Mebendazole Impedes the Proliferation and Migration of Pancreatic Cancer Cells through SK1 Inhibition Dependent Pathway
Abstract
1. Introduction
2. Results
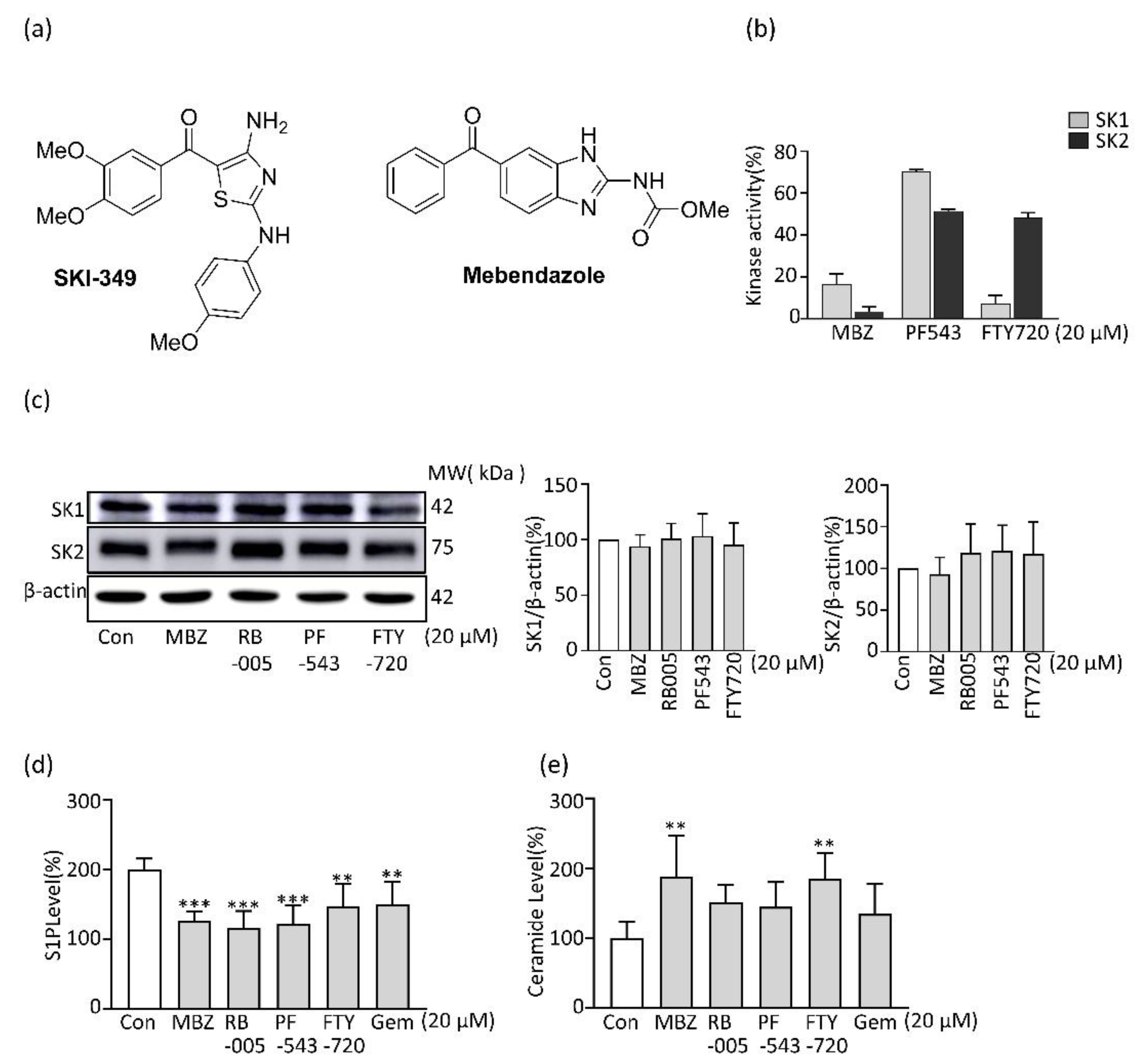
2.1. MBZ Inhibits SK Activity and Thereby Reduces the Production of S1P in PDAC Cells
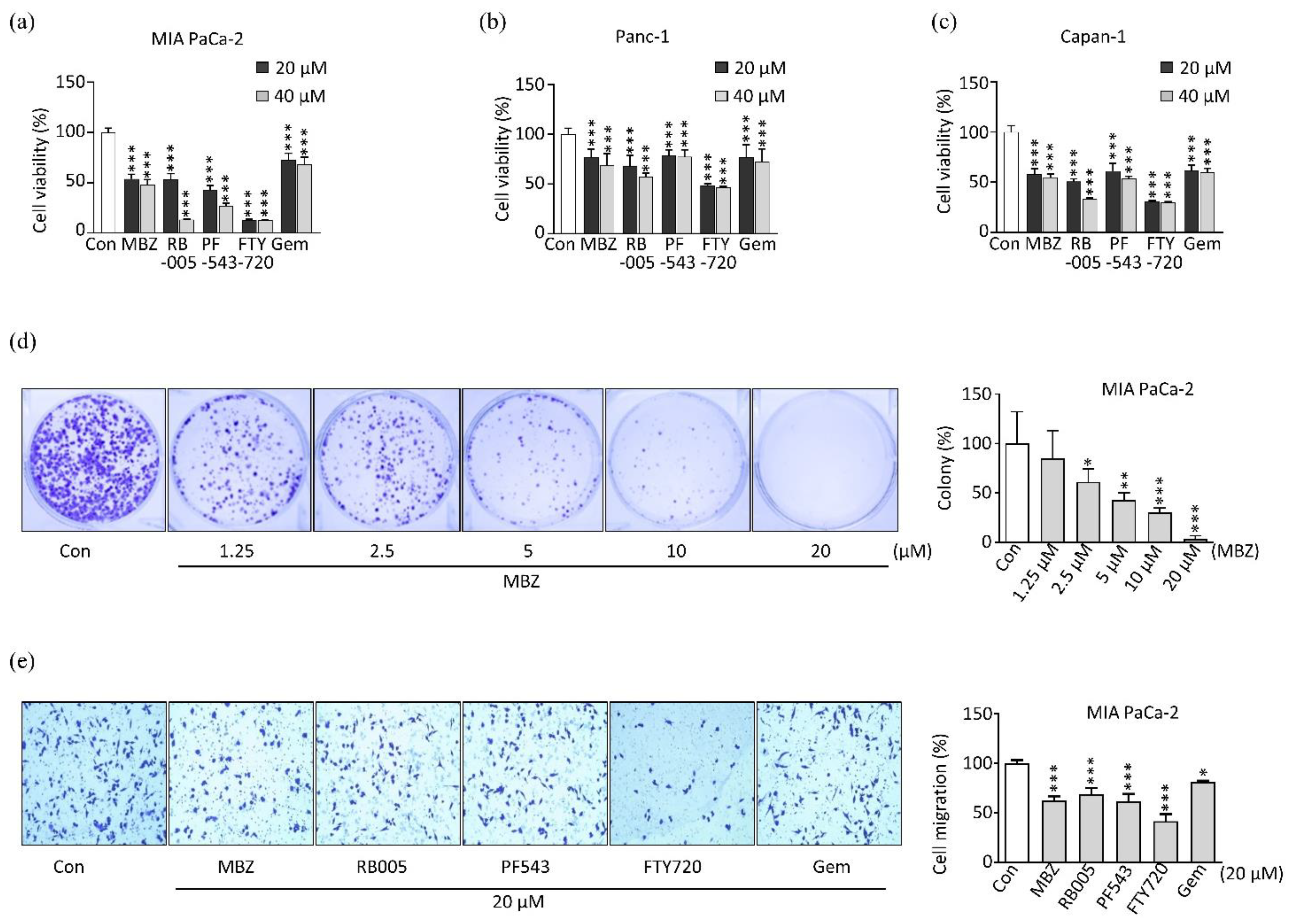
2.2. MBZ Inhibits the Viability, Proliferation, and Migration of Pancreatic Cancer Cell Lines
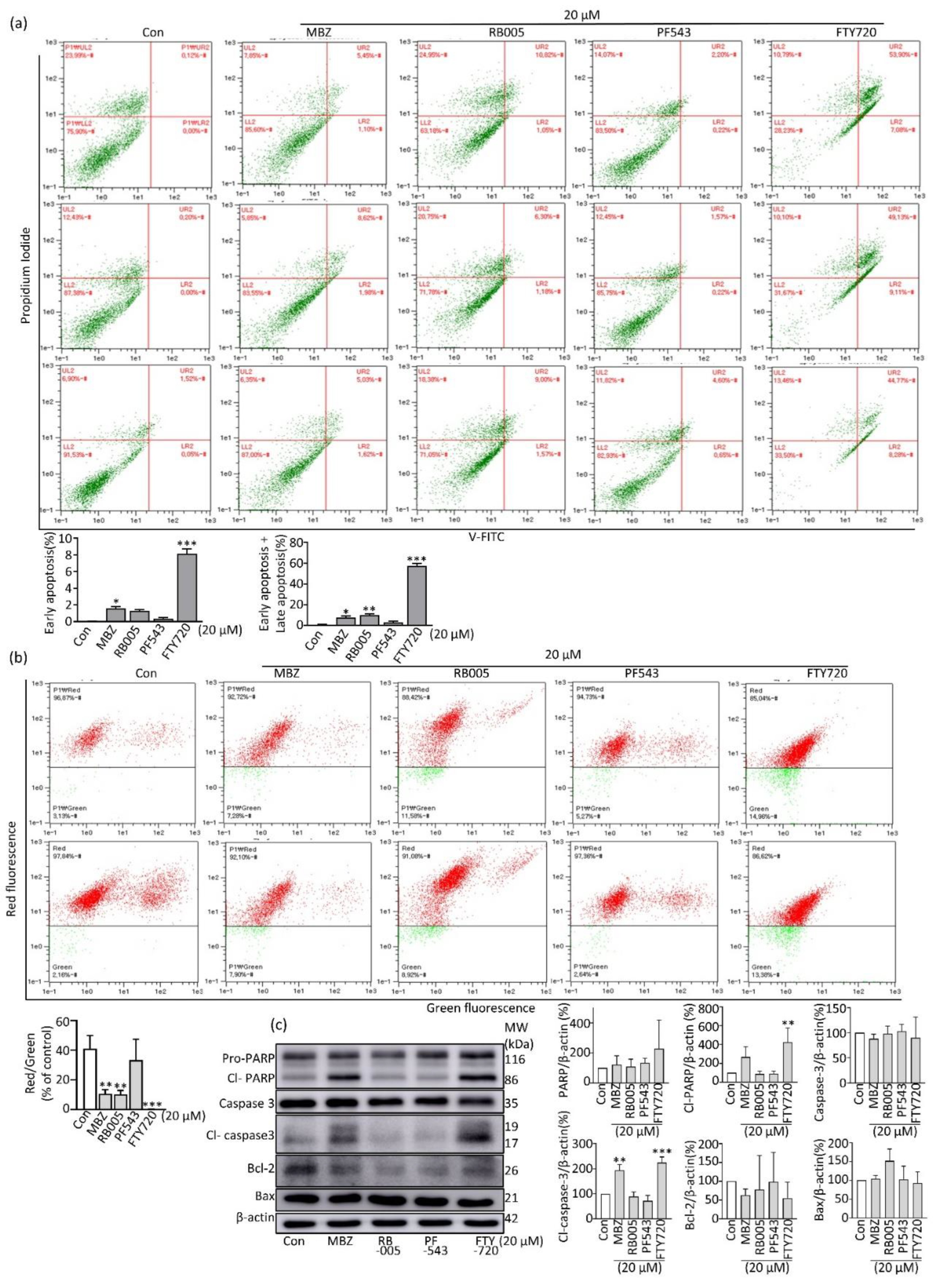
2.3. MBZ Induces Apoptosis in Pancreatic Cancer Cells through the Intrinsic Mitochondrial Pathway
2.4. MBZ Partially Reverses Apoptosis Inhibited by S1P via the JAK2/STAT3/Bcl-2 Pathway
2.5. MBZ Prevents PDAC Cell Migration via Suppression of the S1P/FAK Signaling Pathway
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents, Antibodies and Chemicals
5.2. Cell Lines
5.3. MTT Assay
5.4. Colony-Forming Assay
5.5. Transwell Assay for Migration
5.6. Annexin V-FITC Assay
5.7. ELISA Assay
5.8. SK Assay
5.9. Immunoblotting
5.10. JC-10 Assay
5.11. Phase Contrast Microscopy for Cell Morphology Change
5.12. S1P Preparation and Cell Treatment
5.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Hau, S.O.; Petersson, A.; Nodin, B.; Karnevi, E.; Boman, K.; Williamsson, C.; Eberhard, J.; Leandersson, K.; Gisselsson, D.; Heby, M.; et al. Chemotherapy, host response and molecular dynamics in periampullary cancer: The CHAMP study. BMC Cancer 2020, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Masiak-Segit, W.; Rawicz-Pruszyński, K.; Skórzewska, M.; Polkowski, W.P. Surgical treatment of pancreatic cancer. Pol. Przegl. Chir. 2018, 90, 45–53. [Google Scholar] [CrossRef]
- Narayanan, S.; Vicent, S.; Ponz-Sarvisé, M. PDAC as an Immune Evasive Disease: Can 3D Model Systems Aid to Tackle This Clinical Problem? Front. Cell Dev. Biol. 2021, 9, 787249. [Google Scholar] [CrossRef]
- Osei-Bordom, D.C.; Serifis, N.; Brown, Z.J.; Hewitt, D.B.; Lawal, G.; Sachdeva, G.; Cloonan, D.J.; Pawlik, T.M. Pancreatic ductal adenocarcinoma: Emerging therapeutic strategies. Surg. Oncol. 2022, 43, 101803. [Google Scholar] [CrossRef]
- Raja Arul, G.L.; Toruner, M.D.; Gatenby, R.A.; Carr, R.M. Ecoevolutionary biology of pancreatic ductal adenocarcinoma. Pancreatology 2022, 22, 730–740. [Google Scholar] [CrossRef]
- Torphy, R.J.; Fujiwara, Y.; Schulick, R.D. Pancreatic cancer treatment: Better, but a long way to go. Surg. Today 2020, 50, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Loveday, B.P.T.; Lipton, L.; Thomson, B.N. Pancreatic cancer: An update on diagnosis and management. Aust. J. Gen. Pract. 2019, 48, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Florio, R.; Carradori, S.; Veschi, S.; Brocco, D.; Genni, T.D.; Cirilli, R.; Casulli, A.; Cama, A.; Lellis, L.D. Screening of Benzimidazole-Based Anthelmintics and Their Enantiomers as Repurposed Drug Candidates in Cancer Therapy. Pharmaceuticals 2021, 14, 372. [Google Scholar] [CrossRef] [PubMed]
- Florio, R.; Veschi, S.; di Giacomo, V.; Pagotto, S.; Carradori, S.; Verginelli, F.; Cirilli, R.; Casulli, A.; Grassadonia, A.; Tinari, N.; et al. The Benzimidazole-Based Anthelmintic Parbendazole: A Repurposed Drug Candidate That Synergizes with Gemcitabine in Pancreatic Cancer. Cancers 2019, 11, 2042. [Google Scholar] [CrossRef]
- Williamson, T.; de Abreu, M.C.; Trembath, D.G.; Brayton, C.; Kang, B.; Mendes, T.B.; de Assumpção, P.P.; Cerutti, J.M.; Riggins, G.J. Mebendazole disrupts stromal desmoplasia and tumorigenesis in two models of pancreatic cancer. Oncotarget 2021, 12, 1326–1338. [Google Scholar] [CrossRef]
- Zhang, L.; Bochkur Dratver, M.; Yazal, T.; Dong, K.; Nguyen, A.; Yu, G.; Dao, A.; Bochkur Dratver, M.; Duhachek-Muggy, S.; Bhat, K.; et al. Mebendazole Potentiates Radiation Therapy in Triple-Negative Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 195–207. [Google Scholar] [CrossRef]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a Candidate for Drug Repurposing in Oncology: An Extensive Review of Current Literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef]
- Dogra, N.; Mukhopadhyay, T. Impairment of the ubiquitin-proteasome pathway by methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate leads to a potent cytotoxic effect in tumor cells: A novel antiproliferative agent with a potential therapeutic implication. J. Biol. Chem. 2012, 287, 30625–30640. [Google Scholar] [CrossRef]
- Dogra, N.; Kumar, A.; Mukhopadhyay, T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci. Rep. 2018, 8, 11926. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, L.; Zhang, J.; He, F.; Wang, H.; Liu, Q.; Shi, D.; Ni, N.; Wagstaff, W.; Chen, C.; et al. Antiparasitic mebendazole (MBZ) effectively overcomes cisplatin resistance in human ovarian cancer cells by inhibiting multiple cancer-associated signaling pathways. Aging 2021, 13, 17407–17427. [Google Scholar] [CrossRef]
- Elayapillai, S.; Ramraj, S.; Benbrook, D.M.; Bieniasz, M.; Wang, L.; Pathuri, G.; Isingizwe, Z.R.; Kennedy, A.L.; Zhao, Y.D.; Lightfoot, S.; et al. Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol. Oncol. 2021, 160, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Beljanski, V.; Knaak, C.; Zhuang, Y.; Smith, C.D. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Investig. New Drugs 2011, 29, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; van Koppen, C.J.; Danneberg, K.; Ter Braak, M.; Meyer Zu Heringdorf, D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 374, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta—Biomembr. 2006, 1758, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Su, Y.J.; Huang, J.A.; Qin, M.B.; Tang, G.D. Sphingosine kinase 1 enhances the proliferation and invasion of human colon cancer LoVo cells through up-regulating FAK pathway and the expression of ICAM-1 and VCAM-1. Zhonghua Zhong Liu Za Zhi 2013, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Goetzl, E.J.; Hla, T.; Igarashi, Y.; Lynch, K.R.; Moolenaar, W.; Pyne, S.; Tigyi, G. International Union of Pharmacology. XXXIV. Lysophospholipid Receptor Nomenclature. Pharmacol. Rev. 2002, 54, 265–269. [Google Scholar] [CrossRef]
- Maceyka, M.; Payne, S.G.; Milstien, S.; Spiegel, S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta 2002, 1585, 193–201. [Google Scholar] [CrossRef]
- Zhang, C.X.; He, H.W.; Shao, R.G. Sphingosine kinase 1 and tumor. Yao Xue Xue Bao 2013, 48, 971–978. [Google Scholar]
- Huwiler, A.; Pfeilschifter, J. Altering the sphingosine-1-phosphate/ceramide balance: A promising approach for tumor therapy. Curr. Pharm. Des. 2006, 12, 4625–4635. [Google Scholar] [CrossRef]
- Huwiler, A.; Zangemeister-Wittke, U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit. Rev. Oncol. Hematol. 2007, 63, 150–159. [Google Scholar] [CrossRef]
- Pitson, S.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Xia, P.; Vadas, M.A.; Wattenberg, B.W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003, 22, 5491–5500. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, B. Sphk1 promotes ulcerative colitis via activating JAK2/STAT3 signaling pathway. Hum. Cell 2020, 33, 57–66. [Google Scholar] [CrossRef]
- Tsai, C.F.; Hsieh, T.H.; Lee, J.N.; Hsu, C.Y.; Wang, Y.C.; Kuo, K.K.; Wu, H.L.; Chiu, C.C.; Tsai, E.M.; Kuo, P.L. Curcumin Suppresses Phthalate-Induced Metastasis and the Proportion of Cancer Stem Cell (CSC)-like Cells via the Inhibition of AhR/ERK/SK1 Signaling in Hepatocellular Carcinoma. J. Agric. Food Chem. 2015, 63, 10388–10398. [Google Scholar] [CrossRef]
- Beljanski, V.; Lewis, C.S.; Smith, C.D. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol. Ther. 2011, 11, 524–534. [Google Scholar] [CrossRef]
- Chang, H.R.; Chavoshan, B.; Park, H. Human monoclonal antibody SK1-mediated cytotoxicity against colon cancer cells. Dis. Colon Rectum 1993, 36, 1152–1157. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Li, W.; Yin, L.; Guo, S.; Xu, X.; Ouyang, Y.; Zhao, Z.; Liu, S.; Tian, Y.; et al. Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene 2016, 35, 5501–5514. [Google Scholar] [CrossRef]
- Ren, X.; Su, C. Sphingosine kinase 1 contributes to doxorubicin resistance and glycolysis in osteosarcoma. Mol. Med. Rep. 2020, 22, 2183–2190. [Google Scholar] [CrossRef]
- Yang, L.; Hu, H.; Deng, Y.; Bai, Y. Role of SPHK1 regulates multi-drug resistance of small cell lung cancer and its clinical significance. Zhongguo Fei Ai Za Zhi 2014, 17, 769–777. [Google Scholar] [CrossRef]
- Hengst, J.A.; Hegde, S.; Paulson, R.F.; Yun, J.K. Development of SKI-349, a dual-targeted inhibitor of sphingosine kinase and microtubule polymerization. Bioorg. Med. Chem. Lett. 2020, 30, 127453. [Google Scholar] [CrossRef]
- Shrestha, J.; Shamshiddinova, M.; Lee, Y.-M.; Oh, Y.S.; Baek, D.J.; Park, E.-Y. SK1 inhibitor RB005 Induces Apoptosis in Colorectal Cancer Cells through SK1 Inhibition Dependent and Independent Pathway. Curr. Mol. Pharmacol. 2021, 15, 570–581. [Google Scholar] [CrossRef]
- Shrestha, J.; Hwang, G.T.; Lee, T.; Kim, S.W.; Oh, Y.S.; Kwon, Y.; Hong, S.W.; Kim, S.; Moon, H.S.; Baek, D.J.; et al. Synthesis and Biological Evaluation of BODIPY-PF-543. Molecules 2019, 24, 4408. [Google Scholar] [CrossRef]
- Kralova, V.; Hanušová, V.; Caltová, K.; Špaček, P.; Hochmalová, M.; Skálová, L.; Rudolf, E. Flubendazole and mebendazole impair migration and epithelial to mesenchymal transition in oral cell lines. Chem. Biol. Interact. 2018, 293, 124–132. [Google Scholar] [CrossRef]
- Pinto, L.C.; Soares, B.M.; Pinheiro Jde, J.; Riggins, G.J.; Assumpção, P.P.; Burbano, R.M.; Montenegro, R.C. The anthelmintic drug mebendazole inhibits growth, migration and invasion in gastric cancer cell model. Toxicol. In Vitro 2015, 29, 2038–2044. [Google Scholar] [CrossRef]
- Hanušová, V.; Skálová, L.; Králová, V.; Matoušková, P. The Effect of Flubendazole on Adhesion and Migration in SW480 and SW620 Colon Cancer Cells. Anti-Cancer Agents Med. Chem. 2018, 18, 837–846. [Google Scholar] [CrossRef]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2018, 175, 168–180. [Google Scholar] [CrossRef]
- Bai, R.Y.; Staedtke, V.; Wanjiku, T.; Rudek, M.A.; Joshi, A.; Gallia, G.L.; Riggins, G.J. Brain Penetration and Efficacy of Different Mebendazole Polymorphs in a Mouse Brain Tumor Model. Clin. Cancer Res. 2015, 21, 3462–3470. [Google Scholar] [CrossRef]
- Williamson, T.; Mendes, T.B.; Joe, N.; Cerutti, J.M.; Riggins, G.J. Mebendazole inhibits tumor growth and prevents lung metastasis in models of advanced thyroid cancer. Endocr. Relat. Cancer 2020, 27, 123–136. [Google Scholar] [CrossRef]
- Olgen, S.; Kotra, L.P. Drug Repurposing in the Development of Anticancer Agents. Curr. Med. Chem. 2019, 26, 5410–5427. [Google Scholar] [CrossRef]
- Yuza, K.; Nakajima, M.; Nagahashi, M.; Tsuchida, J.; Hirose, Y.; Miura, K.; Tajima, Y.; Abe, M.; Sakimura, K.; Takabe, K.; et al. Different Roles of Sphingosine Kinase 1 and 2 in Pancreatic Cancer Progression. J. Surg. Res. 2018, 232, 186–194. [Google Scholar] [CrossRef]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef]
- Venkata, J.K.; An, N.; Stuart, R.; Costa, L.J.; Cai, H.; Coker, W.; Song, J.H.; Gibbs, K.; Matson, T.; Garrett-Mayer, E.; et al. Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood 2014, 124, 1915–1925. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250. [Google Scholar] [CrossRef]
- Salama, M.F.; Carroll, B.; Adada, M.; Pulkoski-Gross, M.; Hannun, Y.A.; Obeid, L.M. A novel role of sphingosine kinase-1 in the invasion and angiogenesis of VHL mutant clear cell renal cell carcinoma. FASEB J. 2015, 29, 2803–2813. [Google Scholar] [CrossRef]
- Roy-Luzarraga, M.; Reynolds, L.E. Suppression of Endothelial Cell FAK Expression Reduces Pancreatic Ductal Adenocarcinoma Metastasis after Gemcitabine Treatment. Cancer Res. 2022, 82, 1909–1925. [Google Scholar] [CrossRef]
- Song, Y.; Tang, M.Y.; Chen, W.; Wang, Z.; Wang, S.L. High JAK2 Protein Expression Predicts a Poor Prognosis in Patients with Resectable Pancreatic Ductal Adenocarcinoma. Dis. Markers 2020, 2020, 7656031. [Google Scholar] [CrossRef]
- Lee, H.; Deng, J.; Kujawski, M.; Yang, C.; Liu, Y.; Herrmann, A.; Kortylewski, M.; Horne, D.; Somlo, G.; Forman, S.; et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat. Med. 2010, 16, 1421–1428. [Google Scholar] [CrossRef]
- Fan, X.; Fu, H.; Xie, N.; Guo, H.; Fu, T.; Shan, Y. Inhibition of JAK2/STAT3 signaling pathway by panaxadiol limits the progression of pancreatic cancer. Aging (Albany N. Y.) 2021, 13, 22830–22842. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, C.-J.; Choi, J.-H.; Kim, J.-W.; Kim, J.-Y.; Nam, J.-S. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J. Exp. Clin. Cancer Res. 2019, 38, 399. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limbu, K.R.; Chhetri, R.B.; Oh, Y.S.; Baek, D.J.; Park, E.-Y. Mebendazole Impedes the Proliferation and Migration of Pancreatic Cancer Cells through SK1 Inhibition Dependent Pathway. Molecules 2022, 27, 8127. https://doi.org/10.3390/molecules27238127
Limbu KR, Chhetri RB, Oh YS, Baek DJ, Park E-Y. Mebendazole Impedes the Proliferation and Migration of Pancreatic Cancer Cells through SK1 Inhibition Dependent Pathway. Molecules. 2022; 27(23):8127. https://doi.org/10.3390/molecules27238127
Chicago/Turabian StyleLimbu, Khem Raj, Rashmi Bhandari Chhetri, Yoon Sin Oh, Dong Jae Baek, and Eun-Young Park. 2022. "Mebendazole Impedes the Proliferation and Migration of Pancreatic Cancer Cells through SK1 Inhibition Dependent Pathway" Molecules 27, no. 23: 8127. https://doi.org/10.3390/molecules27238127
APA StyleLimbu, K. R., Chhetri, R. B., Oh, Y. S., Baek, D. J., & Park, E.-Y. (2022). Mebendazole Impedes the Proliferation and Migration of Pancreatic Cancer Cells through SK1 Inhibition Dependent Pathway. Molecules, 27(23), 8127. https://doi.org/10.3390/molecules27238127





