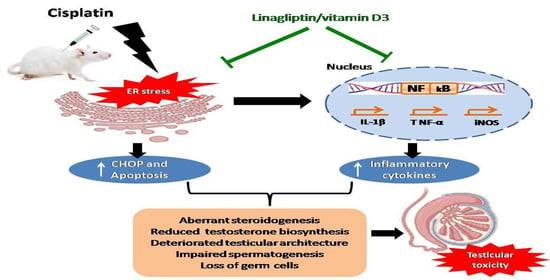
Linagliptin and Vitamin D3 Synergistically Rescue Testicular Steroidogenesis and Spermatogenesis in Cisplatin-Exposed Rats: The Crosstalk of Endoplasmic Reticulum Stress with NF-κB/iNOS Activation
Abstract
1. Introduction
2. Material and Methods
2.1. Drugs and Chemicals
2.2. Animals and Experimental Design
2.3. Hormonal Analysis
2.4. Measurement of Proinflammatory Cytokines
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Measurement of ER Stress Markers
2.7. Histological Analysis
2.8. Morphometric Analysis
2.9. Immunohistochemical Staining
2.10. Statistical Analysis
3. Results
3.1. Effect of Vitamin D3 and/or Linagliptin on Body and Testicular Weights of Cisplatin-Treated Rats
3.2. Effect of Vitamin D3 and/or Linagliptin on Testicular Architecture in Cisplatin-Treated Rats
3.3. Effect of Vitamin D3 and/or Linagliptin on Spermatogenesis and Somatic Populations in Cisplatin-Treated Rats
3.4. Effect of Vitamin D3 and/or Linagliptin on Gonadal Hormone Levels in Cisplatin-Treated Rats
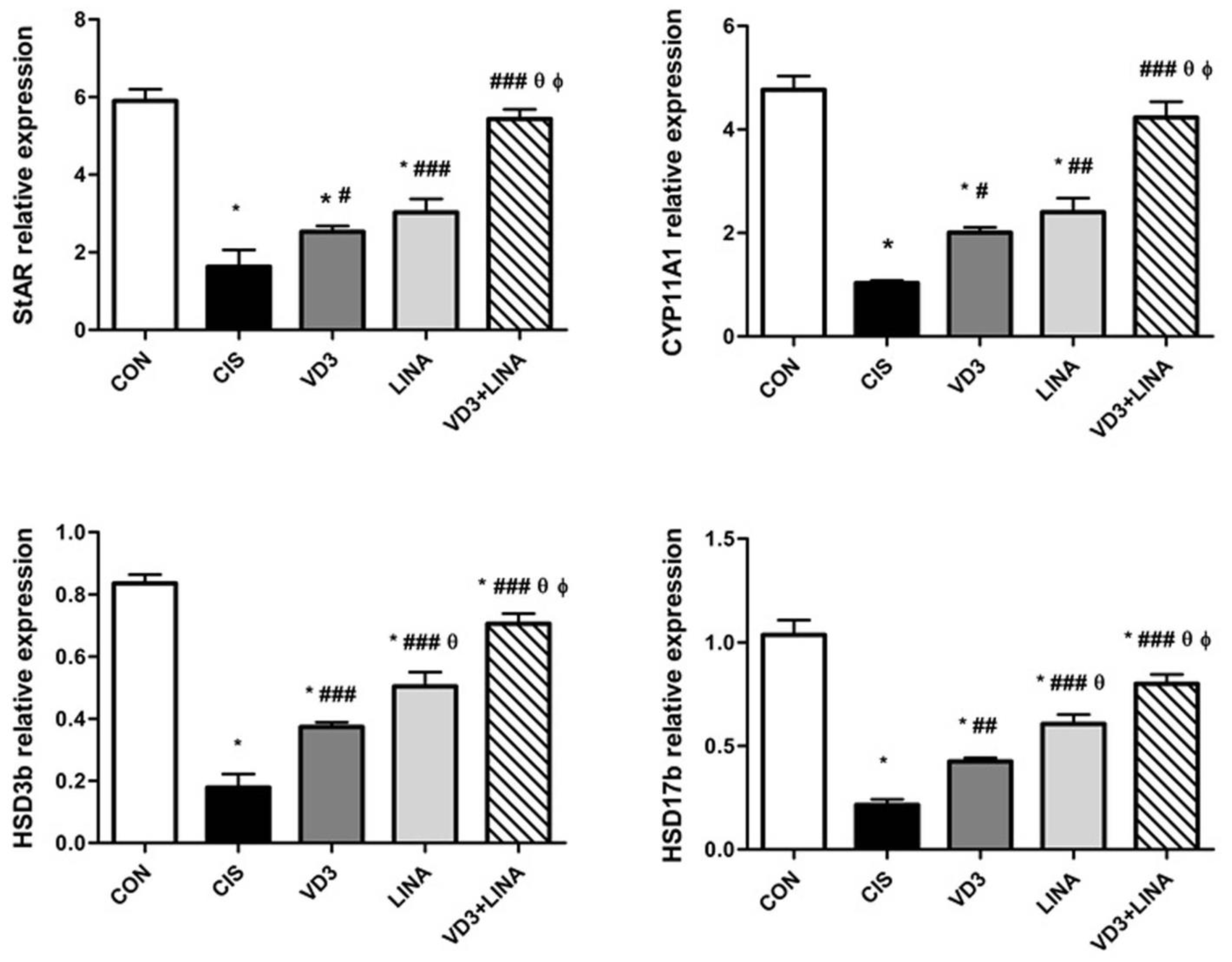
3.5. Effect of Vitamin D3 and/or Linagliptin on Expressions of Steroidogenesis-Related Genes in Testes of Cisplatin-Treated Rats
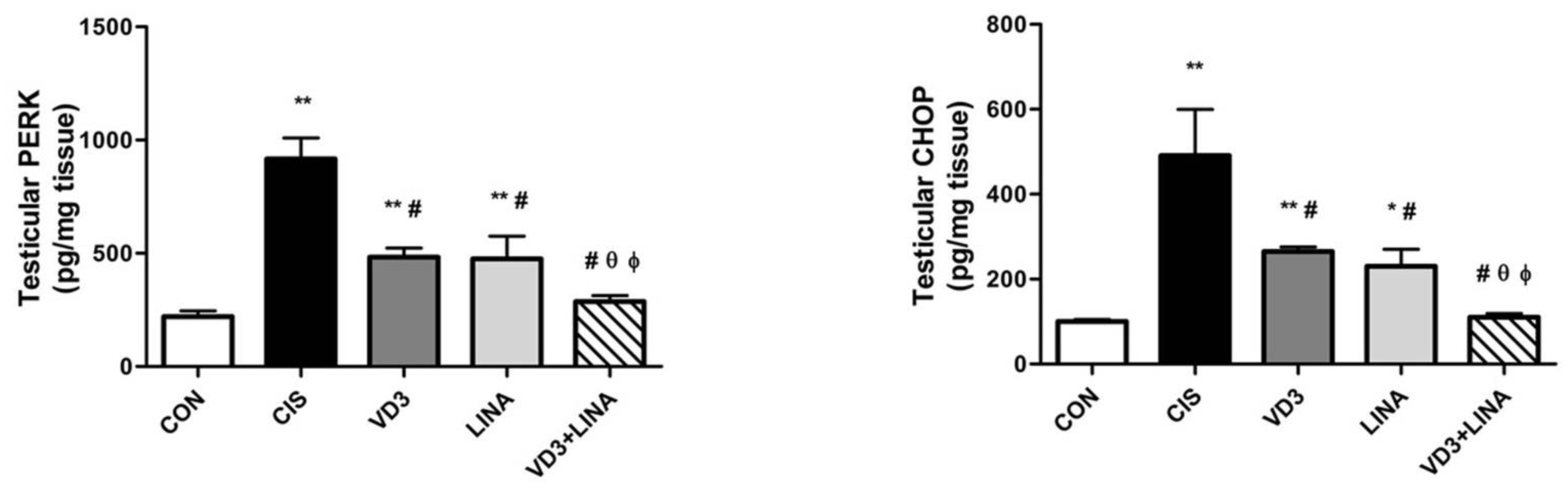
3.6. Effect of Vitamin D3 and/or Linagliptin on Testicular Endoplasmic Reticulum Stress in Cisplatin-Treated Rats
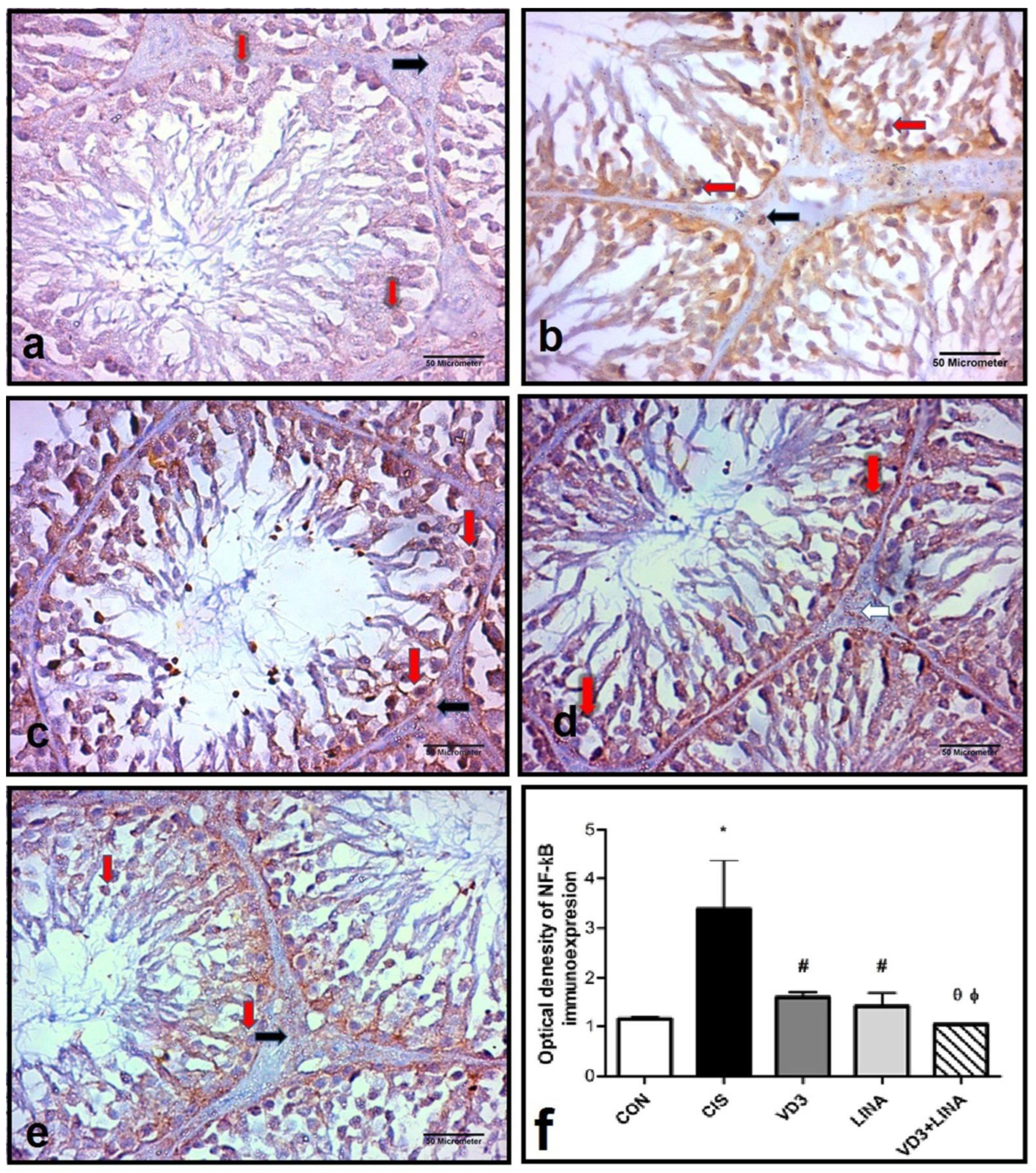
3.7. Effect of Vitamin D3 and/or Linagliptin Therapy on NF-κB Expression in Testicular Tissue of Cisplatin-Treated Rats
3.8. Effect of Vitamin D3 and/or Linagliptin Therapy on Inflammatory Status in Testicular Tissue of Cisplatin-Treated Rats
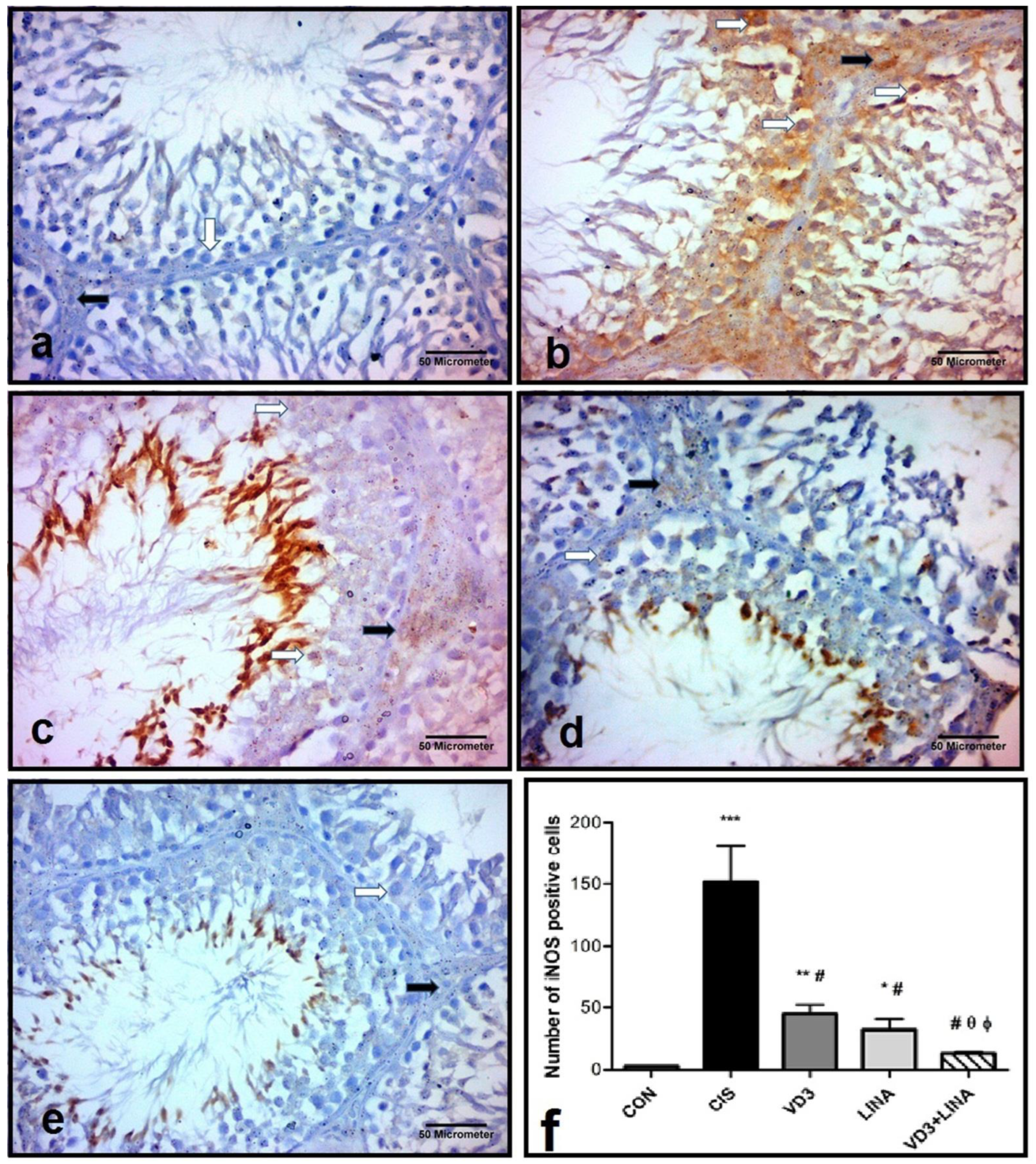
3.9. Effect of Vitamin D3 and/or Linagliptin Therapy on iNOS Expression in Testicular Tissue of Cisplatin-Treated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scott, H.M.; Mason, J.I.; Sharpe, R.M. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr. Rev. 2009, 30, 883–925. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 55–65. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- TTharmalingam, M.D.; Matilionyte, G.; Wallace, W.H.B.; Stukenborg, J.-B.; Jahnukainen, K.; Oliver, E.; Goriely, A.; Lane, S.; Guo, J.; Cairns, B.; et al. Cisplatin and carboplatin result in similar gonadotoxicity in immature human testis with implications for fertility preservation in childhood cancer. BMC Med. 2020, 18, 374. [Google Scholar] [CrossRef]
- Karna, K.K.; Shin, Y.S.; Choi, B.R.; Kim, H.K.; Park, J.K. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J. Men S Health 2020, 38, 484–494. [Google Scholar] [CrossRef]
- Liu, G.L.; Yu, F.; Dai, D.Z.; Zhang, G.L.; Zhang, C.; Dai, Y. Endoplasmic reticulum stress mediating downregulated StAR and 3-beta-HSD and low plasma testosterone caused by hypoxia is attenuated by CPU86017-RS and nifedipine. J. Biomed. Sci. 2012, 19, 4. [Google Scholar] [CrossRef]
- Huang, D.; Wei, W.; Xie, F.; Zhu, X.; Zheng, L.; Lv, Z. Steroidogenesis decline accompanied with reduced antioxidation and endoplasmic reticulum stress in mice testes during ageing. Andrologia 2018, 50, e12816. [Google Scholar] [CrossRef]
- Gao, L.; Gao, D.; Zhang, J.; Li, C.; Wu, M.; Xiao, Y.; Yang, L.; Ma, T.; Wang, X.; Zhang, M.; et al. Age-related endoplasmic reticulum stress represses testosterone synthesis via attenuation of the circadian clock in Leydig cells. Theriogenology 2022, 189, 137–149. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Luo, C.; Cai, J.; Zhou, X.; Xiao, X.; Liu, S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 6129–6140. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Samavedam, U.; Robinson, N. Crosstalk Between ER Stress, Autophagy and Inflammation. Front. Med. (Lausanne) 2021, 8, 758311. [Google Scholar] [CrossRef]
- Soni, K.K.; Zhang, L.T.; You, J.H.; Lee, S.W.; Kim, C.Y.; Cui, W.S.; Chae, H.J.; Kim, H.K.; Park, J.K. The effects of MOTILIPERM on cisplatin induced testicular toxicity in Sprague-Dawley rats. Cancer Cell Int. 2015, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A. Resveratrol improves sperm parameter and testicular apoptosis in cisplatin-treated rats: Effects on ERK1/2, JNK, and Akt pathways. Syst. Biol. Reprod. Med. 2019, 65, 236–249. [Google Scholar] [CrossRef]
- Jensen, M.B.; Nielsen, J.E.; Jorgensen, A.; Meyts, E.R.-D.; Kristensen, D.M.; Jørgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, Y.H.; Xu, S.; Ji, Y.L.; Zhang, C.; Wang, H.; Yu, D.-X.; Xu, D.-X. Vitamin D deficiency impairs testicular development and spermatogenesis in mice. Reprod. Toxicol. 2017, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther. Adv. Endocrinol. Metab. 2013, 4, 95–105. [Google Scholar] [CrossRef]
- Elrashidy, R.A.; Hasan, R.A. Stromal cell-derived factor-1alpha predominantly mediates the ameliorative effect of linagliptin against cisplatin-induced testicular injury in adult male rats. Cytokine 2020, 136, 155260. [Google Scholar] [CrossRef]
- Kaya, K.; Ciftci, O.; Cetin, A.; Dogan, H.; Basak, N. Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia 2015, 47, 793–800. [Google Scholar] [CrossRef]
- Beytur, A.; Ciftci, O.; Oguz, F.; Oguzturk, H.; Yilmaz, F. Montelukast attenuates side effects of cisplatin including testicular, spermatological, and hormonal damage in male rats. Cancer Chemother. Pharmacol. 2012, 69, 207–213. [Google Scholar] [CrossRef]
- Wahba, N.S.; Abdel-Ghany, R.H.; Ghareib, S.A.; Abdel-Aal, M.; Alsemeh, A.E. Vitamin D3 potentiates the renoprotective effects of vildagliptin in a rat model of fructose/salt-induced insulin resistance. Eur. J. Pharm. Sci. 2020, 144, 105196. [Google Scholar] [CrossRef]
- Yoshida, A.; Miura, K.; Shirai, M. Evaluation of seminiferous tubule scores obtained through testicular biopsy examinations of nonobstructive azoospermic men. Fertil. Steril. 1997, 68, 514–518. [Google Scholar] [CrossRef]
- Karimi, S.; Hosseinimehr, S.J.; Mohammadi, H.R.; Khalatbary, A.R.; Amiri, F.T. Zatariamultiflora ameliorates cisplatin-induced testicular damage via suppression of oxidative stress and apoptosis in a mice model. Iran. J. Basic Med. Sci. 2018, 21, 607–614. [Google Scholar] [PubMed]
- Elrashidy, R.A.; Hasan, R.A. Cilostazol preconditioning alleviates cyclophosphamide-induced cardiotoxicity in male rats: Mechanistic insights into SIRT1 signaling pathway. Life Sci. 2021, 266, 118822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Cao, Y.Q.; Wu, J.N.; Chen, M.; Cha, X.Y. Expression of nitric oxide synthase in human gastric carcinoma and its relation to p53, PCNA. World J. Gastroenterol. 2005, 11, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Brydoy, M.; Fossa, S.D.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Wentzel-Larsen, T.; Dahl, O. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin-based chemotherapy. Eur. Urol. 2010, 58, 134–140. [Google Scholar] [CrossRef]
- Brydoy, M.; Fossa, S.D.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Bjoro, T.; Wentzel-Larsen, T.; Dahl, O. Sperm counts and endocrinological markers of spermatogenesis in long-term survivors of testicular cancer. Br. J. Cancer 2012, 107, 1833–1839. [Google Scholar] [CrossRef]
- Mesbahzadeh, B.; Hassanzadeh-Taheri, M.; Aliparast, M.S.; Baniasadi, P.; Hosseini, M. The protective effect of crocin on cisplatin-induced testicular impairment in rats. BMC Urol. 2021, 21, 117. [Google Scholar] [CrossRef]
- Demir, M.; Altindag, F. Sinapic acid ameliorates cisplatin-induced disruptions in testicular steroidogenesis and spermatogenesis by modulating androgen receptor, proliferating cell nuclear antigen and apoptosis in male rats. Andrologia 2022, 54, e14369. [Google Scholar] [CrossRef]
- Beattie, M.C.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B.R. Leydig cell aging and hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef]
- Hofer, D.; Munzker, J.; Schwetz, V.; Ulbing, M.; Hutz, K.; Stiegler, P.; Zigeuner, R.; Pieber, T.R.; Müller, H.; Obermayer-Pietsch, B. Testicular synthesis and vitamin D action. Clin. Endocrinol. Metab. 2014, 99, 3766–3773. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 mediated regulation of steroidogenesis mitigates testicular activity in an aged rat model. J. Steroid Biochem. Mol. Biol. 2019, 190, 64–75. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Wang, Q.Z.; Guo, F.; Huang, F.; Ji, L.; An, T.; Qin, G. Vitamin D3 Activates Phosphatidylinositol-3-Kinase/Protein Kinase B via Insulin-Like Growth Factor-1 to Improve Testicular Function in Diabetic Rats. J. Diabetes Res. 2019, 2019, 7894950. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wang, Q.; Hao, Y.; Ma, X.; Wu, L.; Du, M.; Li, W.; Wu, Y.; Guo, F.; Ma, S.; et al. Vitamin D supplement improved testicular function in diabetic rats. Biochem. Biophys. Res. Commun. 2016, 473, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci. Rep. 2019, 9, 14103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Qi, Y.W.; Hu, C.Y.; Chen, S.H.; Liu, Y. Advanced glycation end products inhibit testosterone secretion by rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress. Int. J. Mol. Med. 2016, 38, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jiang, F.; Zhang, M.; Luo, D.; Shao, S.; Zhao, J.; Gao, L.; Zuo, C.; Guan, Q. HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J. Cell. Mol. Med. 2019, 23, 3140–3150. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Zhu, B.B.; Lin, Z.J.; Chen, J.; Lu, X.; Wang, H.; Zhang, C.; Chen, Y.-H.; Xu, D.-X. Long-term 1-nitropyrene exposure induces endoplasmic reticulum stress and inhibits steroidogenesis in mice testes. Chemosphere 2020, 251, 126336. [Google Scholar] [CrossRef]
- Soni, K.K.; Kim, H.K.; Choi, B.R.; Karna, K.K.; You, J.H.; Cha, J.S.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Park, J.K. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: Reactive oxygen species and endoplasmic reticulum stress. Drug Des. Dev. Ther. 2016, 10, 3959–3968. [Google Scholar] [CrossRef]
- Guan, B.J.; Krokowski, D.; Majumder, M.; Schmotzer, C.L.; Kimball, S.R.; Merrick, W.C.; Koromilas, A.E.; Hatzoglou, M. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eIF2alpha. J. Biol. Chem. 2014, 289, 12593–12611. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Shaban, M.S.; Albert, B.V.; Gokcen, A.; Kracht, M. The Crosstalk of Endoplasmic Reticulum (ER) Stress Pathways with NF-kappaB: Complex Mechanisms Relevant for Cancer, Inflammation and Infection. Biomedicines 2018, 6, 58. [Google Scholar] [CrossRef]
- Tam, A.B.; Mercado, E.L.; Hoffmann, A.; Niwa, M. ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS ONE 2012, 7, e45078. [Google Scholar]
- Willy, J.; Young-Baird, S.; Stevens, J.L.; Masuoka, H.C.; Wek, R.C. CHOP links endoplasmic reticulum stress to NF-kappaB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol. Biol. Cell 2015, 26, 2190–2204. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yue, H.; Wang, L.; Ding, C.; Wu, L.; Wu, Y.; Gao, F.; Qin, G. Vitamin D supplement ameliorates hippocampal metabolism in diabetic rats. Biochem. Biophys. Res. Commun. 2017, 490, 239–246. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, O.S.; Soliman, G.M.; Elesawy, R.O. Linagliptin, the dipeptidyl peptidase-4 enzyme inhibitor, lessens CHOP and GRP78 biomarkers levels in cisplatin-induced neurobehavioral deficits: A possible restorative gateway. J. Biochem. Mol. Toxicol. 2020, 39, e22541. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, G.; Ersoy, O.; Cerkezkayabekir, A.; Topcu-Tarladacalisir, Y. Sitagliptin and fucoidan prevent apoptosis and reducing ER stress in diabetic rat testes. Andrologia 2021, 53, e13858. [Google Scholar] [CrossRef]
- Delfino, F.; Walker, W.H. Stage-specific nuclear expression of NF-kappaB in mammalian testis. Mol. Endocrinol. 1998, 12, 1696–1707. [Google Scholar] [PubMed]
- Pentikainen, V.; Suomalainen, L.; Erkkila, K.; Martelin, E.; Parvinen, M.; Pentikainen, M.O.; Dunkel, L. Nuclear factor-kappa B activation in human testicular apoptosis. Am. J. Pathol. 2002, 160, 205–218. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, H.B. Involvement of nuclear factor-kappa B on corticosterone- induced rat Leydig cell apoptosis. Asian J. Androl. 2006, 8, 693–702. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Wang, Q.; Guo, F.; Huang, F.; Ji, L.; An, T.; Qin, G. Vitamin D3 supplementation improves testicular function in diabetic rats through peroxisome proliferator-activated receptor-gamma/transforming growth factor-beta 1/nuclear factor-kappa B. J. Diabetes Investig. 2019, 10, 261–271. [Google Scholar] [CrossRef]
- Al Zoubi, S.; Chen, J.; Murphy, C.; Martin, L.; Chiazza, F.; Collotta, D.; Yaqoob, M.M.; Collino, M.; Thiemermann, C. Linagliptin Attenuates the Cardiac Dysfunction Associated With Experimental Sepsis in Mice With Pre-existing Type 2 Diabetes by Inhibiting NF-kappaB. Front. Immunol. 2018, 9, 2996. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Schlatt, S.; Gerdprasert, O.; Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Inducible nitric oxide synthase in the rat testis: Evidence for potential roles in both normal function and inflammation-mediated infertility. Biol. Reprod. 2000, 63, 1285–1293. [Google Scholar] [CrossRef]
- Kostic, T.S.; Andric, S.A.; Maric, D.; Stojilkovic, S.S.; Kovacevic, R. Involvement of inducible nitric oxide synthase in stress-impaired testicular steroidogenesis. J. Endocrinol. 1999, 163, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Yadav, S.K.; Prakash, J.; Westfall, S.; Ghosh, A.; Agarwal, N.K.; Singh, S.P. Increase in the expression of inducible nitric oxide synthase on exposure to bisphenol A: A possible cause for decline in steroidogenesis in male mice. Environ. Toxicol. Pharmacol. 2015, 39, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Hsieh, Y.J.; Lu, C.W.; Lee, C.J.; Hsu, B.G. Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species. Int. J. Mol. Sci. 2021, 22, 1119. [Google Scholar] [CrossRef]



| Initial Body Weight (g) | Final Body Weight (g) | Total Testicular Weight (g) | |
|---|---|---|---|
| CON | 231.8 ± 4.12 | 246.3 ± 5.58 | 2.83 ± 0.026 |
| CIS | 226.3 ± 4.88 | 188.8 ± 7.07 ** | 2.08 ± 0.05 ** |
| VD3 | 226.3 ± 3.19 | 199.0 ± 3.27 ** | 2.33 ± 0.032 **,# |
| LINA | 235.0 ± 4.29 | 206.8 ± 3. 21 ** | 2.63 ± 0.035 *,##,θ |
| VD3+LINA | 236.8 ± 2.90 | 210.3 ± 5.19 **,# | 2.65 ± 0.05 *,##,θ |
| Seminiferous Tubules Diameter (μm) | Germinal Epithelial Height (μm) | Basement Membrane Thickness (μm) | Fibrotic Area % | |
|---|---|---|---|---|
| CON | 430.2 ± 11.9 | 115.8 ± 3.6 | 2.37 ± 0.25 | 2.24 ± 0.24 |
| CIS | 246.5 ± 10.3 ** | 58 ± 2.5 ** | 16.6 ± 1.6 ** | 18.9 ± 1.05 ** |
| VD3 | 331.3 ± 11.6 **,# | 80.6 ± 2.7 ** | 6.22 ± 0.54 *,# | 6.58 ± 0.69 **,# |
| LINA | 380.4 ± 8.62 *,#,θ | 92.7 ± 2.3 **,θ | 5.92 ± 0.45 *,# | 5.49 ± 0.26 *,# |
| VD3+LINA | 427.4 ± 11.8 #,θθ,ϕ | 110 ± 2.1 #,θθ,ϕ | 2.46 ± 0.31 #,θ,ϕ | 2.82 ± 0.44 #,θ,ϕ |
| Johnsen Score (JS) Per Tubule | No. of Sertoli Cells Per Tubule | No. of Leydig Cells Per Field | |
|---|---|---|---|
| CON | 8.95 ± 0.19 | 13.27 ± 0.49 | 16.90 ± 0.53 |
| CIS | 3.70 ± 0.31 ** | 12.91 ± 0.48 | 14.10 ± 1.03 |
| VD3 | 7.70 ± 0.32 *,# | 13.09 ± 0.89 | 15.70 ± 0.92 |
| LINA | 7.55 ± 0.25 *,# | 13.0 ± 0.50 | 16.70 ± 0.58 |
| VD3+LINA | 8.40 ± 0.19 # | 13.18 ± 0.57 | 16.70 ± 0.59 |
| Serum LH Levels (ng/mL) | Serum Testosterone Levels (ng/mL) | Testicular Testosterone Levels (ng/g Tissue) | |
|---|---|---|---|
| CON | 90.83 ± 5.83 | 3.29 ± 0.19 | 16.81 ± 0.21 |
| CIS | 82.17 ± 3.05 | 0.81 ± 0.015 ** | 4.05 ± 0.47 ** |
| VD3 | 82.67 ± 3.69 | 1.57 ± 0.20 **,# | 6.60 ± 0.54 **,# |
| LINA | 87.00 ± 6.16 | 1.90 ± 0.17 **,## | 7.80 ± 0.41 **,## |
| VD3+LINA | 87.17 ± 5.68 | 3.02 ± 0.23 ###,θ,ϕ | 13.8 ± 1.07 *,###,θ,ϕ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elrashidy, R.A.; Zakaria, E.M.; Elmaghraby, A.M.; Abd El Aziz, R.E.M.; Abdelgalil, R.M.; Megahed, R.M.; Elshiech, A.A.; Salama, D.E.A.; Ibrahim, S.E. Linagliptin and Vitamin D3 Synergistically Rescue Testicular Steroidogenesis and Spermatogenesis in Cisplatin-Exposed Rats: The Crosstalk of Endoplasmic Reticulum Stress with NF-κB/iNOS Activation. Molecules 2022, 27, 7299. https://doi.org/10.3390/molecules27217299
Elrashidy RA, Zakaria EM, Elmaghraby AM, Abd El Aziz REM, Abdelgalil RM, Megahed RM, Elshiech AA, Salama DEA, Ibrahim SE. Linagliptin and Vitamin D3 Synergistically Rescue Testicular Steroidogenesis and Spermatogenesis in Cisplatin-Exposed Rats: The Crosstalk of Endoplasmic Reticulum Stress with NF-κB/iNOS Activation. Molecules. 2022; 27(21):7299. https://doi.org/10.3390/molecules27217299
Chicago/Turabian StyleElrashidy, Rania A., Esraa M. Zakaria, Asmaa M. Elmaghraby, Rasha E. M. Abd El Aziz, Ranya M. Abdelgalil, Rehab M. Megahed, Asmaa A. Elshiech, Doaa E. A. Salama, and Samah E. Ibrahim. 2022. "Linagliptin and Vitamin D3 Synergistically Rescue Testicular Steroidogenesis and Spermatogenesis in Cisplatin-Exposed Rats: The Crosstalk of Endoplasmic Reticulum Stress with NF-κB/iNOS Activation" Molecules 27, no. 21: 7299. https://doi.org/10.3390/molecules27217299
APA StyleElrashidy, R. A., Zakaria, E. M., Elmaghraby, A. M., Abd El Aziz, R. E. M., Abdelgalil, R. M., Megahed, R. M., Elshiech, A. A., Salama, D. E. A., & Ibrahim, S. E. (2022). Linagliptin and Vitamin D3 Synergistically Rescue Testicular Steroidogenesis and Spermatogenesis in Cisplatin-Exposed Rats: The Crosstalk of Endoplasmic Reticulum Stress with NF-κB/iNOS Activation. Molecules, 27(21), 7299. https://doi.org/10.3390/molecules27217299