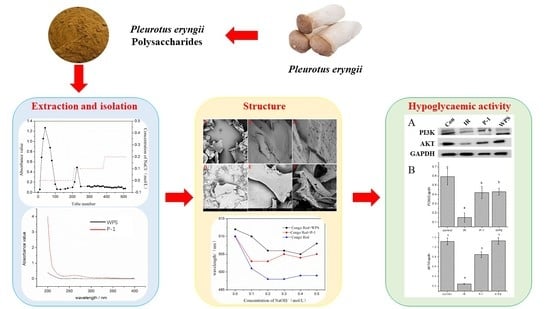
Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Regents
2.2. Extraction and Isolation of P. eryngii Polysaccharides
2.3. Physicochemical Properties of PEP
2.3.1. Total Sugar Content
2.3.2. Protein Content
2.3.3. Uronic Acid Content
2.4. Chemical Characterization of WPS and P-1
2.4.1. Ultraviolet Full-Wavelength Scan of WPS and P-1
2.4.2. FT-IR Analysis of WPS and P-1
2.4.3. 1D-NMR Analysis
2.4.4. Analysis of Monosaccharide Composition with GC
2.4.5. Determination of Molecular Weight
2.4.6. Morphological Analysis
2.4.7. Congo Red Experiment
2.5. Determination of Antioxidant
2.5.1. DPPH Free-Radical Scavenging Activity
2.5.2. Hydroxyl Radical-Scavenging Activity
2.5.3. Superoxide Anion-Scavenging Activity
2.6. Determination of Hypoglycemic Activity
2.6.1. Determination of In Vitro α-Glucosidase Inhibitory Activity
2.6.2. Cell Culture and IR-HepG2 Cell Model Construction
2.6.3. Effect of PEP on IR-HepG2 Cells
2.6.4. Western Blot
2.7. Statistical Analysis
3. Results
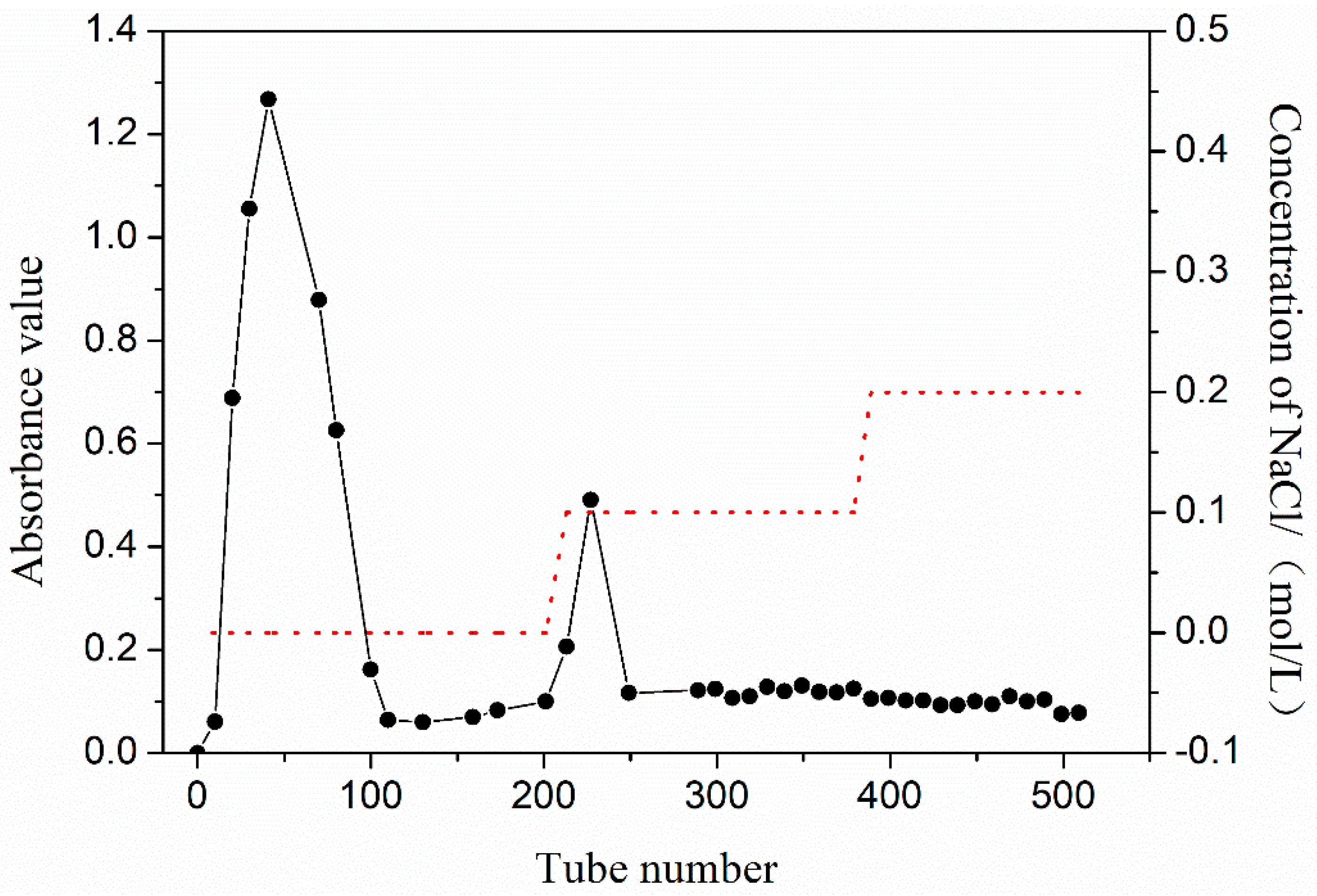
3.1. Separation and Purification of PEP
3.2. Determination of Certain Chemical Components in PEP
3.3. Ultraviolet Full-Wavelength Scanning
3.4. FT-IR Analysis
3.5. NMR Analysis of WPS and P-1
3.6. Analysis of Monosaccharide Composition by GC
3.7. Molecular Weight (Mw)
3.8. Morphological Analysis
3.9. Congo Red Experiment
3.10. Antioxidant Activity of WPS and P-1
3.11. Effects of WPS and P-1 on α-Glucosidase Activities
3.12. Effect of WPS and P-1 on IR-HepG2 Cells
3.13. Effect of WPS and P-1 on the Protein Expression of PI3K-AKT Signaling Pathway in IR-HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Sha, O.; Xu, W.; Wang, S. Hypolipidaemic and hypoglycaemic activities of polysaccharide from Pleurotus eryngii in Kunming mice. Int. J. Biol. Macromol. 2016, 93, 1206–1209. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.M.R. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohyd. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef]
- Khan, A.; Gani, A.; Masoodi, F.A.; Mushtaq, U.; Naik, A.S. Structural, rheological, antioxidant, and functional properties of β–glucan extracted from edible mushrooms Agaricus bisporus, Pleurotus ostreatus and Coprinus attrimentarius. Bioact. Carbohydr. Diet. Fibre 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem. 2016, 197, 240–249. [Google Scholar] [CrossRef]
- Zhu, K.; Nie, S.; Li, C.; Gong, D.; Xie, M. Ganoderma atrum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohyd. Polym. 2014, 103, 520–527. [Google Scholar] [CrossRef]
- Gong, P.; Cui, D.; Guo, Y.; Wang, M.; Wang, Z.; Huang, Z.; Yang, W.; Chen, F.; Chen, X. A novel polysaccharide obtained from Siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the TLR4-NF-kappa B pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Chen, F.; Cui, D.; Wang, M. Advances in the in vitro digestion and fermentation of polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 4970–4982. [Google Scholar] [CrossRef]
- Ren, D.; Wang, N.; Guo, J.; Yuan, L.; Yang, X. Chemical characterization of Pleurotus eryngii polysaccharide and its tumor-inhibitory effects against human hepatoblastoma HepG-2 cells. Carbohydr. Polym. 2016, 138, 123–133. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, J.; Tang, Q.; Liu, Y.; Zhou, S.; Yang, Y.; Zhang, Z.; Di, W.U. Physicochemical Analysis and Bioactivity Investigation of Polysaccharides Separated from Pleurotus eryngii by Ultrafiltration. J. Food Sci. Biotechnol. 2017, 36, 74–79. [Google Scholar]
- Zhang, C.; Li, J.; Wang, J.; Song, X.; Jia, L. Antihyperlipidaemic and hepatoprotective activities of acidic and enzymatic hydrolysis exopolysaccharides from Pleurotus eryngii SI-04. BMC Complementary Altern. Med. 2017, 17, 403. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Jeong, S.C.; Gu, Y.A.; Islam, R.; Song, C.H. Antitumor and Immunomodulating Activities of Endo-biopolymers Obtained from a Submerged Culture of Pleurotus eryngii. Food Sci. Biotechnol. 2010, 19, 399–404. [Google Scholar] [CrossRef]
- Xu, N.; Ren, Z.; Zhang, J.; Song, X.; Gao, Z.; Jing, H.; Li, S.; Wang, S.; Jia, L. Antioxidant and anti-hyperlipidemic effects of mycelia zinc polysaccharides by Pleurotus eryngii var. tuoliensis. Int. J. Biol. Macromol. 2017, 95, 204–214. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Taylor, K.A.C.C. A colorimetric method for the quantitation of galacturonic acid. Appl. Biochem. Biotechnol. 1993, 43, 51–54. [Google Scholar] [CrossRef]
- Zheng, C.; Dong, Q.; Chen, H.; Cong, Q.; Ding, K. Structural characterization of a polysaccharide from Chrysanthemum morifolium flowers and its antioxidant activity. Carbohyd. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Yuan, G.; Pan, Y.; Che, H. Physicochemical characterisation and α-amylase inhibitory activity of tea polysaccharides under simulated salivary, gastric and intestinal conditions. Int. J. Food Sci. Technol. 2017, 53, 423–429. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Liu, Y.; Liang, J.; Zhang, Z. Gymnemic Acid Alleviates Type 2 Diabetes Mellitus and Suppresses Endoplasmic Reticulum Stress in Vivo and in Vitro. J. Agric. Food Chem. 2019, 67, 3662–3669. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of β-pinitol on insulinresistance through the PI3K/Aktsignaling pathway in type 2 diabetes mellitus rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.; Bourne, E.; Stacey, M.; Whiffen, D. Infra-red spectra of carbohydrates. Part I. Some derivatives of D-glucopyranose. J. Chem. Soc. (Resumed) 1954, 4670, 171–176. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Zhao, M.; Liu, Y.; Wang, W.; Jiang, Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr. Res. 2006, 341, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Jia, W.; Gong, G.; Zhao, Y.; Li, G.; Zhou, J.; Li, X.; Zhao, Y.; Ma, W. Extraction, isolation, characterisation, antioxidant and anti-fatigue activities of Pleurotus eryngii polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 2492–2508. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Liu, M.; Wang, Q.; Li, Y. Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. Int. J. Biol. Macromol. 2018, 112, 326–332. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohyd. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohyd. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef]
- Nitschke, J.; Modick, H.; Busch, E.; Rekowski, R.W.V.; Altenbach, H.J.; Mölleken, H. A new colorimetric method to quantify β-1,3-1,6-glucans in comparison with total β-1,3-glucans in edible mushrooms. Food Chem. 2011, 127, 791–796. [Google Scholar] [CrossRef]
- Chen, L.; Long, R.; Huang, G.; Huang, H. Extraction and antioxidant activities in vivo of pumpkin polysaccharide. Ind. Crops Prod. 2020, 146, 112199. [Google Scholar] [CrossRef]
- Chi, M.; Ye, Y.; Zhang, X.D.; Chen, J. Insulin induces drug resistance in melanoma through activation of the PI3K/Akt pathway. Drug Des. Dev. Ther. 2014, 2, 255–262. [Google Scholar]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Wang, J.; Wang, X.; Hu, B.; Lv, F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int. J. Biol. Macromol. 2015, 81, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Wang, D.W.; Chen, Y.T.; Liu, T.T.; Zhang, S.S.; Fan, H.X.; Liu, H.C.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, L.; Qu, Y.; Qu, X.; Mu, M.; Zhang, M.; Muneer, G.; Zhou, Y.; Sun, L. Analyses of active antioxidant polysaccharides from four edible mushrooms. Int. J. Biol. Macromol. 2019, 123, 945–956. [Google Scholar] [CrossRef]
- Biscaia, S.; Carbonero, E.; Bellan, D.; Borges, B.; Costa, C.; Rossi, G.; Gonçalves, J.; Melo, C.; Lívero, F.; Ruthes, A.C. Safe therapeutics of murine melanoma model using a novel antineoplasic, the partially methylated mannogalactan from Pleurotus eryngii. Carbohyd. Polym. 2017, 178, 95–104. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohyd. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Xu, M.; Fu, L.; Sun, P.-L. Structural elucidation of a novel mannogalactan isolated from the fruiting bodies of Pleurotus geesteranus. Carbohyd. Polym. 2013, 92, 236–240. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef]
- Claus-Desbonnet, H.; Nikly, E.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S.; Pierre, G.; Benbassat, N.; Katsarov, P.; Michaud, P.; Lukova, P.; et al. Polysaccharides and Their Derivatives as Potential Antiviral Molecules. Viruses 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Rizkyana, A.D.; Ho, T.C.; Roy, V.C.; Park, J.-S.; Kiddane, A.T.; Kim, G.-D.; Chun, B.-S. Sulfation and characterization of polysaccharides from Oyster mushroom (Pleurotus ostreatus) extracted using subcritical water. J. Supercrit. Fluids 2022, 179, 105412. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated modification, characterization and bioactivities of an acidic polysaccharide fraction from an edible mushroom Pleurotus eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Investigation of chemical and biological properties of an acidic polysaccharide fraction from Pleurotus eous (Berk.) Sacc. Food Biosci. 2021, 42, 101209. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Xie, B. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J. Agric. Food Chem. 2004, 52, 3333–3336. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Wang, P.; Wang, M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol. 2020, 162, 1897–1905. [Google Scholar] [CrossRef]
- Politi, M.; Groves, P.; Chávez, M.I.; Cañada, F.J.; Jiménez-Barbero, J. Useful applications of DOSY experiments for the study of mushroom polysaccharides. Carbohydr. Res. 2006, 341, 84–89. [Google Scholar] [CrossRef]
- Qu, Y.; Yan, J.; Zhang, X.; Song, C.; Zhang, M.; Mayo, K.H.; Sun, L.; Cheng, H.; Zhou, Y. Structure and antioxidant activity of six mushroom-derived heterogalactans. Int. J. Biol. Macromol. 2022, 209, 1439–1449. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhao, M.; Qi, H. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohydr. Polym. 2014, 112, 578–582. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martinez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, Y.; Nie, Y.; Lu, X.; Sun, Y.; Yang, X. Chemical composition of Pleurotus eryngii polysaccharides and their inhibitory effects on high-fructose diet-induced insulin resistance and oxidative stress in mice. Food Funct. 2014, 5, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gutiérrez, R.; Salcido-Montenegro, A.; González-González, J.G. Early clinical expressions of insulin resistance: The real enemy to look for. Diabetes Ther. 2018, 9, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.F.; Olefsky, J.M. Defective insulin receptor function in down-regulated HepG2 cells. Endocrinology 1990, 127, 1706–1717. [Google Scholar] [CrossRef]
- Mao, Z.-J.; Lin, M.; Zhang, X.; Qin, L.-P. Combined use of astragalus polysaccharide and berberine attenuates insulin resistance in IR-HepG2 cells via regulation of the gluconeogenesis signaling pathway. Front. Pharmacol. 2019, 10, 1508. [Google Scholar] [CrossRef]
- Sogame, Y.; Kitamura, A.; Yabuki, M.; Komuro, S. Liver uptake of Biguanides in rats. Biomed. Pharmacother. 2011, 65, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohyd. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Liu, G.; Yao, J.; Zhang, Q. The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity. Int. J. Biol. Macromol. 2017, 105, 686–692. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Fan, L.; Ai, L.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohyd. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]
- Gil, A.; Smiderle, F.R.; Morales, D.; Govers, C.; Soler-Rivas, C. Water-Soluble Polysaccharide Extracts from the Oyster Culinary-Medicinal Mushroom Pleurotus ostreatus (Agaricomycetes) with HMGCR Inhibitory Activity. Int. J. Med. Mushrooms 2017, 19, 879–892. [Google Scholar]
- Ning, J.; Zhang, W.; Yi, Y.; Yang, G.; Wu, Z.; Yi, J.; Kong, F. Synthesis of β-(1→ 6)-branched β-(1→ 3) glucohexaose and its analogues containing an α-(1→ 3) linked bond with antitumor activity. Bioorganic Med. Chem. 2003, 11, 2193–2203. [Google Scholar] [CrossRef]
- Wang, C.; Cui, H.; Wang, Y.; Wang, Z.; Li, Z.; Chen, M.; Li, F. Bidirectional immunomodulatory activities of polysaccharides purified from Pleurotus nebrodensis. Inflammation 2014, 37, 83–93. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zhao, P.; Ban, X.; Zhi, D.; Li, G.; Wang, F.; Yang, X.; Huai, L. Recognization of receptors on bone marrow-derived dendritic cells bound with Pholiota nameko polysaccharides. Int. J. Biol. Macromol. 2015, 72, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-J.; Lv, Q.-Q.; Zhang, B.; Chen, H.-Q. Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem. Carbohyd. Polym. 2019, 212, 89–101. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Long, H.; Guo, Y.; Wang, S.; Chen, F.; Chen, X. Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii. Molecules 2022, 27, 7140. https://doi.org/10.3390/molecules27207140
Gong P, Long H, Guo Y, Wang S, Chen F, Chen X. Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii. Molecules. 2022; 27(20):7140. https://doi.org/10.3390/molecules27207140
Chicago/Turabian StyleGong, Pin, Hui Long, Yuxi Guo, Siyuan Wang, Fuxin Chen, and Xuefeng Chen. 2022. "Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii" Molecules 27, no. 20: 7140. https://doi.org/10.3390/molecules27207140
APA StyleGong, P., Long, H., Guo, Y., Wang, S., Chen, F., & Chen, X. (2022). Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus eryngii. Molecules, 27(20), 7140. https://doi.org/10.3390/molecules27207140