Thermal Activation of Coal Gangue with Low Al/Si Ratio as Supplementary Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
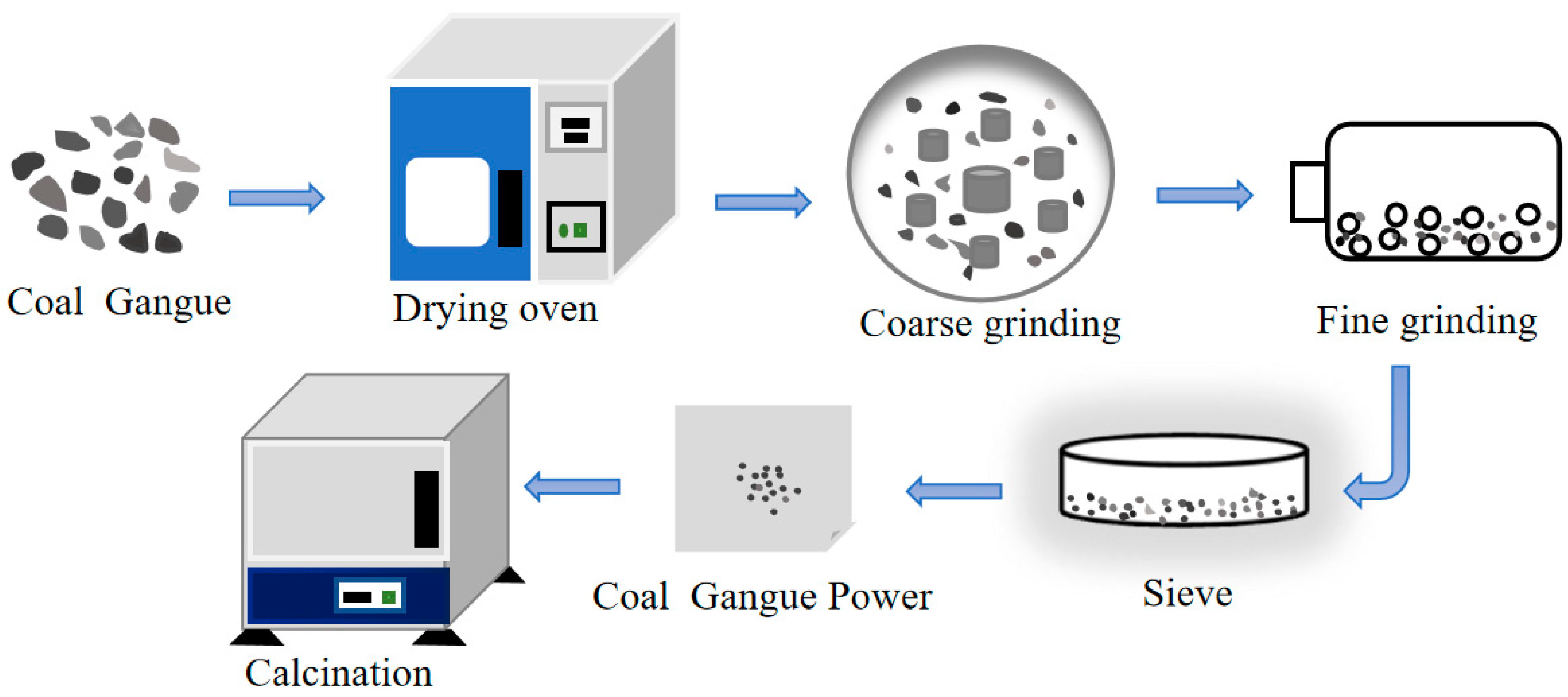
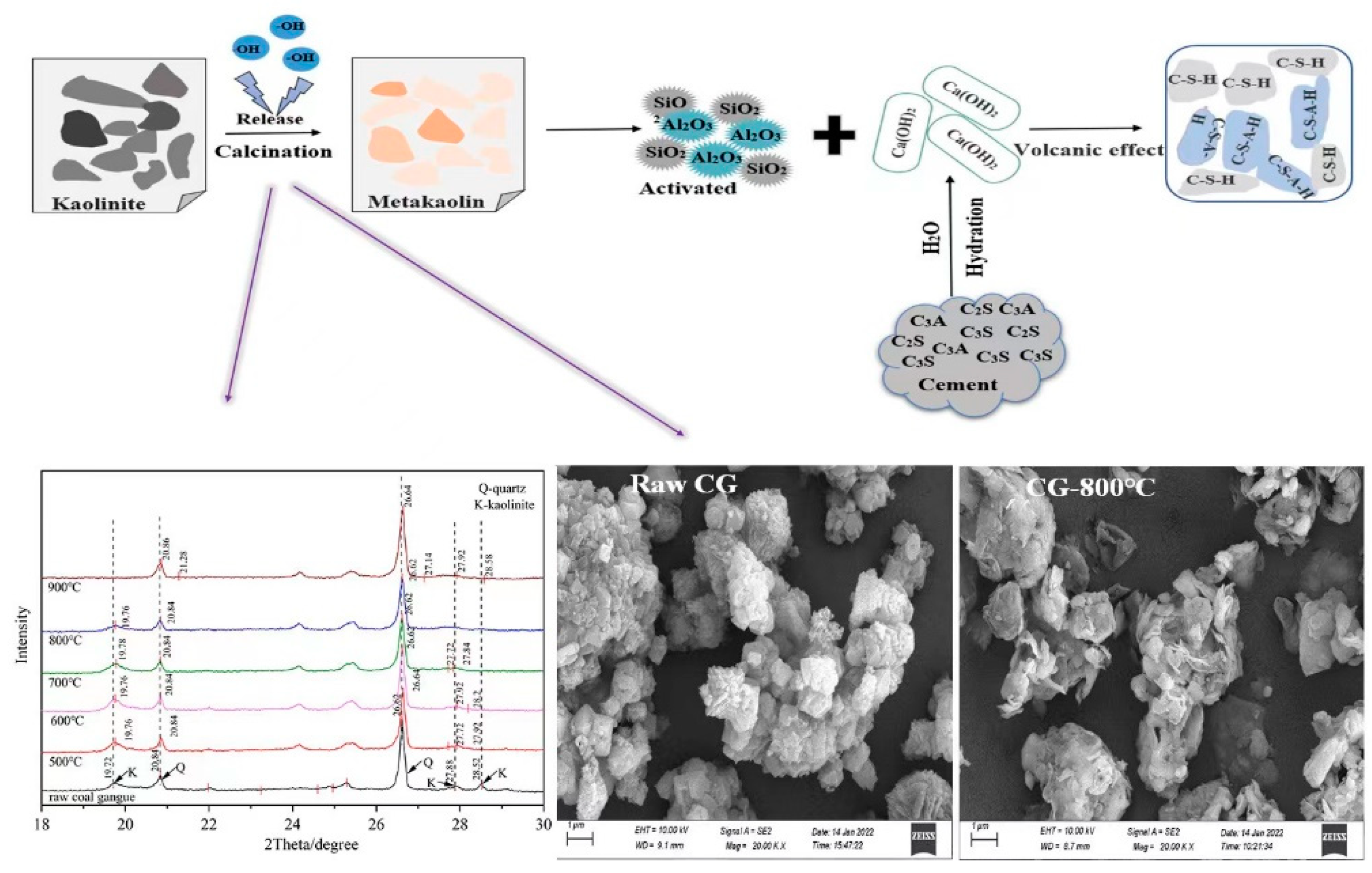
2.2. Thermal Activation of CG
2.3. Characteristics of Activated CG
2.3.1. Composition and Microstructure Analysis
2.3.2. Hole Structure and Micromorphological Analysis
2.4. The Physical Property of Activated CG
3. Results and Discussion
3.1. The Characteristics of the Raw and Calcined CG
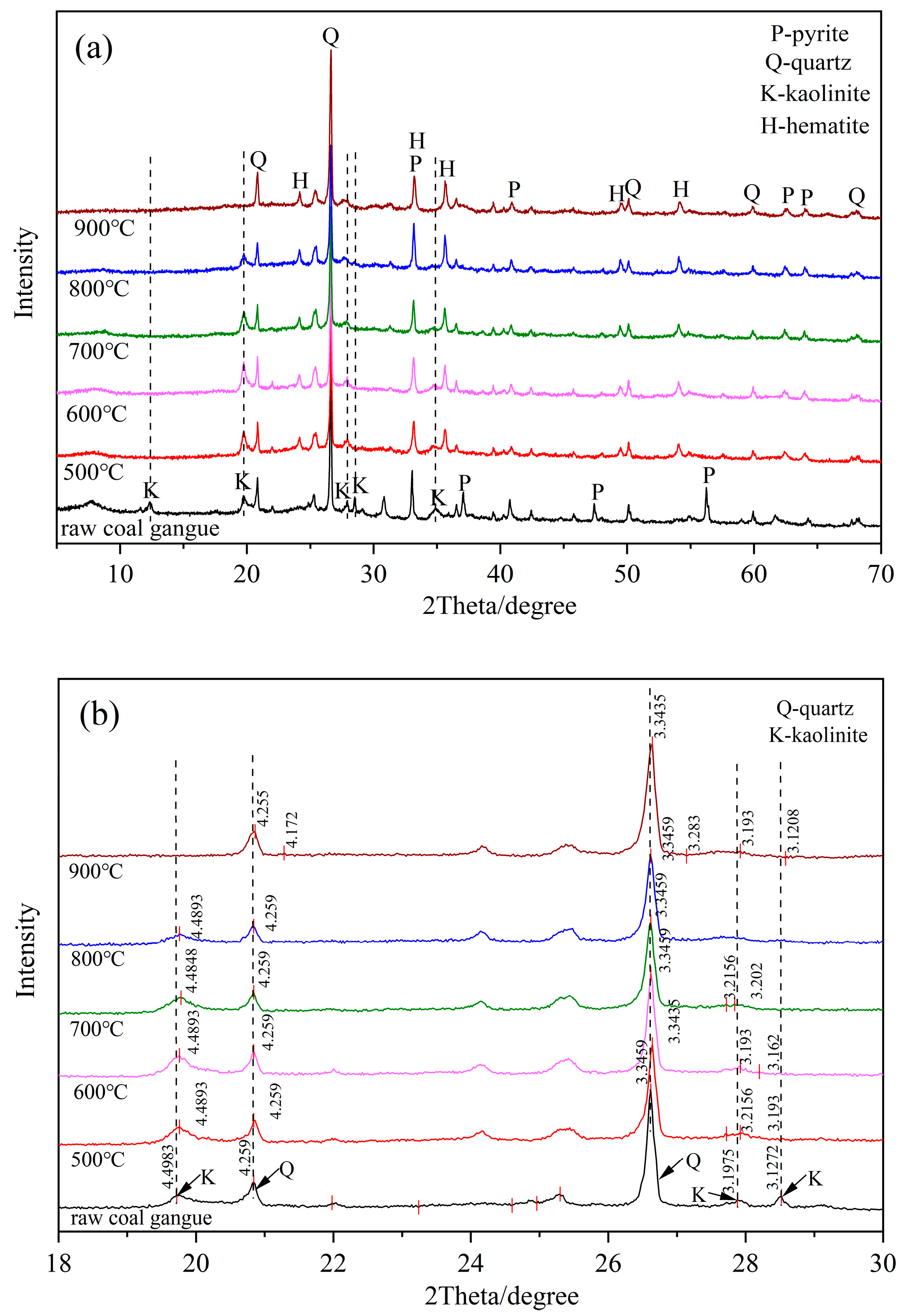
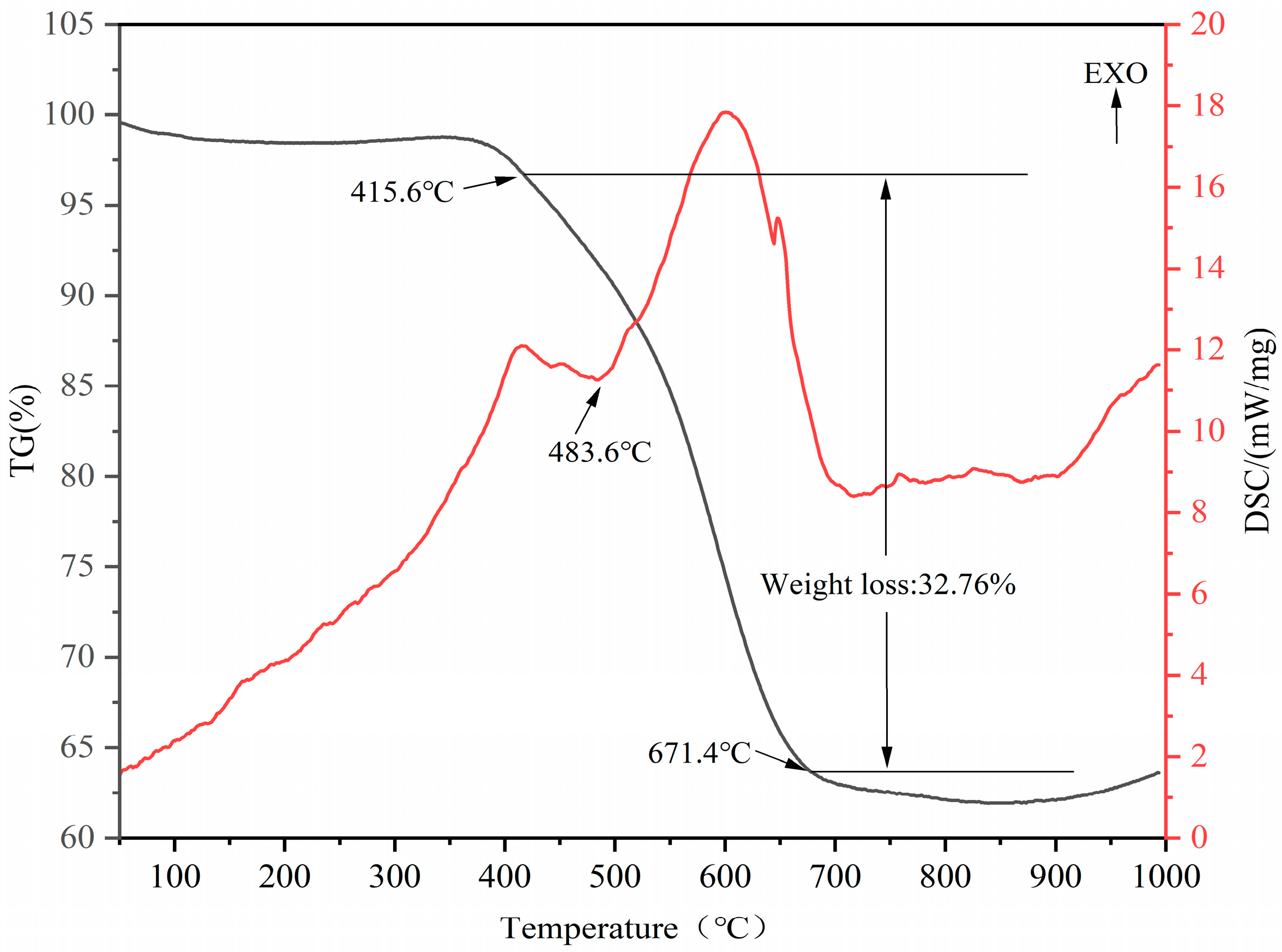
3.1.1. Mineral Phase Transition Analysis for the Thermal Decomposition
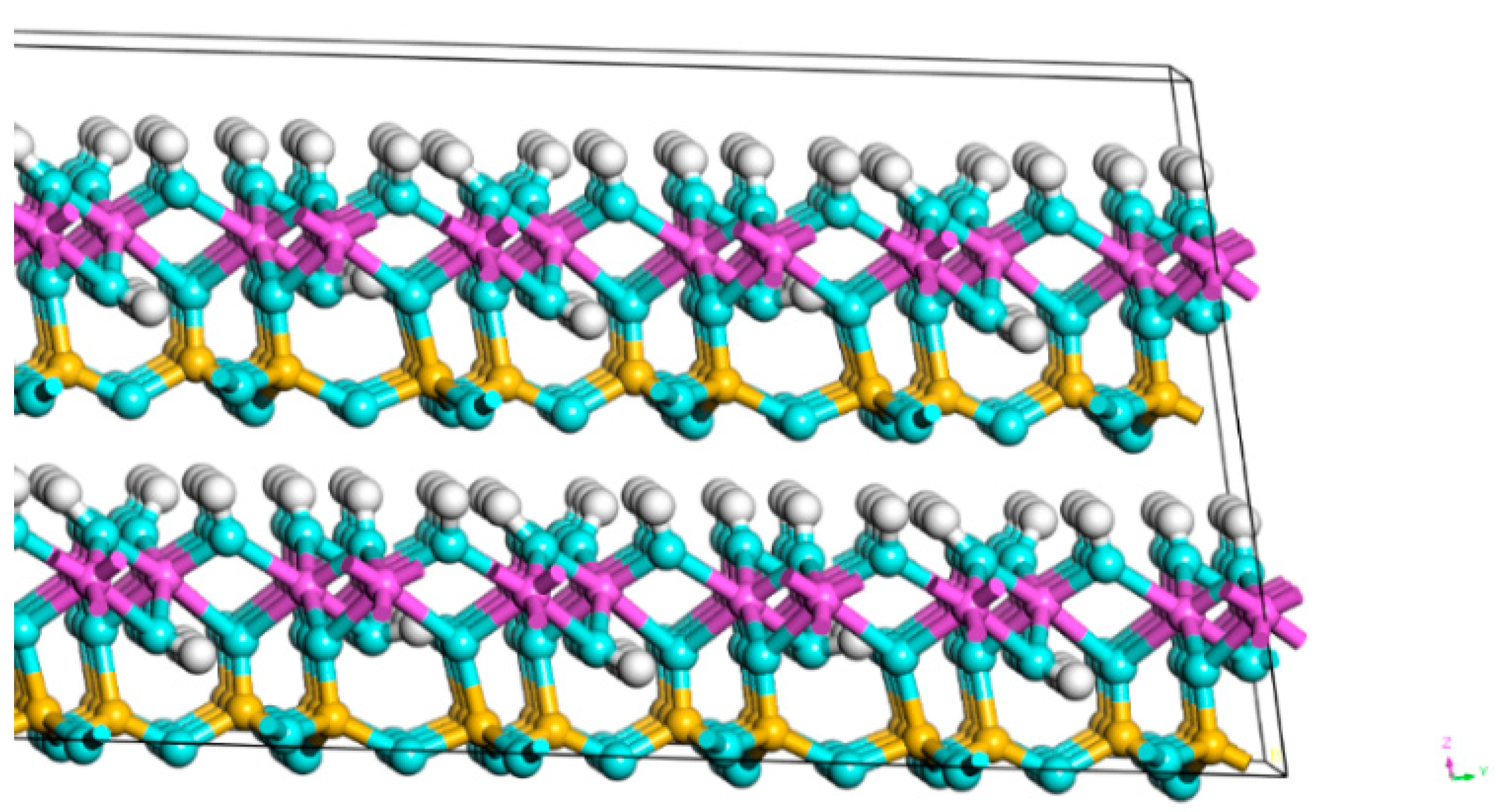
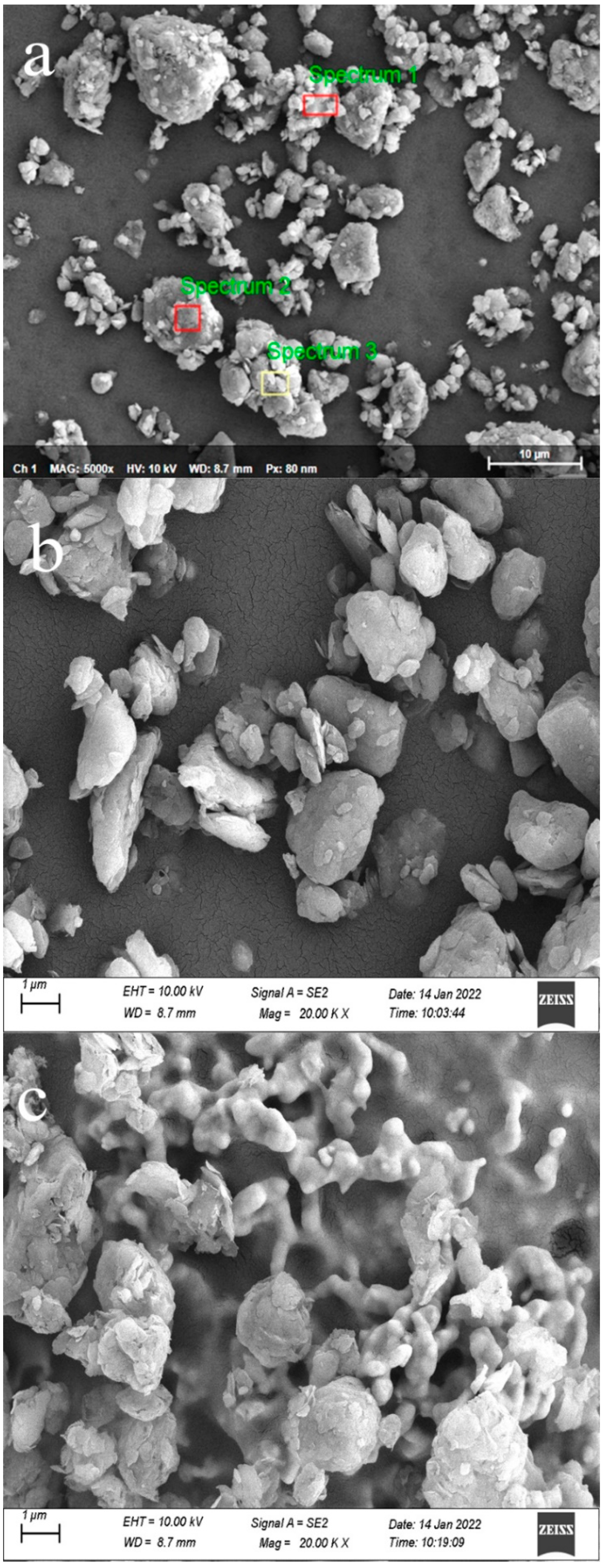
3.1.2. Micromorphological and Compositional Analysis
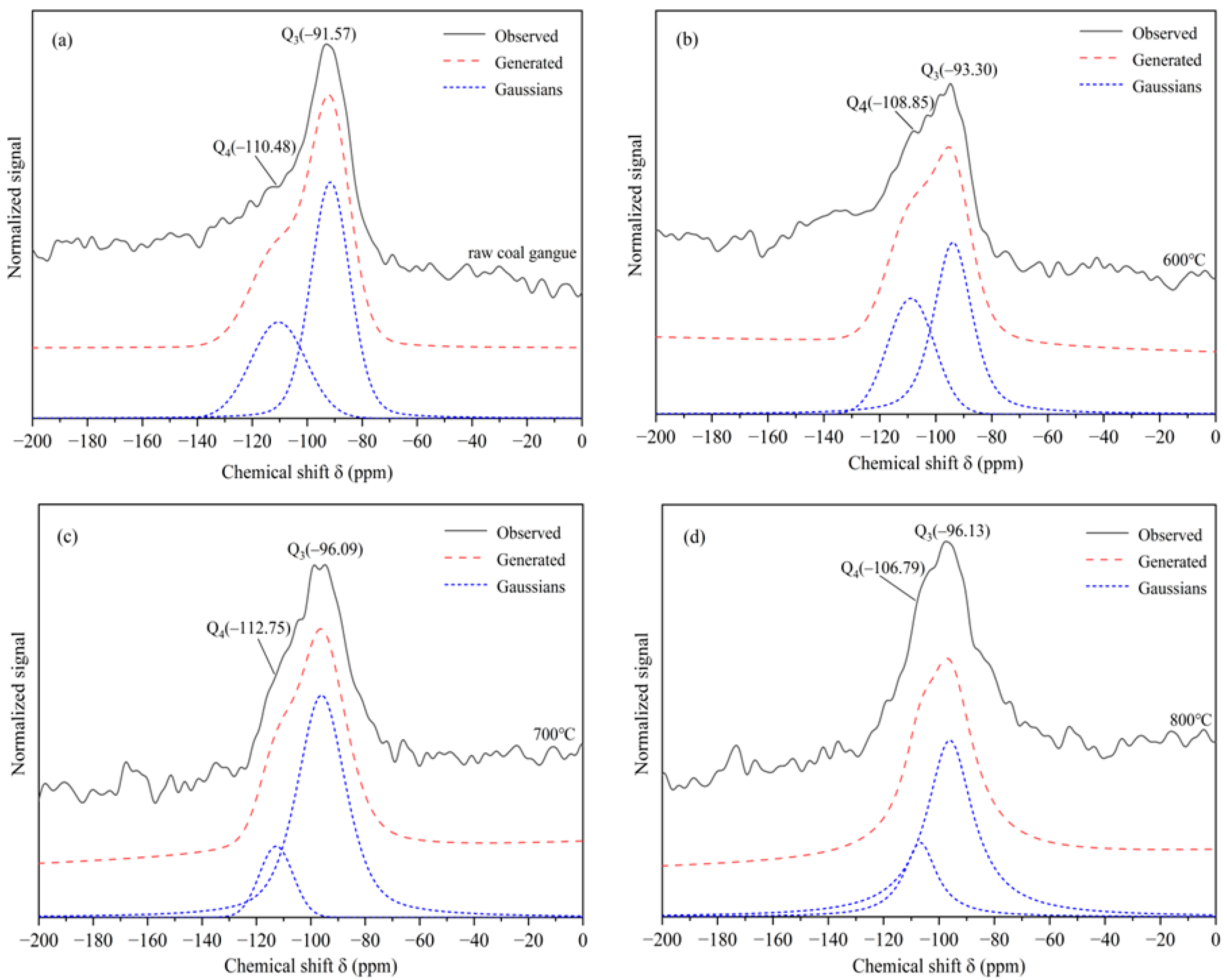
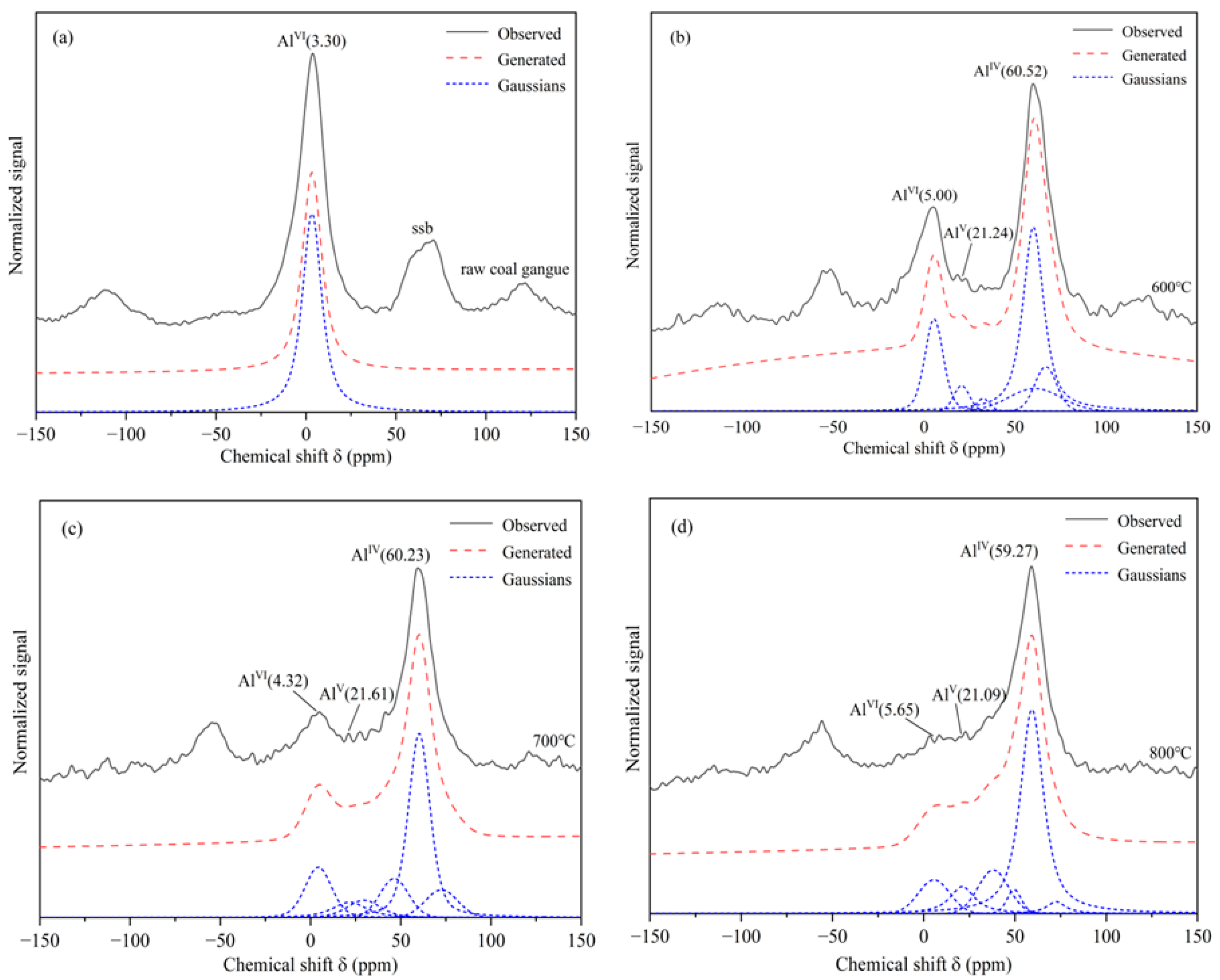
3.1.3. Characterization by NMR
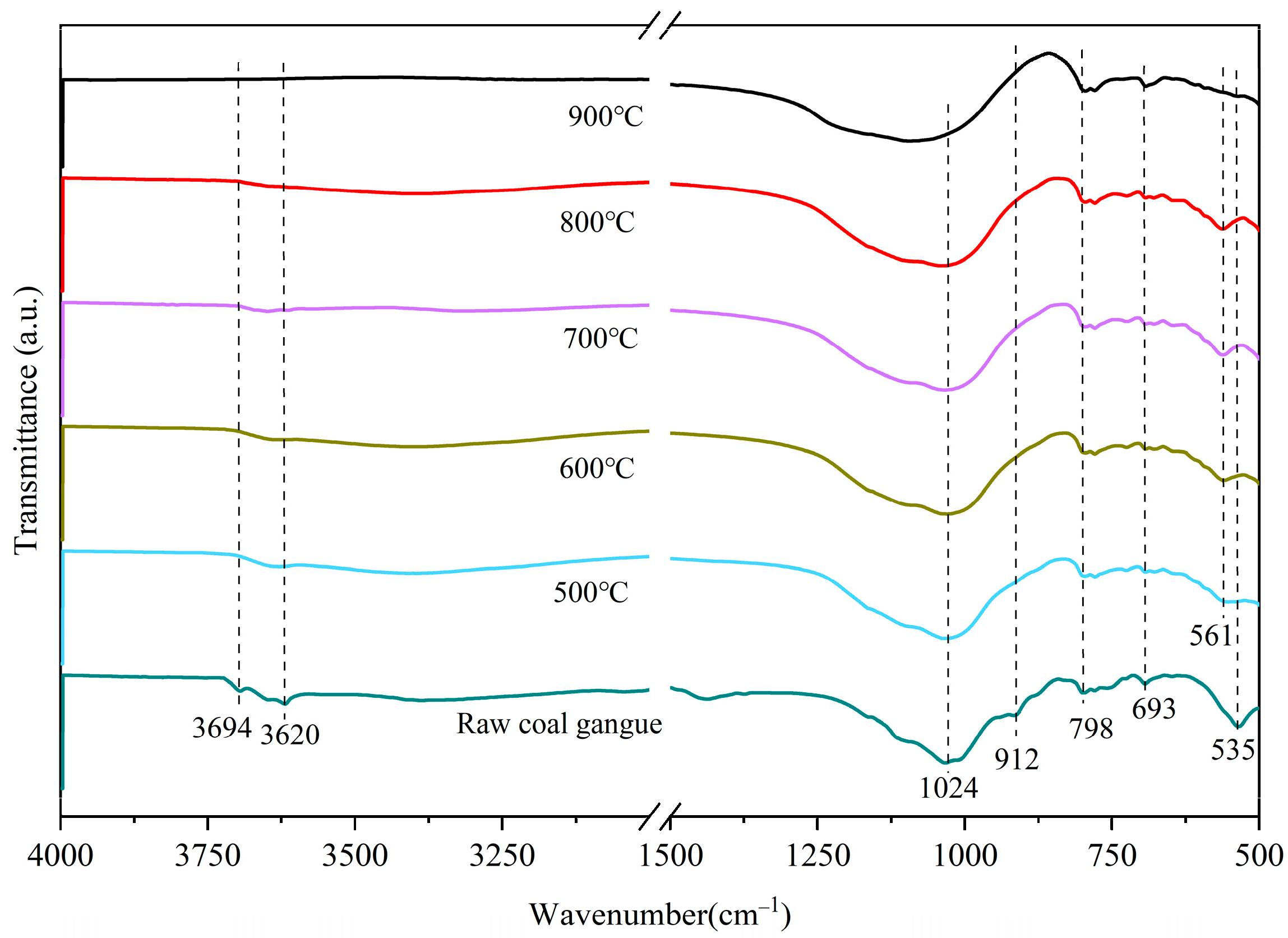
3.1.4. Characterization by IR Spectroscopy
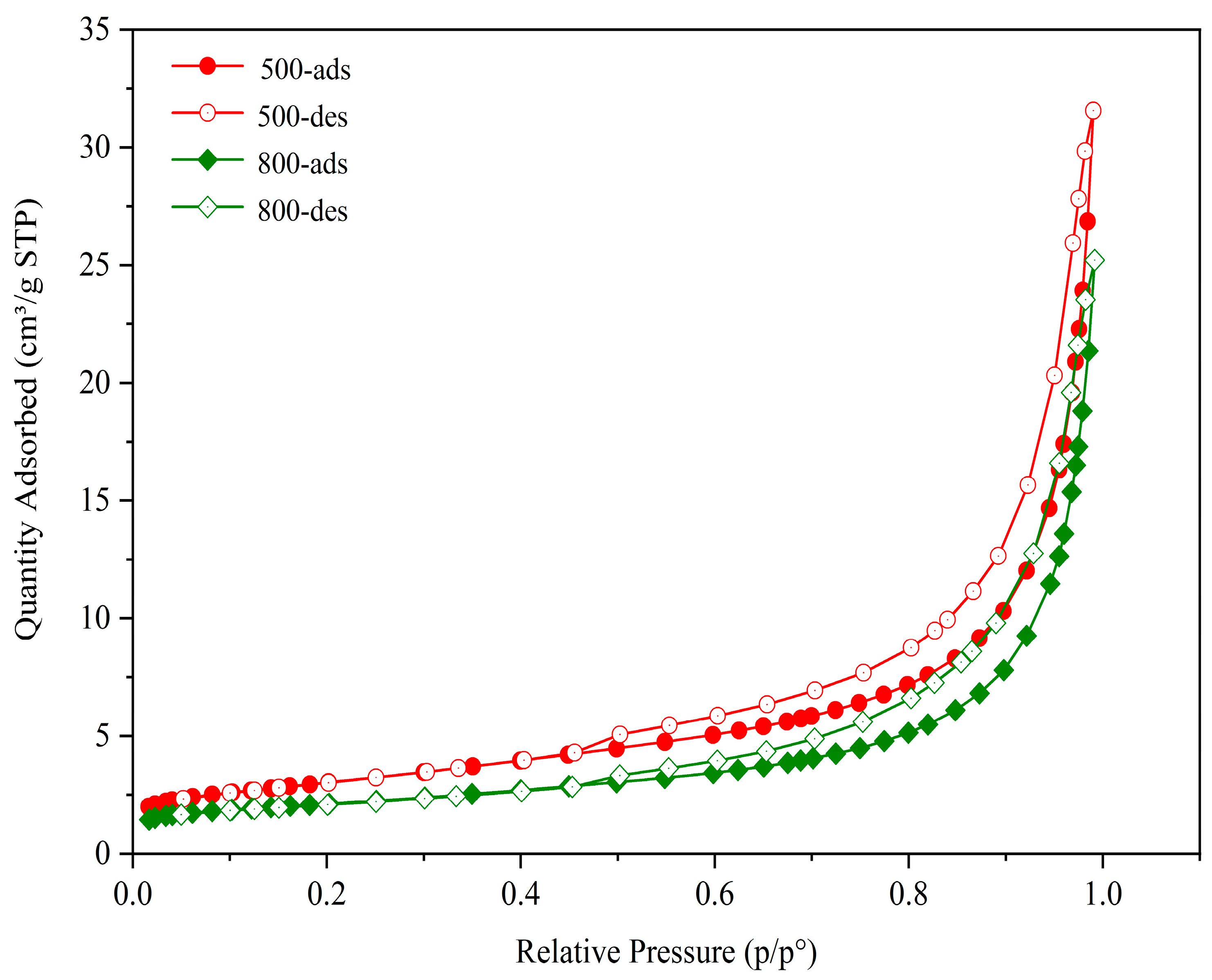
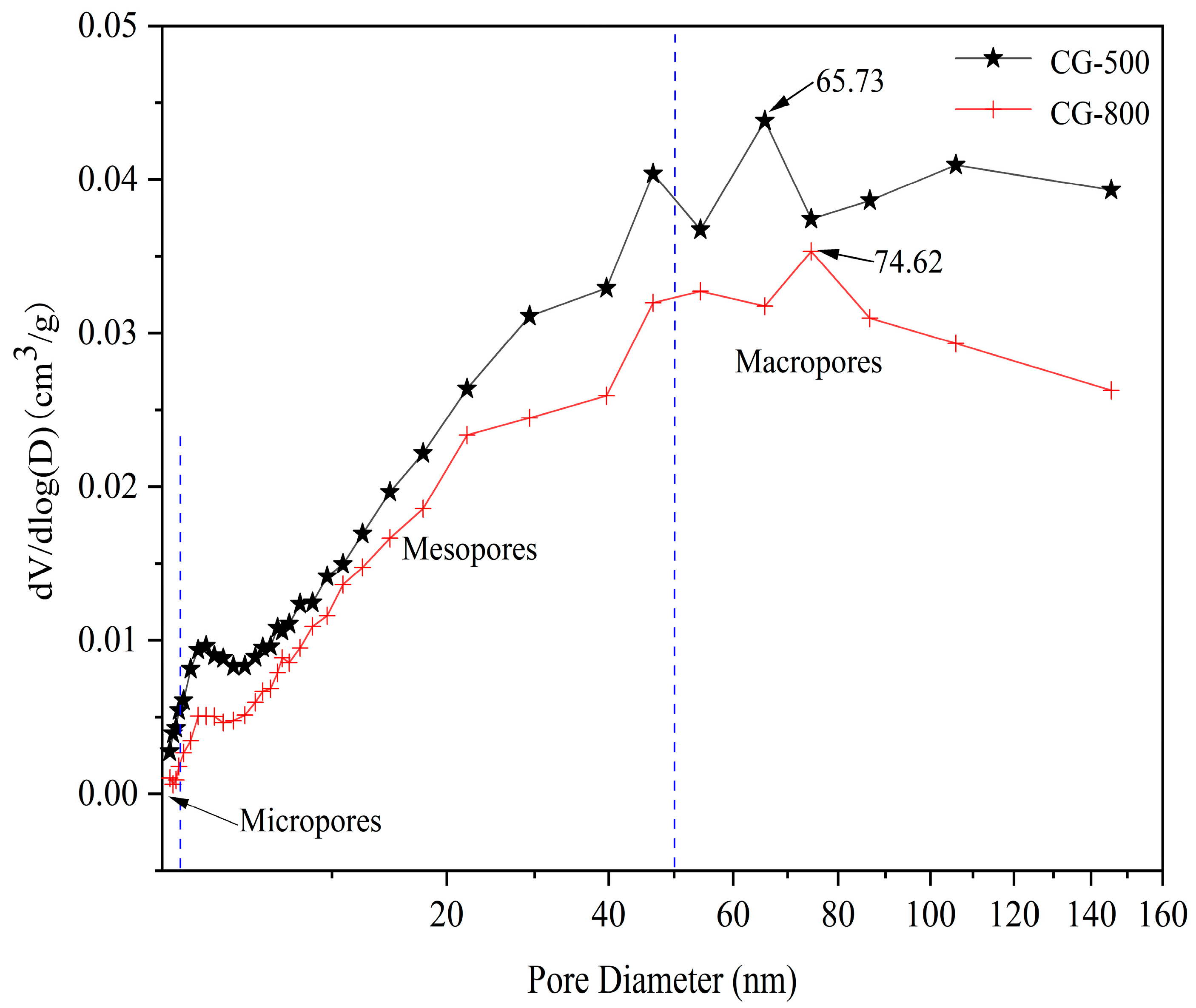
3.1.5. Specific Surface Area and Pore Structure
3.2. The Physical Performance of Cementitious Materials
3.2.1. Water Demand of Normal Consistency
3.2.2. Setting Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, Y.; Shi, X.; Li, Y.; Zhang, X. Co-Spontaneous Combustion of Coal and Gangue: Thermal Behavior, Kinetic Characteristics and Interaction Mechanism. Fuel 2022, 315, 123275. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Cheng, H.; Jiang, C.; Zheng, L. Remediation of Soil Mercury by Modified Vermiculite-Montmorillonite and Its Effect on the Growth of Brassica chinensis, L. Molecules 2022, 27, 5340. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, B.; Xiao, Y.; Ma, L.; Wang, C.-P.; Lai-wang, B.; Shu, C.-M. Combustion Properties of Coal Gangue Using Thermogravimetry–Fourier Transform Infrared Spectroscopy. Appl. Therm. Eng. 2017, 116, 244–252. [Google Scholar] [CrossRef]
- Deng, D.; Cen, W. Environmental effect of coal gangue stack area. China Min. Mag. 1999, 8, 90–94. [Google Scholar]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental Characterization of Burnt Coal Gangue Banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, H.; Zhu, S. Mercury Emission from Spontaneously Ignited Coal Gangue Hill in Wuda Coalfield, Inner Mongolia, China. Fuel 2016, 182, 525–530. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Peng, S.; Zhou, C. Synthesis of Calcium Silicate Hydrate from Coal Gangue for Cr(VI) and Cu(II) Removal from Aqueous Solution. Molecules 2021, 26, 6192. [Google Scholar] [CrossRef]
- Pardal, X.; Pochard, I.; Nonat, A. Experimental Study of Si–Al Substitution in Calcium-Silicate-Hydrate (C-S-H) Prepared under Equilibrium Conditions. Cem. Concr. Res. 2009, 39, 637–643. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D. Production of γ-Al2O3 from Kaolin. Open J. Phys. Chem. 2011, 1, 23–27. [Google Scholar] [CrossRef]
- Gong, B.; Tian, C.; Xiong, Z.; Zhao, Y.; Zhang, J. Mineral Changes and Trace Element Releases during Extraction of Alumina from High Aluminum Fly Ash in Inner Mongolia, China. Int. J. Coal Geol. 2016, 166, 96–107. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved Extraction of Alumina from Coal Gangue by Surface Mechanically Grinding Modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Z. Analysis of Coal Gangue Used to Produce Green Materials. Sci. Technol. Eng. 2007, 7, 90–94. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS). Mineral Commodity Summaries 2012–2018—Cement; U.S. Geological Survey: Reston, VA, USA, 2018.
- Shi, Z.; Geiker, M.R.; De Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of Calcium on Chloride Binding in Hydrated Portland Cement–Metakaolin–Limestone Blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Yan, Z.; Fang, T.; Wang, R. Transformation Behavior of Mineral Composition and Trace Elements during Coal Gangue Combustion. Fuel 2012, 97, 644–650. [Google Scholar] [CrossRef]
- Chakraborty, A. New Data on Thermal Effects of Kaolinite in the High Temperature Region. J. Therm. Anal. Calorim. 2003, 71, 799–808. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.; Sun, H.; Ni, W. Improvement on Pozzolanic Reactivity of Coal Gangue by Integrated Thermal and Chemical Activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Yang, Q.; Lü, M.; Luo, Y. Effects of Surface-Activated Coal Gangue Aggregates on Properties of Cement-Based Materials. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2013, 28, 1118–1121. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Hao, Z. The Thermal Activation Process of Coal Gangue Selected from Zhungeer in China. J. Therm. Anal. Calorim. 2016, 126, 1559–1566. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, J.; Zhang, C.; Chen, J.; Liu, C. Effect of Particle Size and Thermal Activation on the Coal Gangue Based Geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Hao, R.; Li, X.; Xu, P.; Liu, Q. Thermal Activation and Structural Transformation Mechanism of Kaolinitic Coal Gangue from Jungar Coalfield, Inner Mongolia, China. Appl. Clay Sci. 2022, 223, 106508. [Google Scholar] [CrossRef]
- Su, Z.; Li, X.; Zhang, Q. Influence of Thermally Activated Coal Gangue Powder on the Structure of the Interfacial Transition Zone in Concrete. J. Clean. Prod. 2022, 363, 132408. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Xu, Z.; Gao, J.; Zhu, X. Performance of Cement-Based Materials Containing Calcined Coal Gangue with Different Calcination Regimes. J. Build. Eng. 2022, 56, 104821. [Google Scholar] [CrossRef]
- Wang, A.; Liu, P.; Mo, L.; Liu, K.; Ma, R.; Guan, Y.; Sun, D. Mechanism of Thermal Activation on Granular Coal Gangue and Its Impact on the Performance of Cement Mortars. J. Build. Eng. 2022, 45, 103616. [Google Scholar] [CrossRef]
- Shi, X.; Cai, Q.; Qi, C.; Zhang, L.; Lu, X.; Zhou, W.; Zhao, B. Co-Utilization of Reactivated Cement Pastes with Coal Gangue. Constr. Build. Mater. 2021, 270, 121423. [Google Scholar] [CrossRef]
- Song, X.; Zhang, K.; Han, J.; Hu, Z. Effect of Thermal Activated Coal Gangue on Pozzolanic Effect and Cement Properties. Mater. Rep. 2011, 25, 118–121+129. [Google Scholar]
- Zhao, Y.; Qiu, J.; Ma, Z.; Sun, X. Eco-Friendly Treatment of Coal Gangue for Its Utilization as Supplementary Cementitious Materials. J. Clean. Prod. 2021, 285, 124834. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Wang, A.; Sun, D.; Liu, K.; Liu, P.; Chu, Y. Valorization of Calcined Coal Gangue as Coarse Aggregate in Concrete. Cem. Concr. Compos. 2021, 121, 104057. [Google Scholar] [CrossRef]
- Long, G.; Li, L.; Li, W.; Ma, K.; Dong, W.; Bai, C.; Zhou, J.L. Enhanced Mechanical Properties and Durability of Coal Gangue Reinforced Cement-Soil Mixture for Foundation Treatments. J. Clean. Prod. 2019, 231, 468–482. [Google Scholar] [CrossRef]
- Zhou, M.; Dou, Y.; Zhang, Y.; Zhang, Y.; Zhang, B. Effects of the Variety and Content of Coal Gangue Coarse Aggregate on the Mechanical Properties of Concrete. Constr. Build. Mater. 2019, 220, 386–395. [Google Scholar] [CrossRef]
- Ni, Q. The Preparation and Application of C55 High Intensity Concrete Mix. West. China Commun. Sci. Technol. 2011, 67, 35–39+104. [Google Scholar] [CrossRef]
- Brindley, G.W.; Nakahira, M. The Kaolinite-Mullite Reaction Series: II, Metakaolin. J. Am. Ceram. Soc. 1959, 42, 314–318. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, J.; Frost, R.L.; Wu, Z. Infrared Transmission and Emission Spectroscopic Study of Selected Chinese Palygorskites. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Z.; Xue, L.; Zhu, S. Physico-mechanical properties variation of rock with phase transformation under high temperature. Chin. J. Rock Mech. Eng. 2013, 32, 935–942. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Li, Y. Mechanism and Structural Analysis of the Thermal Activation of Coal-Gangue. Adv. Mater. Res. 2012, 356–360, 1807–1812. [Google Scholar] [CrossRef]
- Gasparini, E.; Tarantino, S.C.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal Dehydroxylation of Kaolinite under Isothermal Conditions. Appl. Clay Sci. 2013, 80–81, 417–425. [Google Scholar] [CrossRef]
- Qiu, J.; Lin, J. Rock Chemistry; Geological Press: Beijing, China, 1991; pp. 16–17. [Google Scholar]
- Wang, H.; Li, C.; Peng, Z.; Zhang, S. Characterization and Thermal Behavior of Kaolin. J. Therm. Anal. Calorim. 2011, 105, 157–160. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, H.; Yang, H.; Liu, Q.; Wang, J.; Yue, G. An Experimental Study on the Primary Fragmentation and Attrition of Limestones in a Fluidized Bed. Fuel Process. Technol. 2010, 91, 1119–1124. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, X. Analysis of thermal activation and phase transformation of coal-gangue. J. Chin. Ceram. Soc. 2007, 35, 1258–1263+1269. [Google Scholar] [CrossRef]
- Yanase, I.; Saito, Y.; Kobayashi, H. Solid-State MAS NMR Investigations for Pentavalent Cation-Replaced Pollucite Compounds with a Negative Thermal Expansion Coefficient. J. Therm. Anal. Calorim. 2017, 129, 1271–1276. [Google Scholar] [CrossRef]
- Watanabe, T.; Shimizu, H.; Nagasawa, K.; Masuda, A.; Saito, H. 29Si- and 27Al-MAS/NMR Study of the Thermal Transformations of Kaolinite. Clay Miner. 1987, 22, 37–48. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Li, D. Application progress of solid 29Si,27Al NMR in the research of cement-based materials. Nucl. Tech. 2014, 37, 48–53. [Google Scholar] [CrossRef]
- Stone, W.E.E.; Torres-Sanchez, R.-M. Nuclear Magnetic Resonance Spectroscopy Applied to Minerals. Part 6.—Structural Iron in Kaolinites as Viewed by Proton Magnetic Resonance. J. Chem. Soc. Faraday Trans. 1 1988, 84, 117–132. [Google Scholar] [CrossRef]
- He, H.; Guo, J.; Zhu, J.; Yuan, P.; Hu, C. 29Si and 27Al MAS NMR Spectra of Mullites from Different Kaolinites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1061–1064. [Google Scholar] [CrossRef]
- Venkatathri, N. Synthesis of AlPO4-31 from Nonaqueous Systems. Mater. Lett. 2004, 58, 241–244. [Google Scholar] [CrossRef]
- Rocha, J.; Klinowski, J. 29Si and 27Al Magic-Angle-Spinning NMR Studies of the Thermal Transformation of Kaolinite. Phys. Chem. Miner. 1990, 17, 179–186. [Google Scholar] [CrossRef]
- Konan, K.L.; Peyratout, C.; Smith, A.; Bonnet, J.-P.; Rossignol, S.; Oyetola, S. Comparison of Surface Properties between Kaolin and Metakaolin in Concentrated Lime Solutions. J. Colloid Interface Sci. 2009, 339, 103–109. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Cheng, F. Industrial Development and Prospect about Comprehensive Utilization of Coal Gangue. CIESC J. 2014, 65, 2443–2453. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Sun, Y.; Zhang, N. Correlation between 29Si Polymerization and Cementitious Activity of Coal Gangue. J. Zhejiang Univ. SCI. A 2009, 10, 1334–1340. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Q.; Ai, B.; Ding, S.; Frost, R.L. Thermal Decomposition of Selected Coal Gangue. J. Therm. Anal. Calorim. 2018, 131, 1413–1422. [Google Scholar] [CrossRef]
- Castellano, M.; Turturro, A.; Riani, P.; Montanari, T.; Finocchio, E.; Ramis, G.; Busca, G. Bulk and Surface Properties of Commercial Kaolins. Appl. Clay Sci. 2010, 48, 446–454. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Prieto, D.M. Water Adsorption and Desorption Isotherms of Chestnut and Wheat Flours. Ind. Crops Prod. 2010, 32, 252–257. [Google Scholar] [CrossRef]
- Zhang, R.; Deng, M.; Mo, L. Effects of Surface Activation on the Microstructure and Performance of Coal Gangue. J. Nanjing Tech Univ. Nat. Sci. Ed. 2019, 41, 161–166. [Google Scholar]
- Zhao, H.; Gou, H. Unfired Bricks Prepared with Red Mud and Calcium Sulfoaluminate Cement: Properties and Environmental Impact. J. Build. Eng. 2021, 38, 102238. [Google Scholar] [CrossRef]
- Xun, X.; Xiaoling, F.; Chenglin, Y. Investigation on Physical Properties, Strength and Phase Evolution of Binary Cementitious Materials Made of CFBC Ash and Lime. Constr. Build. Mater. 2020, 265, 120302. [Google Scholar] [CrossRef]
- Ma, B.; Ma, M.; Shen, X.; Li, X.; Wu, X. Compatibility between a Polycarboxylate Superplasticizer and the Belite-Rich Sulfoaluminate Cement: Setting Time and the Hydration Properties. Constr. Build. Mater. 2014, 51, 47–54. [Google Scholar] [CrossRef]
- Sheng, G.; Li, Q.; Zhai, J. Investigation on the Hydration of CFBC Fly Ash. Fuel 2012, 98, 61–66. [Google Scholar] [CrossRef]
- Sun, C.; Yin, W. Review on Research Status on Flotation Principles of Silicate Minerals. Conserv. Util. Miner. Resour. 2001, 17–22. [Google Scholar] [CrossRef]
- Cho, H.; Oh, D.; Kim, K. A Study on Removal Characteristics of Heavy Metals from Aqueous Solution by Fly Ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef]
- Qi, T.; Feng, G.; Guo, R.; Zhang, Y.; Ren, A.; Kang, L.; Guo, J. Experimental study on the changes of coal paste backfilling material performance during hydration process. J. Min. Saf. Eng. 2015, 32, 42–48. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
| SiO2 | Al2O3 | CaO | Fe2O3 | SO3 | MgO | |
|---|---|---|---|---|---|---|
| 500 °C-CG | 43.4 | 10.3 | 2.57 | 31.5 | 0.16 | / |
| 800 °C-CG | 40.5 | 10.6 | 3.61 | 32.2 | 0.15 | 0.21 |
| Cement | / | / | / | / | 2.79 | 3.52 |
| Oxide | Content (wt %) | Elements | Content (wt %) | ||
|---|---|---|---|---|---|
| CG | Point-1 | Point-2 | Point-3 | ||
| SiO2 | 35.90 | O | 48.01 | 48.85 | 45.89 |
| Al2O3 | 12.70 | Mg | 3.19 | 0.91 | 1.19 |
| CaO | 4.06 | Al | 8.45 | 13.94 | 16.55 |
| Fe2O3 | 26.40 | Si | 22.17 | 30.06 | 33.92 |
| MgO | 0.30 | Ca | 10.41 | 0.59 | 0.21 |
| K2O | 3.30 | Fe | 7.78 | 5.66 | 2.24 |
| Al/Si(mol/mol) | 0.42 | / | 0.40 | 0.49 | 0.50 |
| Calcination Condition | Qn Relative Intensity/% | |
|---|---|---|
| Q4 | Q3 | |
| Un-calcined CG | 34.27 | 65.73 |
| Calcined under 600 °C | 39.76 | 60.23 |
| Calcined under 700 °C | 15.15 | 84.85 |
| Calcined under 800 °C | 22.98 | 77.02 |
| Calcination Condition | Al3+ Relative Intensity/% | ||
|---|---|---|---|
| Al Ⅵ | Al Ⅴ | Al Ⅳ | |
| Un-calcined CG | 94.44 | / | / |
| Calcined under 600 °C | 12.85 | 6.11 | 34.30 |
| Calcined under 700 °C | 15.28 | 7.30 | 49.29 |
| Calcined under 800 °C | 9.77 | 9.11 | 60.09 |
| Calcination Temperature/°C | BET Surface Area/(m2/g) | Total Pore Volume/(cm3/g) |
|---|---|---|
| 500 | 10.77 | 0.0031 |
| 800 | 7.54 | 0.0022 |
| Calcination Temperature/°C | Water Demand of the Normal Consistency of Cement/% | Setting Time/Min | |
|---|---|---|---|
| Initial | Final | ||
| Reference sample | 28.80 | 261 | 287 |
| 500 | 35.44 | 318 | 350 |
| 800 | 36.00 | 328 | 368 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Wu, H.; Wang, P.; Xu, F.; Ding, S. Thermal Activation of Coal Gangue with Low Al/Si Ratio as Supplementary Cementitious Materials. Molecules 2022, 27, 7268. https://doi.org/10.3390/molecules27217268
Yuan X, Wu H, Wang P, Xu F, Ding S. Thermal Activation of Coal Gangue with Low Al/Si Ratio as Supplementary Cementitious Materials. Molecules. 2022; 27(21):7268. https://doi.org/10.3390/molecules27217268
Chicago/Turabian StyleYuan, Xianli, Hong Wu, Ping Wang, Fen Xu, and Shuang Ding. 2022. "Thermal Activation of Coal Gangue with Low Al/Si Ratio as Supplementary Cementitious Materials" Molecules 27, no. 21: 7268. https://doi.org/10.3390/molecules27217268
APA StyleYuan, X., Wu, H., Wang, P., Xu, F., & Ding, S. (2022). Thermal Activation of Coal Gangue with Low Al/Si Ratio as Supplementary Cementitious Materials. Molecules, 27(21), 7268. https://doi.org/10.3390/molecules27217268







