inc., Pullman, WA, USA). The surface color of coated and uncoated cheese samples was determined using a colorimeter (PCE-CSM 7; PCE Instruments, UK), and the parameters L*, a*, and b* values were recorded at room temperature. The whiteness index was calculated through Equation (1). Each cheese sample (10 g) was homogenized with distilled water (100 mL) for 1 min using a homogenizer (Ultra-Turrax T25; IKA Labortechnik, Staufen, Germany), and pH was measured by a digital pH-meter (Seven Compact S210; Mettler-Toledo, Switzerland).
Hardness Determination
The hardness value of Kasar cheese samples was determined using a texture analyzer (Brookfield AMETEK CT3-4500; Middleboro, MA, USA). Cheese samples were subjected to a compression test at 6–8 °C using a stainless steel, 2-cm-long cylindrical TA39 probe. The test conditions were 10 mm penetration distance, 3 g trigger load, and 5 mm/s speed. The results were expressed in Newton (N).
3.6.3. Sensory Evaluation
The sensory properties of cheese samples were examined after 1 day of storage with the contribution of six semi-trained panelists in cheese-product evaluation at the Department of Food Engineering, Sakarya University. The panelists rated each sample for color, odor, flavor/taste, texture, and overall acceptability, using a 9-point hedonic scale, 1: dislike extremely, 5: neither like nor dislike and 9: like extremely. Cheese samples receiving overall scores of at least 5 were considered acceptable.
3.7. Statistical Analysis
Data analysis by ANOVA and Duncan’s multiple range test (level of confidence 95%, p < 0.05) was performed using SPSS 20.0 Statistics Software (SPSS, Chicago, IL, USA). All experiments were performed in triplicate and values were expressed as mean ± standard deviation.
4. Conclusions
A new nanoemulsion-based edible coating containing myrtle essential oil was developed using ultrasound treatment. Myrtle essential oil showed a potent antibacterial activity against L. monocytogenes in in vitro analyses. The antibacterial effect of myrtle essential oil against L. monocytogenes increased significantly with ultrasound treatment. The ability of myrtle essential oil nanoemulsion to damage the morphology of L. monocytogenes was clearly demonstrated by FESEM. Nanoemulsion coatings containing 0.5% myrtle essential oil showed bacteriostatic activity against target bacteria in cheese, while nanoemulsion coatings containing 1.0% and 2.0% essential oil had bactericidal effects. It was determined that the essential oil concentration is an important factor in inhibiting L. monocytogenes. Sodium alginate-based nanoemulsion coatings containing myrtle essential oil did not have a negative effect on the physicochemical properties of cheeses, such as pH, color, and hardness. In addition, the sensory evaluation results indicated that the color and appearance attributes were improved by coating the cheese samples. Coated cheese samples were considered to be acceptable at all myrtle essential oil concentrations. Consequently, nanoemulsion-based alginate edible coatings containing myrtle essential oil may be a promising alternative to synthetic additives to increase food safety.
Author Contributions
Conceptualization, G.P.Y., E.S. and H.S.; Methodology, G.P.Y., E.S. and H.S.; Validation, G.P.Y., E.S. and H.S.; Formal Analysis, G.P.Y., E.S. and H.S.; Investigation, G.P.Y., E.S. and H.S.; Data Curation, G.P.Y., E.S. and H.S.; Writing—Original Draft Preparation, G.P.Y., E.S. and H.S.; Writing—Review and Editing, G.P.Y.; Supervision, G.P.Y.; Resources, G.P.Y.; Project Administration, G.P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Scientific Research Project Unit of Sakarya University (Grant No: 2021-9-33-138).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gerard, A.; El-Haajjaji, A.; Niyonzima, E.; Daube, G.; Sindic, M. Prevalence and survival of Listeria monocytogenes in various types of cheese—A review. Int. J. Dairy Technol. 2018, 1, 825–843. [Google Scholar] [CrossRef]
- Wemmwnhove, E.; Wells-Bennik, M.H.J.; Zwietering, M.H. A model to predict the fate of Listeria monocytogenes in different cheese types—A major role for undissociated lactic acid in addition to pH, water activity, and temperature. Int. J. Food Microbiol. 2021, 357, 109350. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Yangılar, F. Chitosan/whey Protein (CWP) Edible Films Efficiency for Controlling Mould Growth and on Microbiological, Chemical and Sensory Properties During Storage of Göbek Kashar Cheese. Korean J. Food Sci. Anim. Resour. 2015, 35, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X. An Overview of Ultrasound-Assisted Food-Grade Nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Kong, I.; Degraeve, P.; Pui, L.P. Polysaccharide-Based Edible Films Incorporated with Essential Oil Nanoemulsions: Physico-Chemical, Mechanical Properties and Its Application in Food Preservation—A Review. Foods 2022, 11, 555. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Salehabadi, A.; Nafchi, A.M.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food- grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Dhifi, W.; Jazi, S.; El Beyrouthy, M.; Sadaka, C.; Mnif, W. Assessing the potential and safety of Myrtus communis flower essential oils as efficient natural preservatives against Listeria monocytogenes growth in minced beef under refrigeration. Food Sci. Nutr. 2020, 8, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Silva, A.C.; García-Díez, J.; Cenci-Goga, B.; Grispoldi, L.; Silva, A.F.; Almeida, J.M. Antimicrobial Activity of Myrtus communis L. and Rosmarinus officinalis L. Essential Oils against Listeria monocytogenes in Cheese. Foods 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Fadil, M.; Benbrahim, K.F.; Rachiq, S.; Ihssane, B.; Lebrazi, S.; Chraibi, M.; Haloui, T.; Farah, A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018, 126, 211–220. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Bajalan, I.; Pirbalouti, A.C. Variation in antibacterial activity and chemical compositions of essential oil from different populations of myrtle. Ind. Crops Prod. 2014, 61, 303–307. [Google Scholar] [CrossRef]
- Usai, M.; Mulas, M.; Marchetti, M. Chemical composition of essential oils of leaves and flowers from five cultivars of myrtle (Myrtus communis L.). J. Essent. Oil Res. 2015, 27, 465–476. [Google Scholar] [CrossRef]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1250. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative study of essential oils extracted from Algerian Myrtus communis L. leaves using microwaves and hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef]
- Akin, M.; Aktumsek, A.; Nostro, A. Antibacterial activity and composition of the essential oils of Eucalyptus camaldulensis Dehn. and Myrtus communis L. growing in Northern Cyprus. Afr. J. Biotechnol. 2010, 9, 531–535. [Google Scholar]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, V.; Vivenzio, G.; Nazzaro, F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizi, A.; Adib, H. Inhibition of Listeria monocytogenes growth in turkey fillets by alginate edible coating with Trachyspermum ammi essential oil nano-emulsion. Int. J. Food Microbiol. 2021, 344, 109104. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Aliahmadi, A.; McClements, D.J.; Rafeti, H. Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT-Food Sci. Technol. 2016, 71, 69–76. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Antibacterial mechanisms of thyme essential oil nanoemulsions against Escherichia coli O157:H7 and Staphylococcus aureus: Alterations in membrane compositions and characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102902. [Google Scholar] [CrossRef]
- Ghaderi, L.; Moghimi, R.; Aliahmadi, A.; McClements, D.J.; Rafati, H. Development of antimicrobial nanoemulsion-based delivery systems against selected pathogenic bacteria using a thymol-rich Thymus daenensis essential oil. J. Appl. Microbiol. 2017, 123, 832–840. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.I.; Taktak, N.E.M. Preparation, characterization, and antimicrobial activity of cinnamon essential oil and cinnamaldehyde nanoemulsions. J. Essent. Oil Res. 2022. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.O.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Sikkema, J.; Bont, J.A.M.; Poolman, B. Interactions of Cyclic Hydrocarbons with Biological Membranes. J. Biol. Chem. 1994, 269, 8022–8023. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion- based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Rahmasari, Y.; Polat Yemiş, G. Characterization of ginger starch-based edible films incorporated with coconut shell liquid smoke by ultrasound treatment and application for ground beef. Meat Sci. 2022, 188, 108799. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef]
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. Invited review: Perspectives on the basis of the rheology and texture properties of cheese. J. Dairy Sci. 2003, 86, 2725–2743. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C.G. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT-Food Sci. Technol. 2022, 153, 112486. [Google Scholar] [CrossRef]
- Berti, S.; Olle Resa, C.P.; Basanta, F.; Gerschenson, L.N.; Jagus, R.J. Edible coatings on Gouda cheese as a barrier against external contamination during ripening. Food Biosci. 2019, 31, 1000447. [Google Scholar] [CrossRef]
- Delgado, F.J.; Gonzalez-Crespo, J.; Cava, R.; Ramirez, R. Changes in microbiology, proteolysis, texture and sensory characteristics of raw goat milk cheeses treated by high-pressure at different stages of maturation. LWT-Food Sci. Technol. 2012, 48, 268–275. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT-Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Özogul, Y.; El Abed, N.; Özogul, F. Antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens and fish spoilage bacteria. Food Chem. 2022, 368, 130831. [Google Scholar] [CrossRef]
- Yazgan, H.; Ozogul, Y.; Kuley, E. Antimicrobial influence of nanoemulsified lemon essential oil and pure lemon essential oil on food-borne pathogens and fish spoilage bacteria. Int. J. Food Microbiol. 2019, 306, 108266. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Sugumar, S.; Ghosh, M.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. SonoChem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef]
- Shi, C.; Sun, Y.; Zheng, Z.; Zhang, X.; Song, K.; Jia, Z.; Chen, Y.; Yang, M.; Liu, X.; Dong, R.; et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem. 2016, 197, 100–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).