Abstract
The factors that affect acceleration and high trans/cis selectivity in the catalytic cyclopropanation reaction of styrene with ethyl diazoacetate by cobalt N-confused porphyrin (NCP) complexes were investigated using density functional theory calculations. The reaction rate was primarily related to the energy gap between the cobalt–carbene adduct intermediates, A and B, which was affected by the NCP skeletons and axial pyridine ligands more than the corresponding porphyrin complex. In addition, high trans/cis stereoselectivity was determined at the TS1 and, in part, in the isomerization process at the carbon-centered radical intermediates, Ctrans and Ccis.
1. Introduction
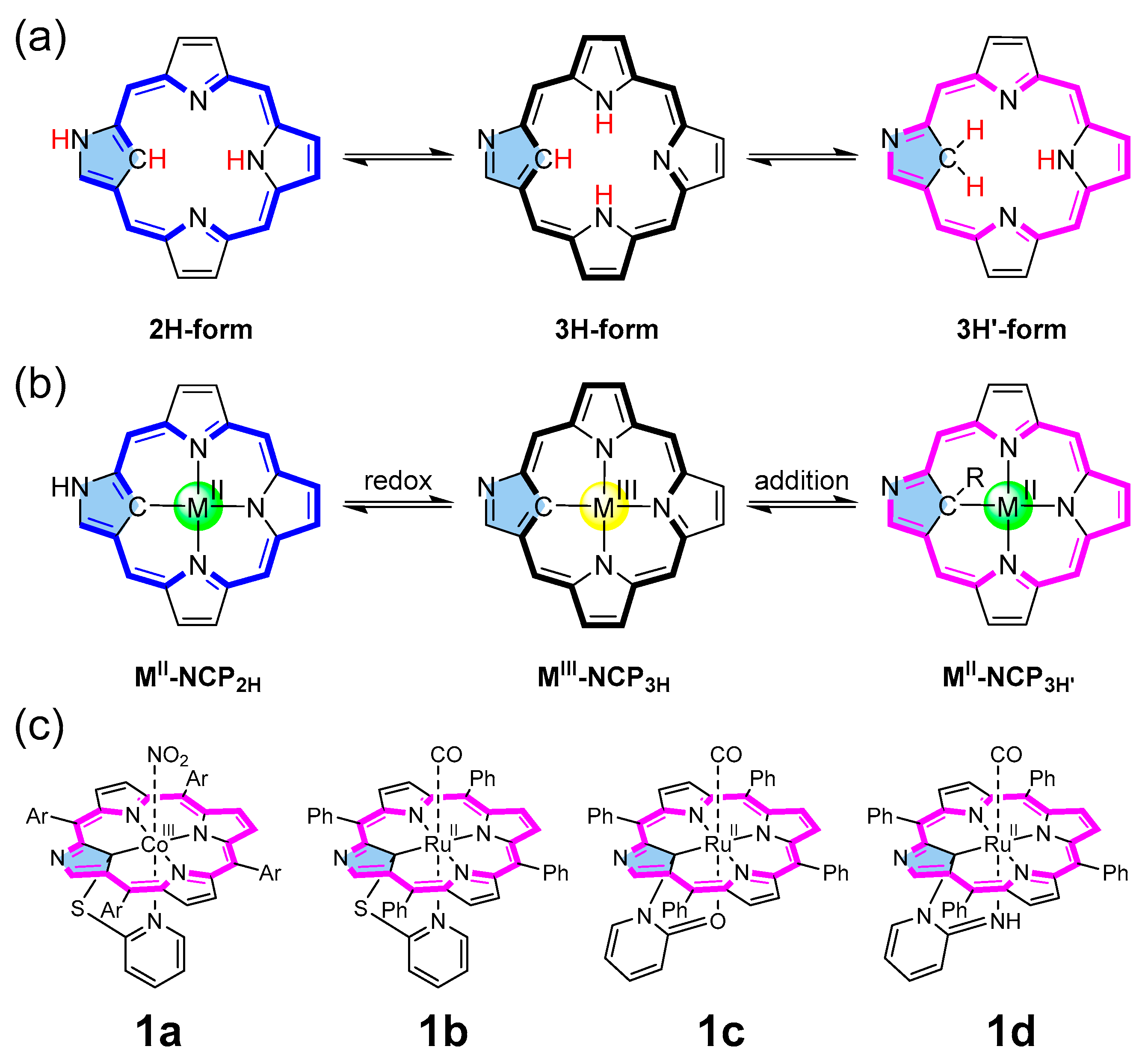
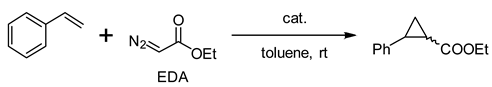
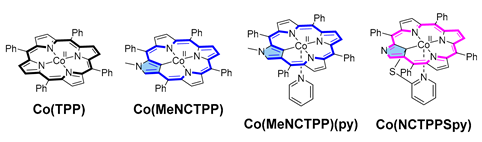
Metalloporphyrins can catalyze fundamental reactions in biological systems and laboratories [1,2]. Various catalytic atom transfer reactions, including carbene, nitrene, and oxygen atoms [3,4,5], have been achieved by synthetic metalloporphyrins. Among such metalloporphyrin-catalyzed reactions, carbene transfer to olefins has been developed to synthesize cyclopropane rings, essential structural motifs found in biologically active natural products and drug molecules [6,7]. So far, a variety of metalloporphyrin complexes (Rh [8,9], Os [10], Fe [11,12], Ru [10,13,14], Ir [15], Co [16,17], etc.) have been demonstrated to be effective for catalytic cyclopropanation reactions. Recently, inspired by the pentacoordinate heme structure [18], we designed and synthesized a series of N-confused porphyrin (NCP) complexes having tethered axial ligands using the reactions of inner-core carbon and 2-heteropyridines (Figure 1c) [19,20]. When the cobalt–NCP complexes (e.g., the reduced form of 1a) were subjected to the cyclopropanation reaction of styrene with ethyl diazoacetate (EDA), enhanced catalytic activity in both the reaction rate and stereoselectivity was observed, compared with the corresponding regular porphyrin. Thus, the structure–activity relationship with these porphyrin complexes remained a question to be solved.
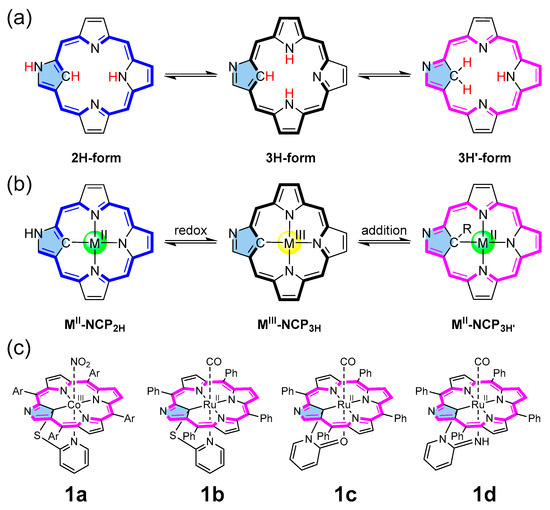
Figure 1.
(a) Tautomeric forms of NCP, (b) redox conversion of metallo-NCP, (c) NCP metal complexes with tethered axial ligands (Ar = Phenyl or 4-MeO-Phenyl).
NCP [21,22] is a porphyrin analog or, more precisely, a porphyrin isomer with an inverted pyrrole ring, which provides an NNNC core for metal coordination [23]. Due to the presence of the carbon atom inside, the introduction of a fifth coordinating ligand becomes facile. In addition, peculiar NH tautomerism between the inside and periphery makes NCP a unique chameleon-type ligand exhibiting different types of NH tautomeric forms (2H- and 3H-form according to the number of hydrogen atoms in the core) (Figure 1a) [24]. The trianionic nature of the 3H- (and 3H′-) form and the σ-donating effect of the inner carbon atom through the carbon–metal bond allow for the stabilization of the high-valent metal ions in the NCP core [25,26]. Due to the intriguing coordination capabilities, NCP metal complexes have been subjected to several catalytic reactions such as cyclopropanation by rhodium and cobalt complexes [27,28], oxygen atom transfer from pyridine N-oxide by rhenium oxo complexes [29], styrene oxidation with PhIO by manganese complex [30], and cyclic carbonate formation between epoxide and carbon dioxide by nickel, palladium, and zinc complexes [31,32].
In particular, the cyclopropanation reaction of styrene with EDA using rhodium(III, IV)–NCP catalysts showed notable catalytic activity in yield and trans/cis (t/c) selectivity compared with that of regular porphyrin (e.g., 92% vs. 71%; t/c = 91/9 vs. 52/48) [27]. Fields et al. reported a similar high selectivity of cobalt(II) N-methylated N-confused tetraphenylporphyrin having an axial pyridine ligand, [Co(MeNCTPP)(py)] (e.g., 85% vs. 67%; t/c = 93/7 vs. 74/26) (Table 1, Entries 1–2) [28]. When we tried the same cyclopropanation reactions with 0.5 mol% of reduced 1a (obtained by treating with aqueous sodium hydrosulfite), the reaction was completed within 5 min at room temperature, resulting in 78% yield and 92/8 of trans/cis selectivity [19]. Compared with the reported Co(MeNCTPP)(py) and the reference cobalt(II) tetraphenylporphyrin complex [Co(TPP)], the catalytic reactivity was significantly enhanced (Table 1, Entries 4–5). Co(MeNCTPP)(py) catalyzed the cyclopropanation reaction similar to Co(NCTPPSpy) at room temperature but with less than half of the turnover number (Table 1, Entries 3–4). On the other hand, Co(TPP) was almost inactive for this cyclopropanation reaction at room temperature. Aiming to clarify the origin of the enhanced catalytic ability of Co(NCTPPSpy), we herein conducted density functional theory (DFT) calculations referring to the proposed mechanism of the cobalt(II) porphyrin-catalyzed cyclopropanation reaction by Zhang and de Bruin [33,34]. In the calculations, we focus on the factors that affect the reaction rate and the trans/cis selectivity of Co(NCTPPSpy) and Co(MeNCTPP)(py) compared with Co(TPP).

Table 1.
Yield and trans/cis selectivity for the cyclopropanation of styrene with EDA 1.
2. Results and Discussion
2.1. Reaction Mechanism
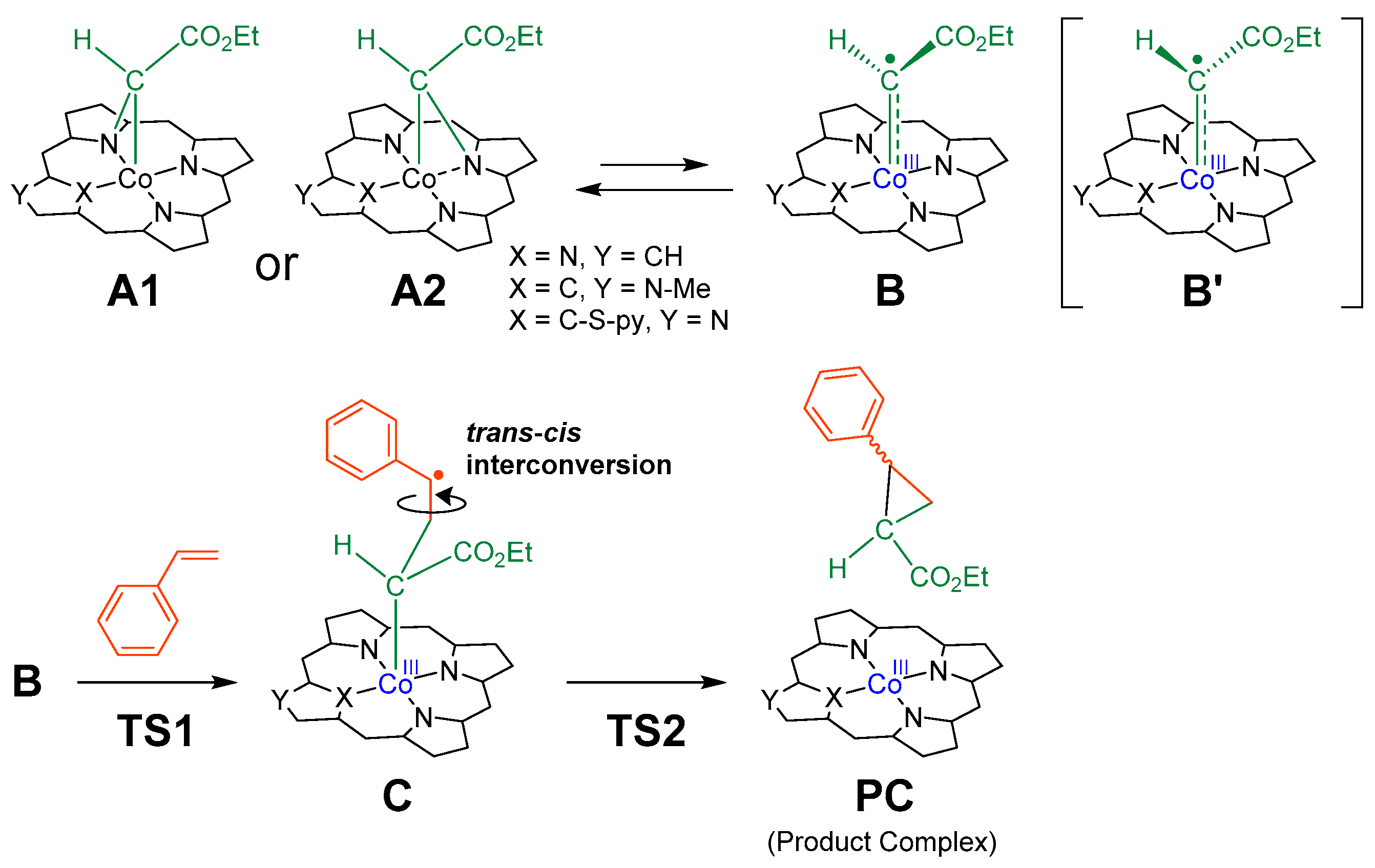
The catalysts used for DFT calculations were Co(TPP), Co(MeNCTPP), Co(MeNCTPP)(py) and Co(NCTPPSpy) (Table 1). Co(MeNCTPP) has the same NCP skeleton as Co(MeNCTPP)(py) without an axial pyridine ligand, and Co(NCTPPSpy) is the supposed structure of the reduced form of 1a. The mechanism adopted for calculating cyclopropanation reactions of styrene with EDA is shown in Scheme 1 [33]. The bridging carbene A (A1 or A2) derived from EDA, and cobalt complex is assumed in equilibrium with radical carbene B (Scheme 1, upper). Styrene contacts B to form a C–C bond, yielding γ-alkyl radical C. C comprises two states, Ctrans and Ccis, leading to the trans and cis configurations of the cyclopropane, respectively. Ctrans and Ccis interconvert mutually by the C–C bond rotation. After the cleavage of the Co–C bond in C, the product complex (PC), comprising Co porphyrin and cyclopropane derivatives (trans and cis), is formed (Scheme 1, bottom).
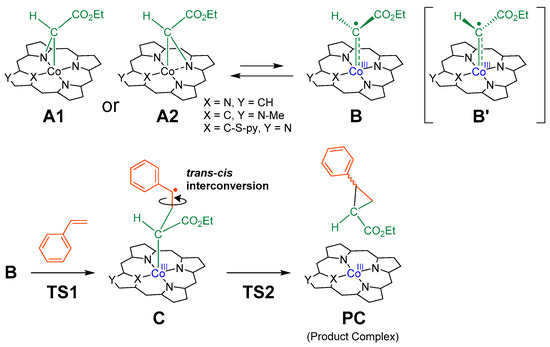
Scheme 1.
A plausible reaction mechanism for cyclopropanation reaction of styrene with EDA by cobalt porphyrin catalysts.
2.2. Energy Diagram for the Reaction with Co(NCTPPSpy)
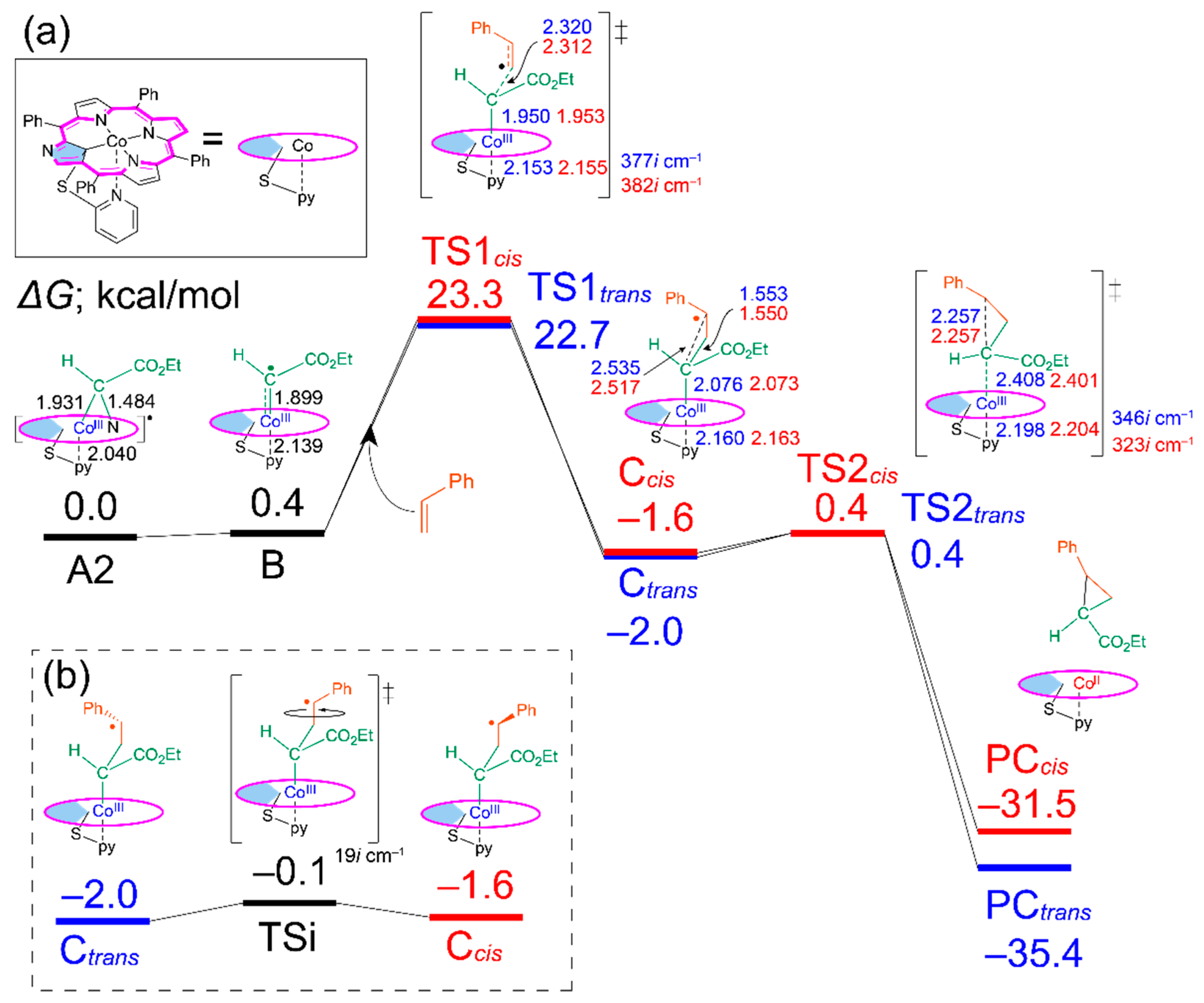
The energy diagrams of the trans and cis products for the catalytic cyclopropanation reaction with various cobalt porphyrins were obtained by DFT calculations. As a representative, the energy diagram with Co(NCTPPSpy) is shown in Figure 2a. The diagrams with other catalysts are shown in Figures S2–S4, and the relative energies of each state are summarized in Table S1 (Supporting Information). In all cases, the reaction pathways involve intermediates (Ctrans and Ccis) and products (PCtrans and PCcis). In the first step, the cyclopropanation reaction initiates with the C–N bond dissociation of A2 (A1 is less stable than A2 by 1.1 kcal/mol, Table S1), resulting in the formation of radical carbene B in the doublet state. (Note: the quartet and sextet states are in higher energy by 28.7 and 35.8 kcal/mol, respectively.) The rotation isomer B’ has a higher energy of 0.6 kcal/mol than B with a rotation barrier (45.1i cm−1) of 4.3 kcal/mol. The dihedral angles (inner C–Co-radical C–carbonyl C) of B, B’ and a transition state are 126.7°, 231.0°, and 174.9°, respectively. Since the energy difference between A2 and B is small (0.4 kcal/mol), we expect that B plays the role of a possible near-attack conformation in the cyclopropanation reaction. The bond formation between B and styrene occurs via TS1 with activation energies of 22.3 and 22.9 kcal/mol for TS1trans and TS1cis, leading to Ctrans and Ccis, respectively (Scheme 1, bottom). An intramolecular radical-radical coupling succeedingly occurs to form the cyclopropane complex PC via TS2. The latter reaction proceeds smoothly because of the small activation energies of 2.4 kcal/mol in TS2trans and 2.0 kcal/mol in TS2cis. Computed results conclude that the rate-determining step of this cyclopropanation reaction is the C–C bond formation between the radical carbene B and styrene, namely at TS1, consistent with the reported studies [33,34].
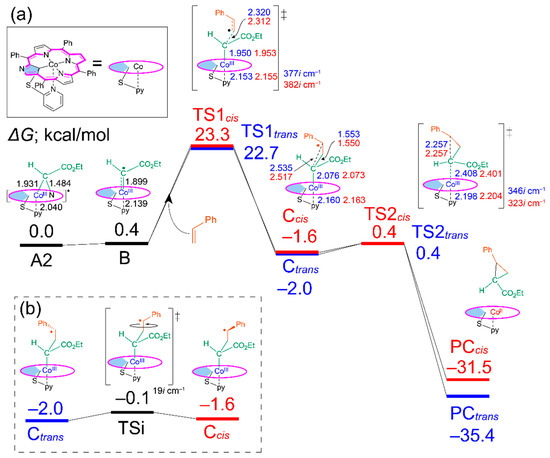
Figure 2.
(a) Energy profile for cyclopropanation reaction catalyzed by Co(NCTPPSpy). (b) Interconversion between Ctrans and Ccis. The relative Gibbs free energies are shown to A2 in kcal/mol. Bond length (Å), Blue: trans route, Red: cis route.
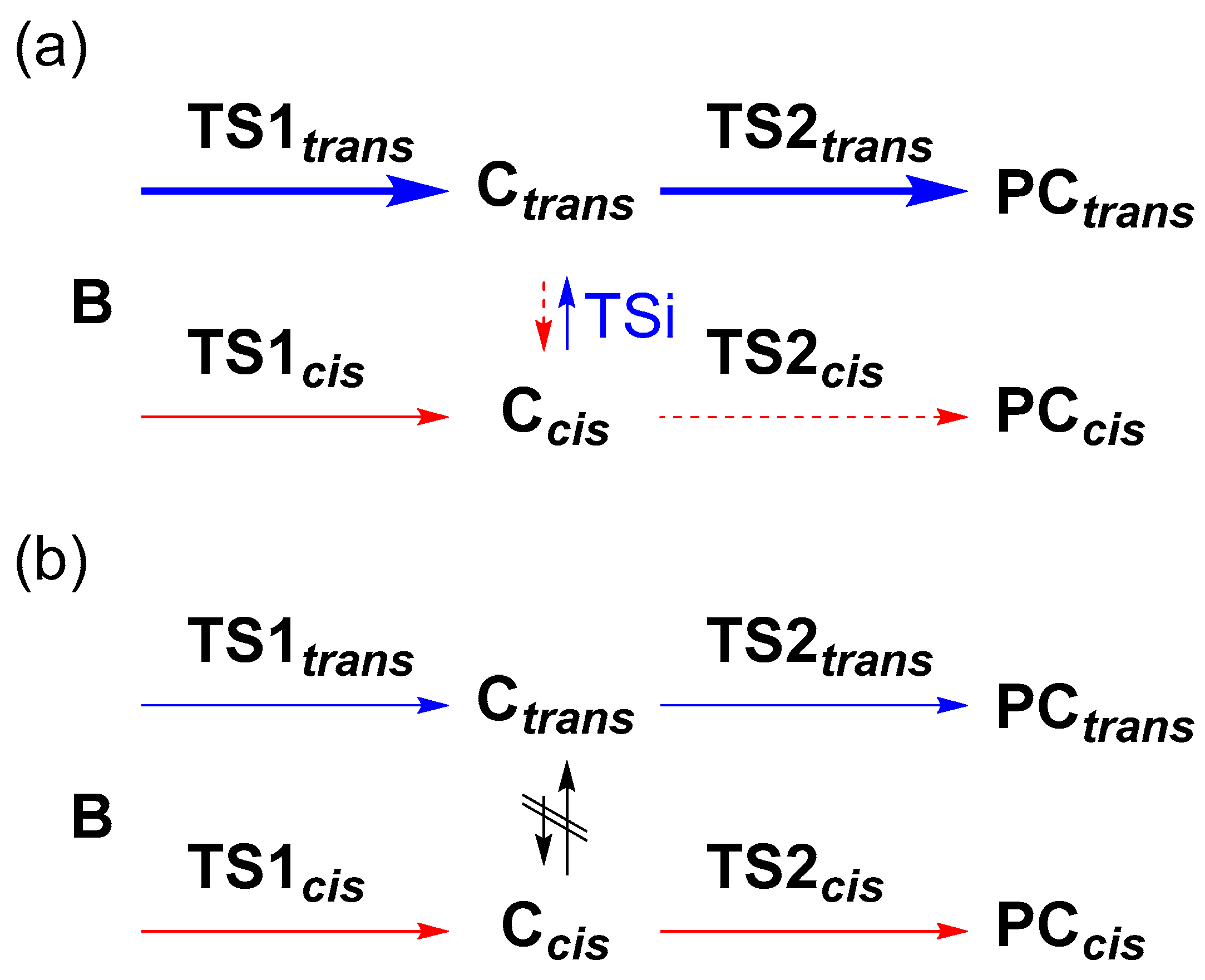
The trans/cis selectivity of cyclopropanation reaction is attributed to the TS1 state in the literature [33]. However, the calculated energy difference (0.6 kcal/mol) between TS1trans and TS1cis seems to be small to quantitively explain the observed trans/cis selectivity (t/c = 92/8, Table 1), which corresponds to 2.3 kcal/mol. Thus, we investigated the possibility of an intervening isomerization pathway at the stage of γ-alkyl radical intermediates, Ccis and Ctrans, with an energy barrier of TSi (Figure 2b). When the energy barrier of the TSi is lower than that of TS2, the trans/cis isomerization could affect the stereoselectivity (Scheme 2a). Conversely, when the height of TS2 is lower than TSi, the trans/cis selectivity is irrelevant to the TSi (Scheme 2b) because of fewer opportunities for interconversion. In this study, we focused on the steps from A to TS1 and the energy barrier of TSi and TS2cis to explain the acceleration effect and the high trans/cis selectivity in the Co-NCP catalyzed cyclopropanation reactions.
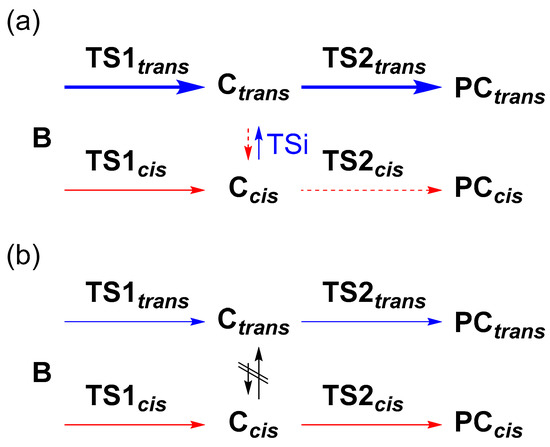
Scheme 2.
Pathways from B to PCtrans and PCcis, (a) with and (b) without TSi. The blue and red lines represent the pathways to PCtrans and PCcis, respectively.
The calculated energy differences between cis and trans forms of transition states and intermediates are much smaller than we expected for discussing the experimental results quantitatively. However, we could see some trends among the catalytic systems examined. In the following sections, we will discuss the factors affecting the reaction rate and trans/cis selectivity based on the calculation and the experimental results.
2.3. Acceleration Effect
The rate of our cyclopropanation reaction depends on the relative Gibbs free energy [ΔG(B–A)] and the activation energy for TS1 from B [Ea(TS1)] (Equation (S4) in the Supporting Information). After TS1, the reaction goes to the product exergonically. The calculated ΔG(B–A) and Ea(TS1trans) values for cobalt porphyrin catalysts are summarized in Table 2. Ea(TS1trans) of Co(NCTPPSpy) (22.3 kcal/mol) is higher than that of Co(TPP) (18.7 kcal/mol). However, the relative energy for TS1trans of Co(NCTPPSpy) (22.7 kcal/mol) from the A state is lower than that of Co(TPP) (23.5 kcal/mol) due to the contribution of ΔG(B–A). The cyclopropanation reaction with Co(NCTPPSpy) catalyst proceeded smoothly at room temperature, whereas Co(TPP) did not show catalytic activity under the same conditions. Therefore, the reaction rates are likely dependent on ΔG(B–A) rather than Ea(TS1), and the smaller ΔG(B–A) may accelerate the reaction. A similar trend was observed with other NCP catalysts. Because A and B are in an equilibrium state (A ⇄ B), a smaller ΔG(B–A) increases the opportunity of the reaction between B and styrene, which results in the acceleration of the cyclopropanation reaction.

Table 2.
The calculated Gibbs free energies ΔG and activation energy Ea (in kcal/mol).
2.4. Structures of A and B
To clarify the factors that afford the much smaller ΔG(B–A) values for Co(MeNCTPP)(py) and Co(NCTPPSpy) than those for Co(TPP) and Co(MeNCTPP), the structures of A and B states are analyzed in detail. For Co(TPP) and Co(MeNCTPP), the changes in the bond length between the cobalt and the carbene atom in A and B are around 0.1 Å (0.091 and 0.122 Å, respectively). The formal valency of the cobalt center in A and B is different, divalent in A and trivalent in B. Owing to the σ-donation from the inner carbon atom of the NCP ligand, the trivalent B state of Co(MeNCTPP) is stabilized to decrease the energy gap between A and B.
On the other hand, for Co(MeNCTPP)(py) and Co(NCTPPSpy), the corresponding bond-length difference in A and B is much smaller, less than 0.05 Å (0.047 and 0.032 Å). The valency of cobalt centers is trivalent in both A and B, and the spin density is delocalized on the NCP skeleton, different from Co(TPP) and Co(MeNCTPP). Trivalent cobalt centers in both states are stabilized by NCP skeletons and the axial pyridine ligands. Consequently, the energy gap between A and B becomes smaller. Stabilizing the trivalent cobalt center by the NCP skeletons and the electron donation from the pyridine ligands are essential factors for accelerating the catalytic cyclopropanation reaction of styrene.
2.5. Trans/Cis Stereoselectivity
In the energy diagram with Co(NCTPPSpy), the activation energies from Ccis to Ctrans via TSi, Ea[TSi(Ccis→Ctrans)], and TS2, Ea(TS2cis), were significantly lower than Ea(TS1trans/cis) (Figure 2). This result suggests that C formed by passing through TS1 is rapidly converted to PC via TS2. However, the possibility of conversion of Ccis to Ctrans via TSi remains because Ea[TSi(Ccis→Ctrans)] is lower than Ea(TS2cis) by 0.5 kcal/mol, and Ctrans is more stable than Ccis by 0.4 kcal/mol. We assume this bypath process is kinetically controlled, and the formed Ctrans goes to PC via TS2trans without reaching the equilibrium between Ccis to Ctrans. Although the reverse process, Ctrans→Ccis via TSi, cannot be ignored because of a small energy barrier difference (0.4 kcal/mol), it might be energetically less favorable. This situation is manifested in the Co(MeNCTPP)(py) case, which shows energy barriers of 3.3 kcal/mol vs. 1.9 kcal/mol, for Ctrans→Ccis and Ccis→Ctrans, respectively (Figure S2). To summarize, a large portion of C goes to PC via TS2 without interconversion. In addition to this pathway, a small part of Ccis isomerizes into more stable Ctrans via TSi, increasing the population of Ctrans. Consequently, Co(NCTPPSpy) and Co(MeNCTPP)(py) exhibit high trans selectivity in the catalytic system. These results are attributed to the energy difference between TS1trans and TS1cis and the isomerization from Ccis to Ctrans via TSi (Scheme 2a). On the other hand, Ea[TSi(Ccis→Ctrans)] is more significant than Ea(TS2cis) by 2.7 kcal/mol for Co(TPP), which loses the opportunity of conversion into Ctrans via TSi (Scheme 2b). Although the energy differences we used in the discussion are too small to correctly evaluate the trans/cis ratios at our calculation level, the selectivity trends agree with the experimental results. Thus, we think that a further high trans/cis selectivity could be realized by controlling Ea(TSi) and Ea(TS2) with suitable substituents in Co(NCTPPSpy) and Co(MeNCTPP)(py).
2.6. Structures of C
Regarding the higher TS2 levels of NCP catalysts than the regular porphyrin, the structures of γ-alkyl radical intermediate C and TS2 were compared in detail. The bond lengths between the cobalt and carbene atoms are similar in all C, [Co(TPP); Ctrans (Ccis): 2.067 (2.060) Å, Co(MeNCTPP); 2.030 (2.030) Å, Co(MeNCTPP)(py); 2.062 (2.062) Å, Co(NCTPPSpy); 2.076 (2.073) Å]. In contrast, those values in TS2 are largely affected by the NCP skeletons and pyridine ligands: NCP complexes show longer bond lengths than that of Co(TPP), [Co(TPP); TS2trans (TS2cis): 2.180 (2.194) Å, Co(MeNCTPP); 2.289 (2.299) Å, Co(MeNCTPP)(py); 2.382 (2.397) Å, Co(NCTPPSpy); 2.408 (2.401) Å]. For Co(NCTPPSpy), the pyridine nitrogen atom strongly contacts the cobalt center compared with Co(MeNCTPP)(py). Due to the electron donation from the axial pyridine ligand to the cobalt center, the structural changes become larger in TS2 for the pyridine-coordinating catalysts. Consequently, the reactions with Co(NCTPPSpy) and Co(MeNCTPP)(py) go through both the TS2 and TSi to exhibit high trans/cis selectivity.
3. Conclusions
The factors affecting acceleration and trans/cis selectivity in the catalytic cyclopropanation reaction of styrene with ethyl diazoacetate by cobalt N-confused porphyrin (NCP) complexes were investigated with DFT calculations in this work. The reaction rates are affected by the energy gap (ΔG(B–A)) between the cobalt–carbene adduct intermediates, A and B, which is largely decreased by the NCP skeletons and axial pyridine ligands. Co(MeNCTPP)(py) and Co(NCTPPSpy) exhibit smaller ΔG(B–A) values than that of Co(TPP), leading to higher reaction rates than Co(TPP), as illustrated experimentally. High trans/cis selectivity originates from the energy difference in TS1 and, in addition, the isomerization of γ-alkyl radical intermediate C. For Co(TPP), the conversion from Ccis to Ctrans is negligible, whereas Co(MeNCTPP)(py) and Co(NCTPPSpy) can take a bypath route to increase the population of Ctrans. Consequently, cyclopropanation products with high trans/cis selectivity were achieved.
This study revealed that minute changes in the porphyrin structure, such as N-confusion, cause a distinct difference in the energy level of intermediates and pathways in the catalytic reaction. Furthermore, because of the structural resemblance to regular porphyrin, NCP metal complexes could also be applied for the cofactor-replacing modification of biocatalysts [35,36]. The research in this direction is currently underway in our laboratory.
4. Materials and Methods
Calculation methods: The DFT calculations were conducted with the Gaussian 09 program package (Rev. E.01) [37]. We used the B3LYP functional [38,39,40] combined with the (15s11p6d) primitive set of Wachters–Hay supplemented with one polarization f-function (α = 1.17) [41,42,43] for cobalt atoms and the D95** basis set [44] for the other atoms. After geometry optimizations, vibrational analyses were performed for all reaction species to confirm stable and transition structures. The spin multiplicity and electronic charge analysis were performed in the doublet state (S = 1/2) and neutral in all calculations, respectively. Energy profiles of calculated pathways are presented as Gibbs free energy changes (in kcal/mol) at 298.15 K.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217266/s1, Figure S1: Optimized structures of reaction species and transition states in Co(NCTPPSpy); Figure S2: (a) Energy profile for cyclopropanation reaction catalyzed by Co(MeNCTPP)(py). (b) Interconversion between Ctrans and Ccis.; Figure S3: (a) Energy profile for cyclopropanation reaction catalyzed by Co(MeNCTPP). (b) Interconversion between Ctrans and Ccis.; Figure S4: (a) Energy profile for cyclopropanation reaction catalyzed by Co(TPP). (b) Interconversion between Ctrans and Ccis.; Table S1: Relative Gibbs free energies ΔG of trans-species with respect to A1 or A2 are in kcal/mol.; Table S2: Calculated Mulliken spin populations in Co(NCTPPSpy).; Table S3: Calculated Mulliken spin populations in Co(MeNCTPP)(py).; Table S4: Calculated Mulliken spin populations in Co(MeNCTPP).; Table S5: Calculated Mulliken spin populations in Co(TPP).; Table S6: Cartesian coordinates of A2 in the doublet state of Co(NCTPPSpy).; Table S7: Cartesian coordinates of B in the doublet state of Co(NCTPPSpy).; Table S8: Cartesian coordinates of TS1trans in the doublet state of Co(NCTPPSpy).; Table S9: Cartesian coordinates of TS1cis in the doublet state of Co(NCTPPSpy).; Table S10: Cartesian coordinates of Ctrans in the doublet state of Co(NCTPPSpy).; Table S11: Cartesian coordinates of Ccis in the doublet state of Co(NCTPPSpy).; Table S12: Cartesian coordinates of TS2trans in the doublet state of Co(NCTPPSpy).; Table S13: Cartesian coordinates of TS2cis in the doublet state of Co(NCTPPSpy).; Table S14: Cartesian coordinates of PCtrans in the doublet state of Co(NCTPPSpy).; Table S15: Cartesian coordinates of PCcis in the doublet state of Co(NCTPPSpy).; Table S16: Cartesian coordinates of TSi in the doublet state of Co(NCTPPSpy).
Author Contributions
Conceptualization, H.F. and T.M.; methodology, M.M. and Y.S.; formal analysis, M.M., T.M., T.T. and Y.S.; investigation, M.M., T.T. and Y.S.; resources, Y.S. and K.Y.; data curation, O.I., M.M. and T.M.; writing—original draft preparation, O.I., M.M., T.M., Y.S. and H.F.; writing—review and editing, H.F.; visualization, O.I., M.M. and T.M.; supervision, H.F.; funding acquisition, H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant numbers JP20H00406 and JP21K18984. Financial support from Qdai-jump Research Program, Kyushu University, is also acknowledged. This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef] [PubMed]
- Barona-Castaño, J.C.; Carmona-Vargas, C.C.; Brocksom, T.J.; De Oliveira, K.T. Porphyrins as Catalysts in Scalable Organic Reactions. Molecules 2016, 21, 310. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.M.Q.; Gonzaga, D.T.G.; Cardoso, M.F.C.; Forezi, L.D.S.M.; Gomes, A.T.P.C.; Da Silva, F.D.C.; Ferreira, V.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Carbene Transfer Reactions Catalysed by Dyes of the Metalloporphyrin Group. Molecules 2018, 23, 792. [Google Scholar] [CrossRef]
- Singh, R.; Mukherjee, A. Metalloporphyrin Catalyzed C–H Amination. ACS Catal. 2019, 9, 3604–3617. [Google Scholar] [CrossRef]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.L.; Brown, K.C.; Bartley, D.W.; Kodadek, T. Mechanism of the Rhodium Porphyrin-Catalyzed Cyclopropanation of Alkenes. Science 1992, 256, 1544–1547. [Google Scholar]
- Ciammaichella, A.; Cardoni, V.; Leoni, A.; Tagliatesta, P. Rhodium Porphyrin Bound to a Merrifield Resin as Heterogeneous Catalyst for the Cyclopropanation Reaction of Olefins. Molecules 2016, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Che, C.-M.; Huang, J.-S. Ruthenium and osmium porphyrin carbene complexes: Synthesis, structure, and connection to the metal-mediated cyclopropanation of alkenes. Coord. Chem. Rev. 2002, 231, 151–164. [Google Scholar] [CrossRef]
- Intrieri, D.; Gac, S.L.; Caselli, A.; Rose, E.; Boitrel, B.; Gallo, E. Highly diastereoselective cyclopropanation of α-methylstyrene catalysed by a C2-symmetrical chiral iron porphyrin complex. Chem. Commun. 2014, 50, 1811–1813. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Tinoco, A.; Steck, V.; Fasan, R.; Zhang, Y. Cyclopropanations via Heme Carbenes: Basic Mechanism and Effects of Carbene Substituent, Protein Axial Ligand, and Porphyrin Substitution. J. Am. Chem. Soc. 2018, 140, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Simonneaux, G.; Maux, P.L. Optically active ruthenium porphyrins: Chiral recognition and asymmetric catalysis. Coord. Chem. Rev. 2002, 228, 43–60. [Google Scholar] [CrossRef]
- Wolf, M.W.; Vargas, D.A.; Lehnert, N. Engineering of RuMb: Toward a Green Catalyst for Carbene Insertion Reactions. Inorg. Chem. 2017, 56, 5623–5635. [Google Scholar] [CrossRef] [PubMed]
- Anding, B.J.; Ellern, A.; Woo, L.K. Olefin Cyclopropanation Catalyzed by Iridium(III) Porphyrin Complexes. Organometallics 2012, 31, 3628–3635. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Y.; Gao, G.-Y.; Zhang, X.P. Diastereoselective and Enantioselective Cyclopropanation of Alkenes Catalyzed by Cobalt Porphyrins. J. Org. Chem. 2003, 68, 8179–8184. [Google Scholar] [CrossRef]
- Chen, Y.; Fields, K.B.; Zhang, X.P. Bromoporphyrins as Versatile Synthons for Modular Construction of Chiral Porphyrins: Cobalt-Catalyzed Highly Enantioselective and Diastereoselective Cyclopropanation. J. Am. Chem. Soc. 2004, 126, 14718–14719. [Google Scholar] [CrossRef] [PubMed]
- De Montellano, P.R.O. Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamamoto, T.; Mashita, S.; Deguchi, Y.; Fukuyama, K.; Ishida, M.; Mori, S.; Furuta, H. N-Confused Porphyrin Metal Complexes with an Axial Pyridine Directly Tethered from an Inner Carbon: A Bioinspired Ligand as a Versatile Platform for Catalysis. Eur. J. Inorg. Chem. 2018, 2018, 203–207. [Google Scholar] [CrossRef]
- Miyazaki, T.; Fukuyama, K.; Mashita, S.; Deguchi, Y.; Yamamoto, T.; Ishida, M.; Mori, S.; Furuta, H. Ruthenium N-Confused Porphyrins: Selective Reactivity for Ambident 2-Heteroatom-Substituted Pyridines Serving as Axial Ligands. ChemPlusChem 2019, 84, 603–607. [Google Scholar] [CrossRef]
- Furuta, H.; Asano, T.; Ogawa, T. “N-Confused Porphyrin”: A New Isomer of Tetraphenylporphyrin. J. Am. Chem. Soc. 1994, 116, 767–768. [Google Scholar] [CrossRef]
- Chmielewski, P.J.; Latos-Grażyński, L.; Rachlewicz, K.; Głowiak, T. Tetra-p-tolylporphyrin with an Inverted Pyrrole Ring: A Novel Isomer of Porphyrin. Angew. Chem. Int. Ed. Engl. 1994, 33, 779–781. [Google Scholar] [CrossRef]
- Toganoh, M.; Furuta, H. Creation from Confusion and Fusion in Porphyrin World—The Last Three Decades of N-Confused Porphyrinoid Chemistry. Chem. Rev. 2022, 122, 8313–8437. [Google Scholar] [CrossRef] [PubMed]
- Furuta, H.; Ishizuka, T.; Osuka, A.; Dejima, H.; Nakagawa, H.; Ishikawa, Y. NH Tautomerism of N-Confused Porphyrin. J. Am. Chem. Soc. 2001, 123, 6207–6208. [Google Scholar] [CrossRef] [PubMed]
- Furuta, H.; Ogawa, T.; Uwatoko, Y.; Araki, K. N-Confused Tetraphenylporphyrin–Silver(III) Complex. Inorg. Chem. 1999, 38, 2676–2682. [Google Scholar] [CrossRef]
- Maeda, H.; Ishikawa, Y.; Matsuda, T.; Osuka, A.; Furuta, H. Control of Cu(II) and Cu(III) States in N-Confused Porphyrin by Protonation/Deprotonation at the Peripheral Nitrogen. J. Am. Chem. Soc. 2003, 125, 11822–11823. [Google Scholar] [CrossRef] [PubMed]
- Niino, T.; Toganoh, M.; Andrioletti, B.; Furuta, H. Rhodium N-confused porphyrin-catalyzed alkene cyclopropanation. Chem. Commun. 2006, 41, 4335–4337. [Google Scholar] [CrossRef]
- Fields, K.B.; Engle, J.T.; Sripothongnak, S.; Kim, C.; Zhang, X.P.; Ziegler, C.J. Cobalt carbaporphyrin-catalyzed cyclopropanation. Chem. Commun. 2011, 47, 749–751. [Google Scholar] [CrossRef]
- Yamamoto, T.; Toganoh, M.; Furuta, H. Cooperation between metal and ligand in oxygen atom transport by N-confused porphyrin oxorhenium(V) complexes. Dalton Trans. 2012, 41, 9154–9157. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.-H.; Mahmood, M.H.; Zou, H.-B.; Yang, S.-B.; Liu, H.-Y. The first manganese N-confused porphyrins catalyzed oxidation of alkene. J. Mol. Catal. A-Chem. 2014, 395, 180–185. [Google Scholar] [CrossRef]
- Dela Cruz, J.B.; Ruamps, M.; Arco, S.; Hung, C.-H. Ni and Pd N-confused porphyrin complexes as catalysts for the synthesis of cyclic carbonates from epoxides and CO2. Dalton Trans. 2019, 48, 7527–7531. [Google Scholar] [CrossRef]
- Ge, Y.; Cheng, G.; Xu, N.; Wang, W.; Ke, H. Zinc 2-N-methyl N-confused porphyrin: An efficient catalyst for the conversion of CO2 into cyclic carbonates. Catal. Sci. Technol. 2019, 9, 4255–4261. [Google Scholar] [CrossRef]
- Dzik, W.I.; Xu, X.; Zhang, X.P.; Reek, J.N.H.; de Bruin, B. ‘Carbene Radicals’ in CoII(por)-Catalyzed Olefin Cyclopropanation. J. Am. Chem. Soc. 2010, 132, 10891–10902. [Google Scholar] [CrossRef]
- Lu, H.; Dzik, W.I.; Xu, X.; Wojtas, L.; de Bruin, B.; Zhang, X.P. Experimental Evidence for Cobalt(III)-Carbene Radicals: Key Intermediates in Cobalt(II)-Based Metalloradical Cyclopropanation. J. Am. Chem. Soc. 2011, 133, 8518–8521. [Google Scholar] [CrossRef] [PubMed]
- Oohora, K.; Meichin, H.; Kihira, Y.; Sugimoto, H.; Shiro, Y.; Hayashi, T. Manganese(V) Porphycene Complex Responsible for Inert C–H Bond Hydroxylation in a Myoglobin Matrix. J. Am. Chem. Soc. 2017, 139, 18460–18463. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, J.P.T.; Yosca, T.H.; Siegler, M.A.; Moënne-Loccoz, P.; Green, M.T.; Goldberg, D.P. Direct Observation of Oxygen Rebound with an Iron-Hydroxide Complex. J. Am. Chem. Soc. 2017, 139, 13640–13643. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian Basis Set for Molecular Wavefunctions Containing Third-Row Atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]
- Hay, P.J. Gaussian basis sets for molecular calculations. The representation of 3d orbitals in transition-metal atoms. J. Chem. Phys. 1977, 66, 4377–4384. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems. Excitation energies of first-row transition metals Sc–Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Dunning, T.H.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1976; Volume 3, pp. 1–27. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).