Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometry (GC-MS)
- carvacrol: EO 2, 3, 4, 6
- caryophyllene oxide: EO 1
- terpineol/sabinene hydrate: EO 5, 7, 8
- thymol: EO 9
2.2. Thin-Layer Chromatography-Direct Bioautography (TLC-DB)
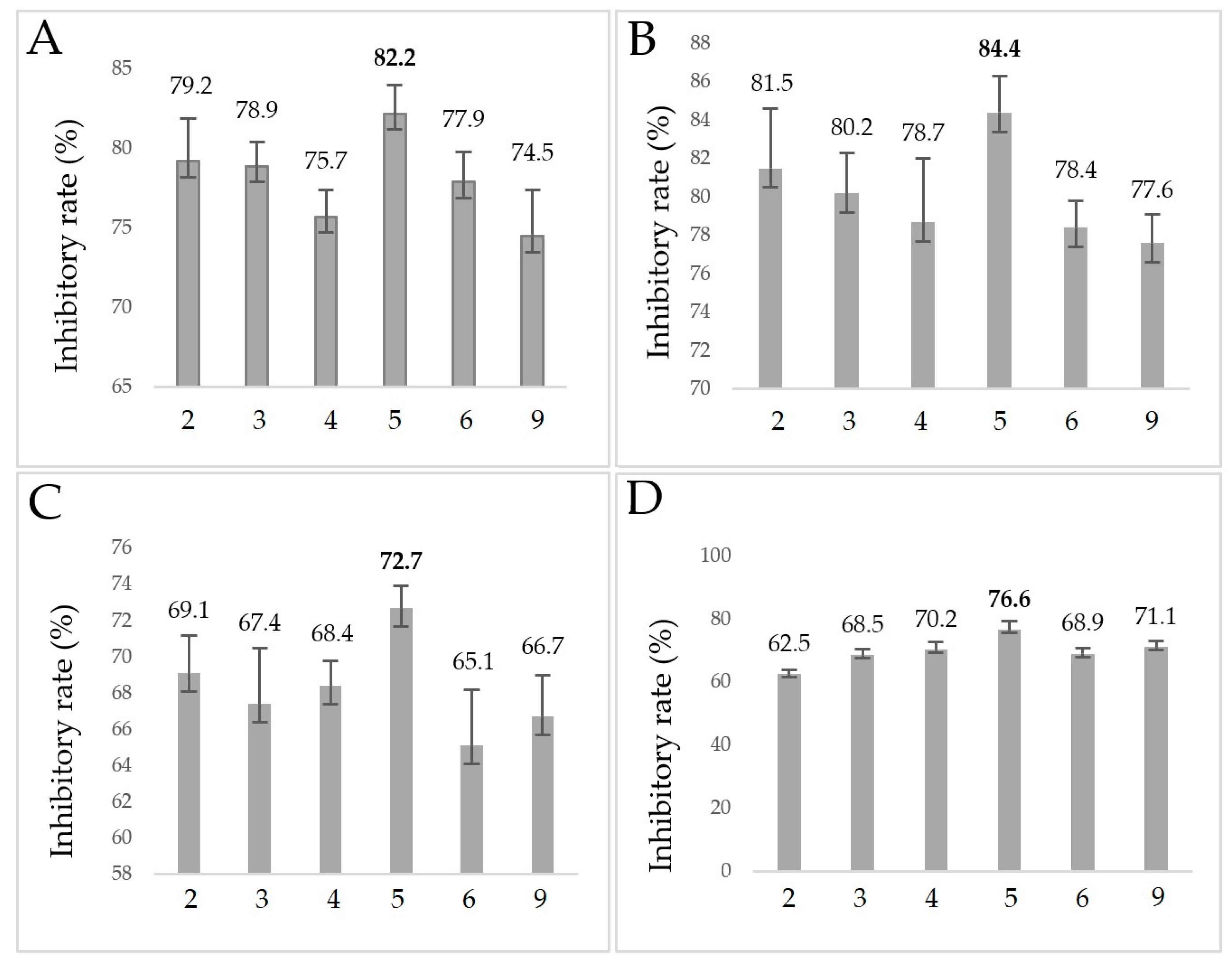
2.3. Minimum Inhibitory Concentration Assay
2.4. Anti-Biofilm Assay
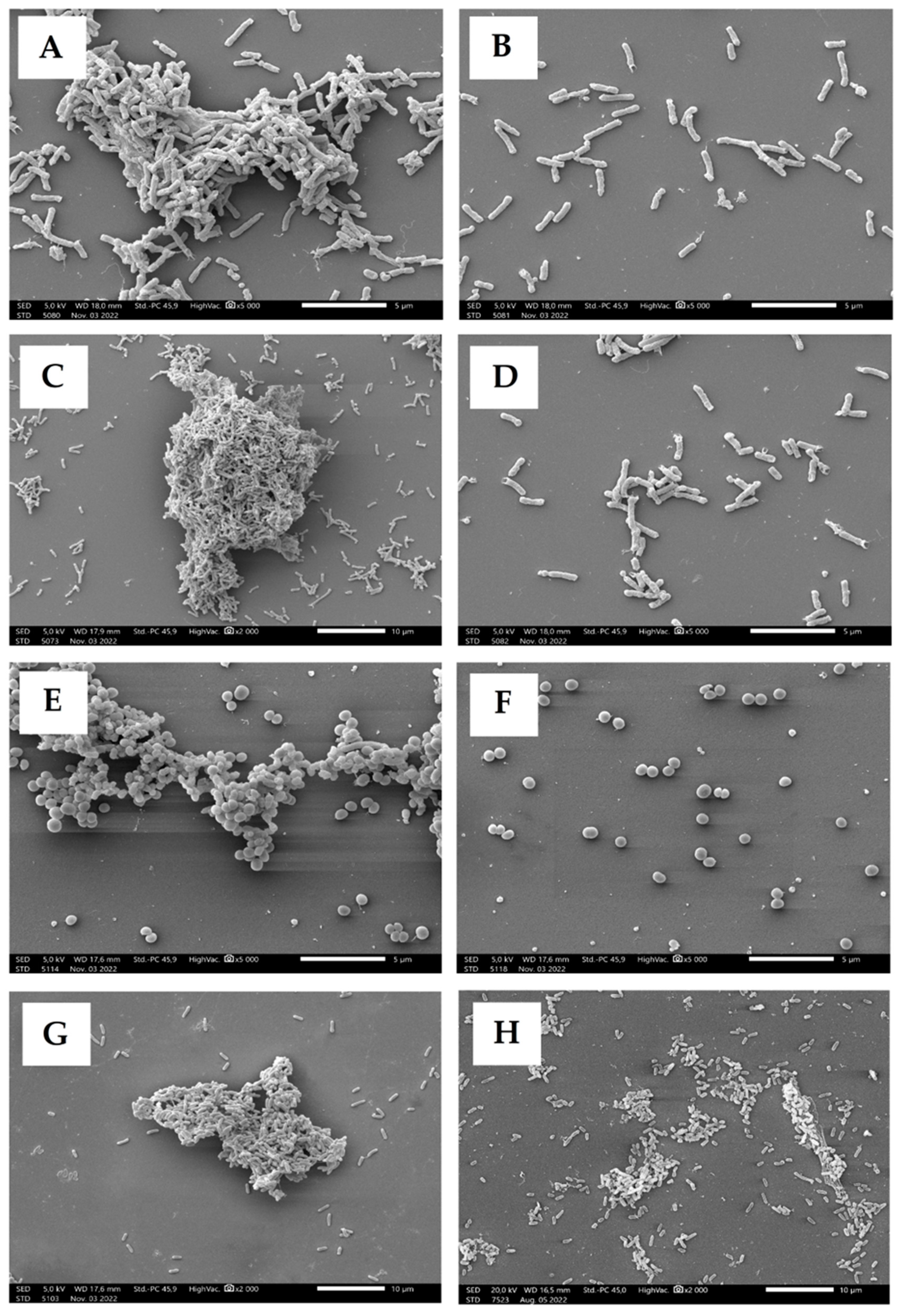
2.5. Scanning Electron Microscopy
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Gas Chromatography-Mass Spectrometry
4.3. Cultivation of Bacterial Strains
4.4. Thin-Layer Chromatography-Direct Bioautography (TLC-DB) Assay
4.5. Minimum Inhibitory Concentrations and Anti-Biofilm Assay
4.6. Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Origanum Tourn. ex L. Available online: http://www.worldfloraonline.org/taxon/wfo-4000027145 (accessed on 13 February 2023).
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phyther. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Erdem, S.A.; Ozturk, S.; Oz, Z.S.; Subasi, E.; Koyuncu, M.; Vlainić, J.; Kosalec, I. Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model. Molecules 2022, 27, 663. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from Oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, P.; Liu, H.; Sun, X.; Liang, J.; Sun, L.; Chen, Y. Hypoglycemic activity of Origanum vulgare L. And its main chemical constituents identified with HPLC-ESI-QTOF-MS. Food Funct. 2021, 12, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Fares, R.; Bazzi, S.; El-Chami, N.; Baydoun, E. The apoptotic and anti-proliferative activity of Origanum majorana extracts on human leukemic cell line. Leuk. Res. 2010, 34, 1052–1056. [Google Scholar] [CrossRef]
- Gong, H.Y.; Liu, W.H.; LV, G.Y.; Zhou, X. Analysis of essential oils of Origanum vulgare from six production areas of China and Pakistan. Rev. Bras. Farmacogn. 2014, 24, 25–32. [Google Scholar] [CrossRef]
- Baj, T.; Sieniawska, E.; Ludwiczuk, A.; Widelski, J.; Kiełtyka-Dadasiewicz, A.; Skalicka-Woźniak, K.; Głowniak, K. Thin-layer chromatography-fingerprint, antioxidant activity, and gas chromatography-mass spectrometry profiling of several Origanum L. species. J. Planar Chromatogr.–Mod. TLC 2017, 30, 386–391. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.H.; Ye, M.; Wang, K.B.; Fan, L.M.; Su, F.W. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, R.; Golus, J.; Przekora, A.; Ludwiczuk, A.; Sieniawska, E.; Ginalska, G. Antimycobacterial activity of cinnamaldehyde in a mycobacterium tuberculosis(H37Ra) model. Molecules 2018, 23, 2381. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, anti-MRSA activity and toxicity of essential oils from Cymbopogon species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Singh, B.N.; Prateeksha, N.; Upreti, D.K.; Singh, B.R.; Defoirdt, T.; Gupta, V.K.; De Souza, A.O.; Singh, H.B.; Barreira, J.C.M.; Ferreira, I.C.F.R.; et al. Bactericidal, quorum quenching and anti-biofilm nanofactories: A new niche for nanotechnologists. Crit. Rev. Biotechnol. 2017, 37, 525–540. [Google Scholar] [CrossRef]
- Sepahi, E.; Tarighi, S.; Ahmadi, F.S.; Bagheri, A. Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J. Microbiol. 2015, 53, 176–180. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, W.; Guo, Y.; Wu, Q.; Wang, F.; Xuan, H. Anti-Biofilm Activities of Chinese Poplar Propolis Essential Oil against Streptococcus mutans. Nutrients 2022, 14, 3290. [Google Scholar] [CrossRef]
- Guimarães Silva Vasconcelos, P.; Medeiros de Almeida Maia, C.; Mendes de Vasconcelos, V.; Paolla Raimundo e Silva, J.; Fechine Tavares, J.; Vieira Pereira, J.; Wanderley Cavalcanti, Y.; Maria Melo de Brito Costa, E. In vitro inhibition of a multispecies oral cavity biofilm by Syzygium aromaticum essential oil. Gerodontology 2022, 39, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Sienkiewicz, M.; Pruss, A.; Łopusiewicz, Ł.; Arszyńska, N.; Wojciechowska-Koszko, I.; Kilanowicz, A.; Kot, B.; Dołęgowska, B. Antibacterial and Anti-Biofilm Activities of Essential Oil Compounds against New DelhiMetallo-β-Lactamase-1-Producing Uropathogenic Klebsiella pneumoniae Strains. Antibiotics 2022, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-biofilm effect of selected essential oils and main components on mono- and polymicrobic bacterial cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical Composition, Enantiomeric Distribution, Antimicrobial and Antioxidant Activities of Origanum majorana L. Essential Oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Nizamlloǧlu, N.M.; Gücüş, M.O.; Bildik Dal, A.E.; Akgül, K. Origanum majorana L. Essential Oil-Coated Paper Acts as an Antimicrobial and Antioxidant Agent against Meat Spoilage. ACS Omega 2022, 7, 9033–9043. [Google Scholar] [CrossRef]
- Silva, E.C.A.d.; Leuthier, L.L.; Almeida Júnior, A.; Nunes, J.M.F.F.; Correia Sampaio, F.; Farias, I.A.P. Physicochemical characteristics and antimicrobial activity of Origanum vulgare L. essential oil and carvacrol on cariogenic bacteria: An in vitro and in silico study. Nat. Prod. Res. 2022, 36, 6410–6413. [Google Scholar] [CrossRef] [PubMed]
- Sarac, N.; Ugur, A. Antimicrobial activities of the essential oils of Origanum onites L., Origanum vulgare L. subspecies hirtum (link) Ietswaart, Satureja thymbra L., and Thymus cilicicus Boiss. & Bal. growing wild in Turkey. J. Med. Food 2008, 11, 568–573. [Google Scholar]
- Alma, M.H.; Mavi, A.; Yildirim, A.; Digrak, M.; Hirata, T. Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol. Pharm. Bull. 2003, 26, 1725–1729. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Shi, L. A Carvacrol-Rich Essential Oil Extracted From Oregano (Origanum vulgare “Hot & Spicy”) Exerts Potent Antibacterial Effects Against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar]
- Baranska, M.; Schulz, H.; Krüger, H.; Quilitzsch, R. Chemotaxonomy of aromatic plants of the genus Origanum via vibrational spectroscopy. Anal. Bioanal. Chem. 2005, 381, 1241–1247. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zouhir, A.; Jridi, T.; Nefzi, A.; Ben Hamida, J.; Sebei, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by antimicrobial peptides (AMPs) and plant essential oils. Pharm. Biol. 2016, 54, 3136–3150. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Niaz, N.; Bano, A.; Khan, B.A.; Zafar, N.; Iqbal, M.; Tahira, R.; Fasim, F. Essential oils showing in vitro anti MRSA and synergistic activity with penicillin group of antibiotics. Pak. J. Pharm. Sci. 2017, 30, 1997–2002. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Eichenberger, E.M.; Thaden, J.T. Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiotics 2019, 8, 37. [Google Scholar] [CrossRef]
- Otsuka, Y. Potent antibiotics active against multidrug-resistant gram-negative bacteria. Chem. Pharm. Bull. 2020, 68, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Balázs, V.L.; Horváth, B.; Kerekes, E.; Ács, K.; Kocsis, B.; Varga, A.; Böszörményi, A.; Nagy, D.U.; Krisch, J.; Széchenyi, A.; et al. Anti-haemophilus activity of selected essential oils detected by TLC-direct bioautography and biofilm inhibition. Molecules 2019, 24, 3301. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qian, C.; Xu, H.; Huang, Y. Antibacterial activity of Artemisia asiatica essential oil against some common respiratory infection causing bacterial strains and its mechanism of action in Haemophilus influenzae. Microb. Pathog. 2018, 114, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Sela, F.; Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Kulevanova, S. Chemical composition and antimicrobial activity of essential oils of Juniperus excelsa Bieb. (Cupressaceae) grown in R. Macedonia. Pharmacognosy Res. 2015, 7, 74–80. [Google Scholar] [PubMed]
- Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Grapefruit essential oils inhibit quorum sensing of Pseudomonas aeruginosa. Food Sci. Technol. Int. 2020, 26, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Van, L.T.; Hagiu, I.; Popovici, A.; Marinescu, F.; Gheorghe, I.; Curutiu, C.; Ditu, L.M.; Holban, A.M.; Sesan, T.E.; Lazar, V. Antimicrobial Efficiency of Some Essential Oils in Antibiotic-Resistant Pseudomonas aeruginosa Isolates. Plants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Horváth, G.; Jámbor, N.; Végh, A.; Böszörményi, A.; Lemberkovics, É.; Héthelyi, É.; Kovácsc, K.; Kocsisc, B. Antimicrobial activity of essential oils: The possibilities of TLC-bioautography. Flavour Fragr. J. 2010, 25, 178–182. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential oil as a potential anti-acne topical nanoemulsion—In vitro and in vivo study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef]
- Lagha, R.; Abdallah, F.B.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef]
- Shamseddine, L.; Chidiac, J.J. Composition’s effect of Origanum syriacum essential oils in the antimicrobial activities for the treatment of denture stomatitis. Odontology 2021, 109, 327–335. [Google Scholar] [CrossRef]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The effect of ten essential oils on several cutaneous drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, T.; Moradi, P.; Mahboubi, M.; Rahimi, E.; Momtaz, H.; Hamedi, B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine 2015, 22, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Pandey, H.; Narvi, S.S.; Agarwal, V. Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa. PLoS ONE 2015, 10, e0135495. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Mohammadzadeh, A.; Zahraei Salehi, T.; Mahmoodi, P. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 2018, 124, 379–388. [Google Scholar] [CrossRef]
- Ramos, S.; Rojas, L.B.; Lucena, M.E.; Meccia, G.; Usubillaga, A. Chemical composition and antibacterial activity of origanum majorana L. Essential oil from the venezuelan andes. J. Essent. Oil Res. 2011, 23, 45–49. [Google Scholar] [CrossRef]
- Gencic, M.; Aksic, J.; Stosic, Z.; Randjelovic, P.; Stojanovic, N.; Radic, Z.; Radulovic, N. Linking the antimicrobial and anti-inflammatory effects of immortelle essential oil with its chemical composition–The interplay between the major and minor constituents. Food Chem. Toxicol. 2021, 158, 112666. [Google Scholar] [CrossRef]
- Hsouna, A.; Halima, N.; Abdelkafi, S.; Hamdi, N. Essential oil from Artemisia phaeolepis: Chemical composition and antibacterial activities. J. Oleo Sci. 2013, 62, 973–980. [Google Scholar] [CrossRef]
- Miladinovic, D.; Dimitrijevic, M.; Krstev, T.; Markovic, M.; Ciric, V. The signifcance of minor components on the antibacterial activity of essential oil via chemometrics. LWT 2021, 136, 110305. [Google Scholar] [CrossRef]
- Humphries, R.M.; Hindler, J.A. Susceptibility Test Methods: Fastidious Bacteria. In Manual of Clinical Microbiology, 10th ed.; James, H.J., Karen, C.C., Guido, F., Michael, A.P., Marie, L.L., Sandra, S.R., David, W.W., Eds.; Wiley: Washington, DC, USA, 2011; Volume 1, pp. 1180–1187. [Google Scholar]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, C.; Liu, Y.; Ma, L.; Zhang, X. Effects of sub-minimum inhibitory concentrations of lemon essential oil on the acid tolerance and biofilm formation of Streptococcus mutans. Arch. Oral Biol. 2018, 87, 235–241. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
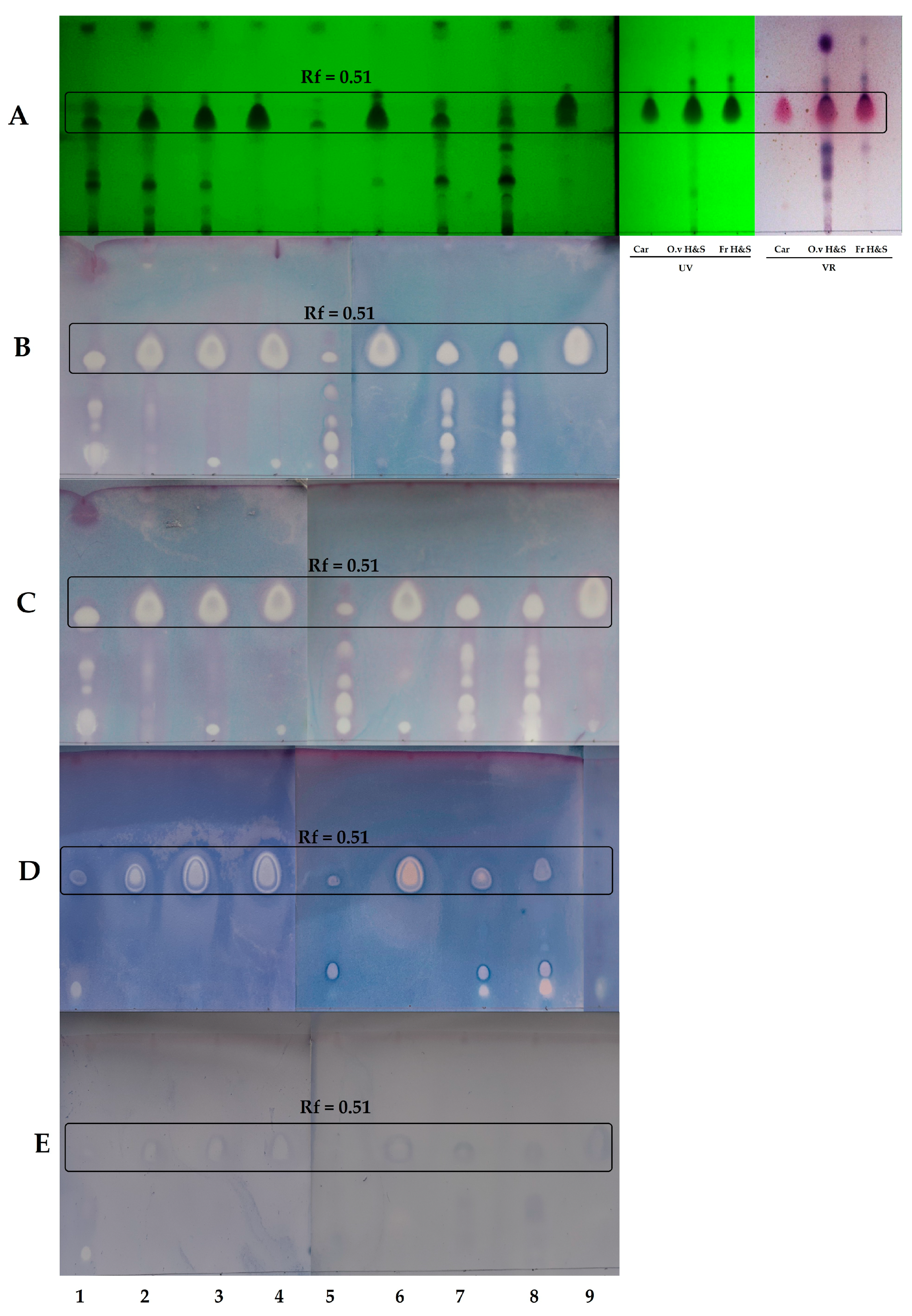
| RIex | RIlit | Compound | 1 * | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 925 | 932 | α-Thujene | - | - | - | 0.1 | - | - | 0.2 | - | 0.6 |
| 933 | 936 | α-Pinene | - | - | 0.3 | 0.6 | - | - | 0.2 | - | 0.5 |
| 973 | 973 | Sabinene | - | - | - | - | - | - | 0.7 | 0.2 | - |
| 978 | 978 | β-Pinene | - | - | - | - | - | - | 0.1 | - | 0.1 |
| 982 | 962 | 1-Octen-3-ol | 0.7 | 0.7 | 0.4 | 0.2 | - | 0.2 | 1.8 | 1.8 | 0.3 |
| 984 | 969 | 3-Octanone | - | 0.2 | 0.1 | - | - | - | 0.2 | - | - |
| 988 | 987 | Myrcene | - | - | 0.9 | 1.3 | - | 0.4 | 0.3 | - | 1.7 |
| 998 | 981 | 3-Octanol | 0.2 | - | - | - | - | 0.2 | 0.2 | - | 0.4 |
| 1007 | 1002 | α-Phellandrene | - | - | - | 0.1 | - | - | - | - | 0.2 |
| 1018 | 1013 | α-Terpinene | - | - | 0.5 | 0.9 | 0.1 | 0.4 | 0.4 | - | 1.9 |
| 1026 | 1015 | p-Cymene | 1.8 | 1.1 | 5.8 | 5.6 | 0.1 | 3.8 | 8.2 | 4.1 | 7.0 |
| 1030 | 1024 | Limonene | - | - | 0.2 | 0.2 | - | - | 0.3 | 0.1 | 0.3 |
| 1033 | 1025 | 1,8-Cineole | 0.1 | 0.2 | 0.5 | - | - | - | 0.4 | 0.2 | 0.2 |
| 1036 | 1029 | (Z)-β-Ocimene | - | - | 0.2 | - | - | - | 0.3 | - | - |
| 1046 | 1041 | (E)-β-Ocimene | - | - | 0.1 | - | - | - | - | - | - |
| 1060 | 1051 | γ-Terpinene | - | - | 2.1 | 2.7 | 0.2 | 1.3 | 0.2 | - | 8.9 |
| 1074 | 1065 | cis-Sabinene hydrate | 4.5 | 1.0 | 0.6 | 0.5 | 6.4 | 0.6 | 3.9 | 2.6 | 0.9 |
| 1087 | 1082 | α-Terpinolene | - | - | - | 0.1 | - | - | - | - | 0.1 |
| 1101 | 1086 | Linalool | 0.3 | 0.3 | 0.2 | - | 3.4 | - | 0.2 | 1.8 | 0.8 |
| 1105 | 1098 | trans-Sabinene hydrate | 1.5 | 0.9 | 0.5 | 0.3 | 37.1 | 0.6 | 26.4 | 28.0 | 1.2 |
| 1129 | 1108 | cis-p-Menth-2-en-1-ol | 0.2 | - | - | - | 1.7 | - | 0.8 | 1.6 | - |
| 1148 | 1116 | trans-p-Menth-2-en-1-ol | 0.1 | - | - | - | 0.7 | - | 0.4 | 0.1 | - |
| 1152 | 1123 | Camphor | - | 0.2 | - | - | - | - | 0.2 | 0.2 | - |
| 1162 | 1132 | Sabina ketone | 0.3 | - | - | - | - | - | 0.2 | 0.9 | - |
| 1179 | 1150 | endo-Borneol | - | 0.4 | 0.2 | 0.1 | - | 0.1 | 0.7 | 0.1 | 0.1 |
| 1183 | 1156 | Neomenthol | 0.3 | - | - | - | - | - | - | 0.2 | - |
| 1186 | 1164 | Terpinen-4-ol | 2.8 | 0.7 | 0.7 | 0.7 | 19.8 | 0.7 | 9.7 | 4.4 | 0.5 |
| 1193 | 1169 | p-Cymen-8-ol | 0.2 | - | - | - | 0.2 | - | 0.3 | 0.5 | - |
| 1201 | 1176 | α-Terpineol | 0.6 | 0.4 | 0.4 | 0.3 | 5.9 | 0.3 | 1.1 | 1.8 | 0.2 |
| 1215 | 1193 | trans-Piperitol | - | - | - | - | 0.4 | - | 0.2 | 0.3 | - |
| 1230 | 1215 | Thymol methyl ether | 0.1 | - | - | - | - | - | - | 0.1 | - |
| 1240 | 1221 | Carvacrol methyl ether | 0.2 | 1.8 | 0.8 | 0.4 | - | - | 3.8 | 0.3 | - |
| 1248 | 1239 | Linalyl acetate | - | - | - | - | 4.5 | - | - | - | 0.2 |
| 1249 | 1242 | Carvone | 0.7 | 0.1 | - | - | - | - | 0.3 | 0.3 | - |
| 1287 | 1270 | Bornyl acetate | - | 0.1 | - | - | - | - | 0.2 | 0.2 | - |
| 1297 | 1289 | Terpinen-4-yl acetate | - | - | - | - | 0.8 | - | - | - | - |
| 1301 | 1290 | Thymol | 0.8 | 0.3 | 0.4 | 0.4 | - | 0.6 | - | 5.7 | 46.9 |
| 1313 | 1300 | Carvacrol | 22.1 | 57.6 | 71.5 | 81.3 | 8.0 | 86.4 | 26.5 | 15.3 | 25.3 |
| 1356 | 1342 | Neryl acetate | - | - | - | - | 0.3 | - | - | - | - |
| 1367 | 1354 | Carvacryl acetate | - | 0.2 | 0.3 | 0.3 | - | 0.3 | - | - | - |
| 1375 | 1362 | Geranyl acetate | - | - | - | - | 0.6 | - | - | - | - |
| 1382 | 1379 | α-Copaene | 0.1 | - | 0.1 | - | - | - | - | 0.3 | - |
| 1391 | 1386 | β-Bourbonene | 1.8 | 0.2 | 0.2 | - | - | - | 1.1 | 0.9 | - |
| 1394 | 1389 | β-Elemene | 0.2 | - | - | - | - | - | 0.2 | 0.1 | - |
| 1429 | 1420 | (E)-β-Caryophyllene | 0.6 | - | 1.2 | 1.5 | 1.2 | 2.0 | - | 0.6 | 0.9 |
| 1438 | 1430 | β-Copaene | 0.2 | 0.1 | - | - | - | - | 0.1 | 0.1 | - |
| 1448 | 1443 | Aromandendrene | - | 0.2 | - | - | - | - | - | - | - |
| 1466 | 1455 | α-Humulene | 0.2 | - | 0.2 | - | - | - | - | - | - |
| 1470 | 1462 | allo-Aromadendrene | 0.7 | - | - | - | - | - | - | 0.8 | - |
| 1499 | 1494 | Valencene | - | 0.1 | 0.1 | - | - | - | - | - | - |
| 1505 | 1496 | α-Muurolene | 0.2 | 0.3 | 0.3 | - | - | - | - | 0.4 | 0.2 |
| 1511 | 1503 | β-Bisabolene | 0.4 | 1.9 | 0.6 | 0.2 | - | - | 0.5 | 0.2 | - |
| 1522 | 1507 | γ-Cadinene | 0.5 | 0.5 | 0.2 | 0.1 | - | - | - | 0.2 | - |
| 1525 | 1520 | δ-Cadinene | - | 0.7 | 0.5 | 0.3 | - | - | - | 0.5 | - |
| 1591 | 1572 | Spathulenol | 11.7 | 1.5 | 0.9 | 0.1 | 1.3 | 5.6 | 1.6 | - | |
| 1596 | 1580 | Caryophyllene oxide | 15.2 | 6.8 | 1.9 | 0.4 | 1.3 | 0.6 | 0.6 | 4.9 | - |
| 1606 | 1592 | Viridiflorol | 0.4 | - | - | - | - | - | 0.1 | 0.2 | - |
| 1625 | 1602 | Humulene epoxide II | 3.1 | 1.0 | 0.2 | 0.3 | - | - | - | 0.7 | - |
| 1669 | 1643 | α-Cadinol | 2.3 | 0.3 | 0.2 | - | - | - | 0.4 | 2.1 | - |
| 1964 | 1951 | Hexadecanoic acid | 1.3 | - | - | - | - | - | - | 0.2 | - |
| 2095 | 2096 | Heneicosane | 0.8 | 8.2 | - | - | - | - | - | - | - |
| Sample Name | Minimum Inhibitory Concentrations (mg/mL) | |||
|---|---|---|---|---|
| H. influenzae | H. parainfluenzae | P. aeruginosa | MRSA | |
| O. vulgare “Hirtum” (2) | 0.15 | 0.15 | 0.3 | 0.6 |
| O. vulgare “Margarita” (3) | 0.15 | 0.15 | 0.3 | 0.6 |
| O. vulgare “Hot & Spicy” (4) | 0.15 | 0.15 | 0.3 | 0.6 |
| O. majorana (5) | 0.15 | 0.15 | 0.15 | 0.3 |
| O. syriacum (I) (6) | 0.15 | 0.15 | 0.3 | 0.6 |
| O. syriacum (II) (9) | 0.15 | 0.15 | 0.3 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piasecki, B.; Balázs, V.L.; Kieltyka-Dadasiewicz, A.; Szabó, P.; Kocsis, B.; Horváth, G.; Ludwiczuk, A. Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens. Molecules 2023, 28, 3044. https://doi.org/10.3390/molecules28073044
Piasecki B, Balázs VL, Kieltyka-Dadasiewicz A, Szabó P, Kocsis B, Horváth G, Ludwiczuk A. Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens. Molecules. 2023; 28(7):3044. https://doi.org/10.3390/molecules28073044
Chicago/Turabian StylePiasecki, Bartłomiej, Viktória L. Balázs, Anna Kieltyka-Dadasiewicz, Péter Szabó, Béla Kocsis, Györgyi Horváth, and Agnieszka Ludwiczuk. 2023. "Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens" Molecules 28, no. 7: 3044. https://doi.org/10.3390/molecules28073044
APA StylePiasecki, B., Balázs, V. L., Kieltyka-Dadasiewicz, A., Szabó, P., Kocsis, B., Horváth, G., & Ludwiczuk, A. (2023). Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens. Molecules, 28(7), 3044. https://doi.org/10.3390/molecules28073044










