Effects of Auricularia auricula Polysaccharides on Gut Microbiota Composition in Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Results
2.1. Monosaccharide Composition and Molecular Weight of AAP
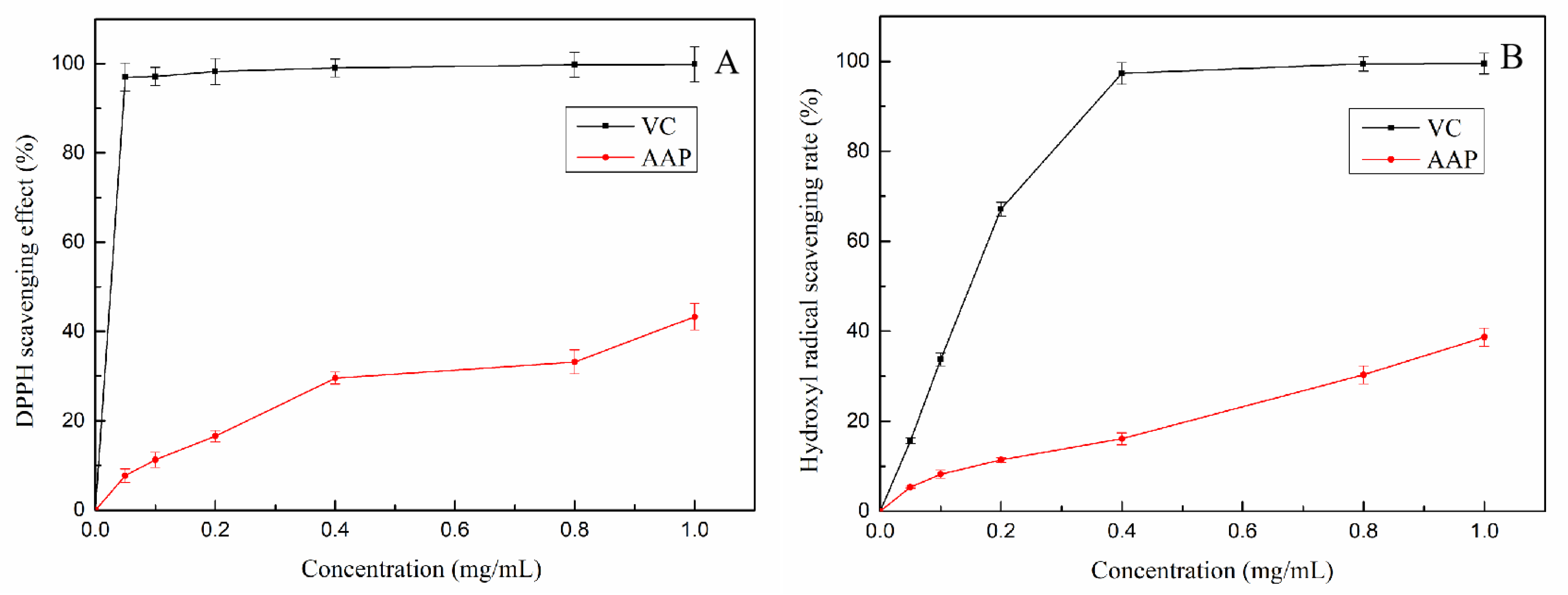
2.2. Antioxidant Activities of AAP In Vitro
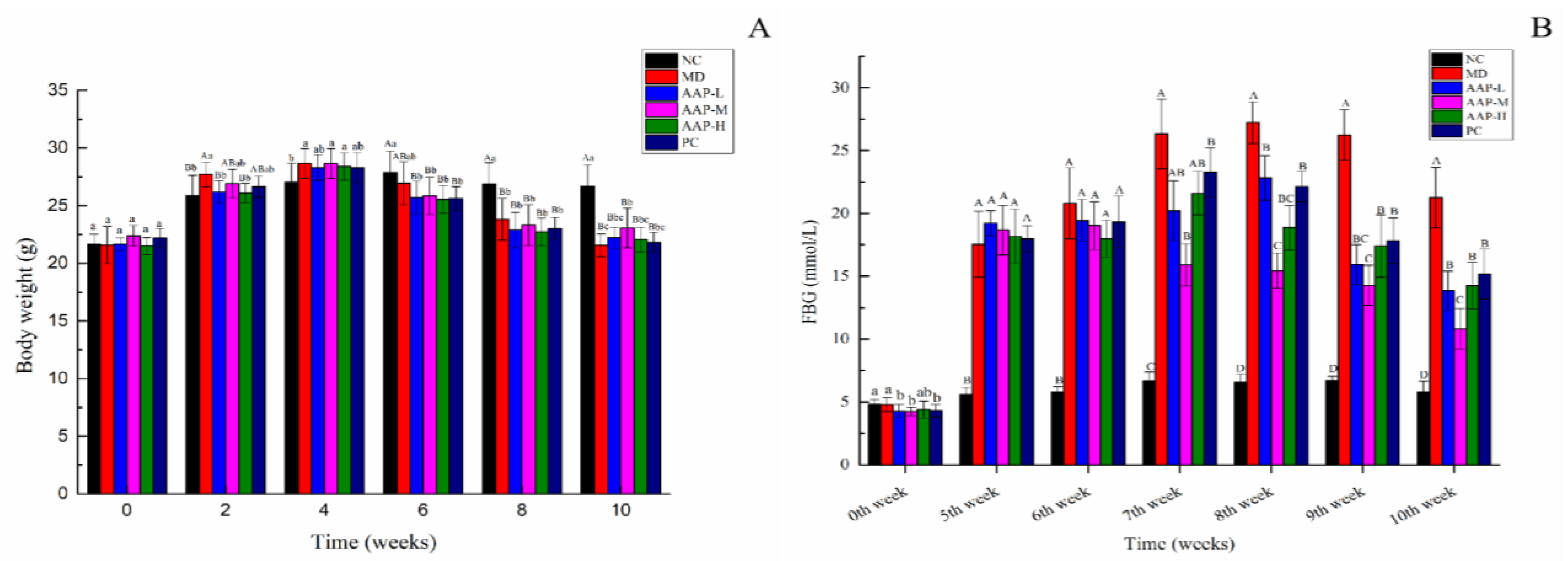
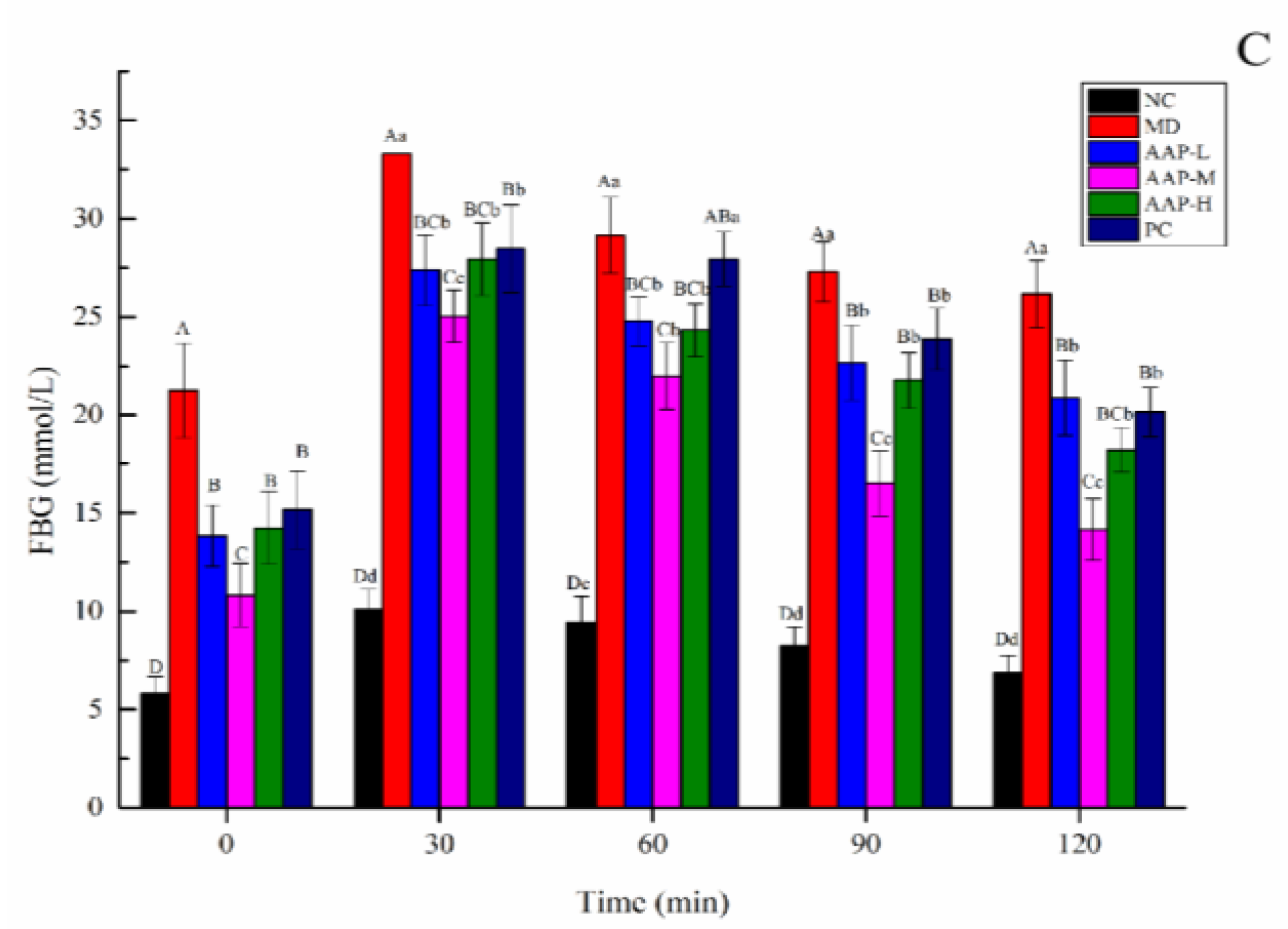
2.3. Effects of AAP on Body Weight, FBG, and OTGG of Mice
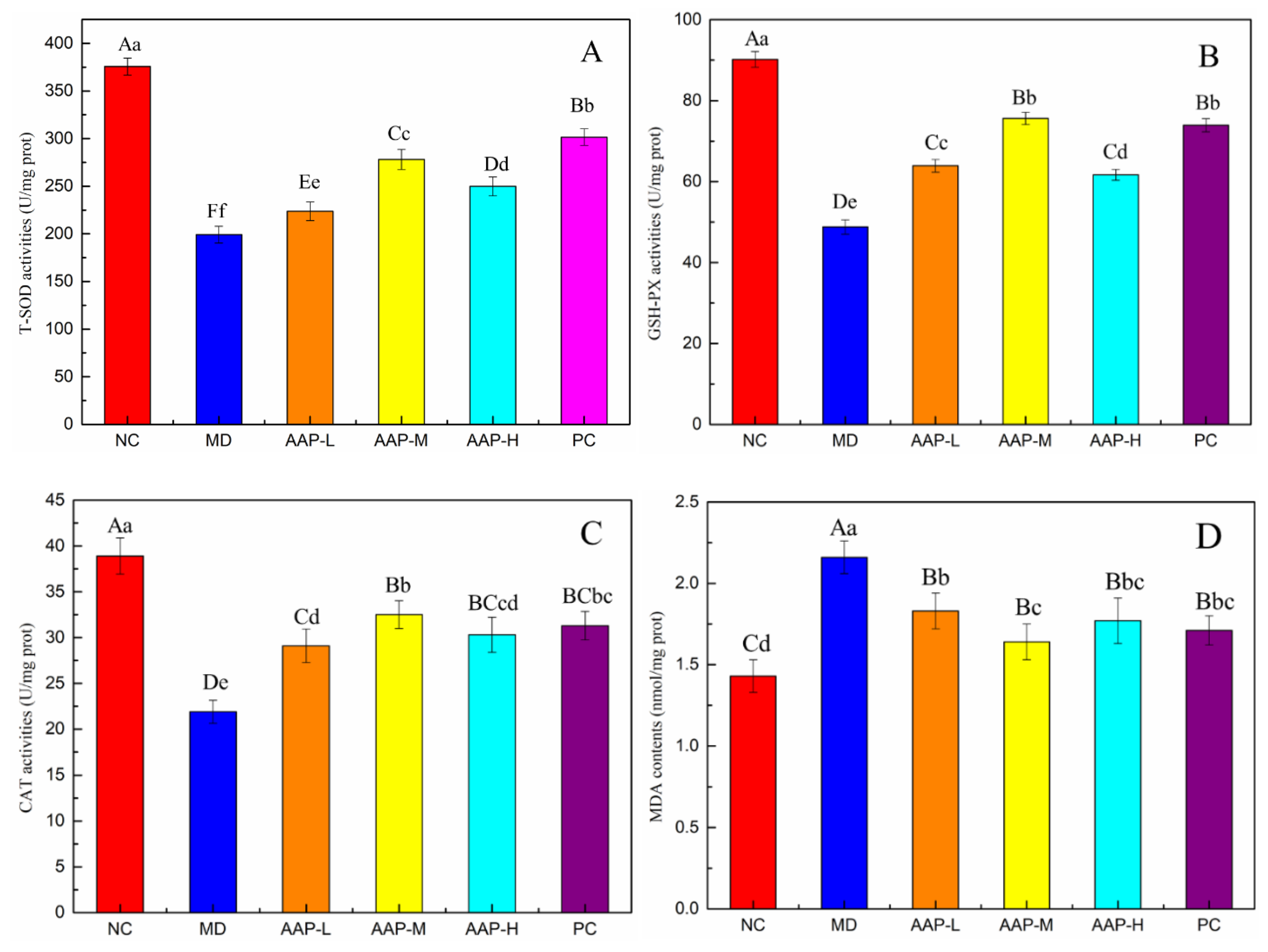
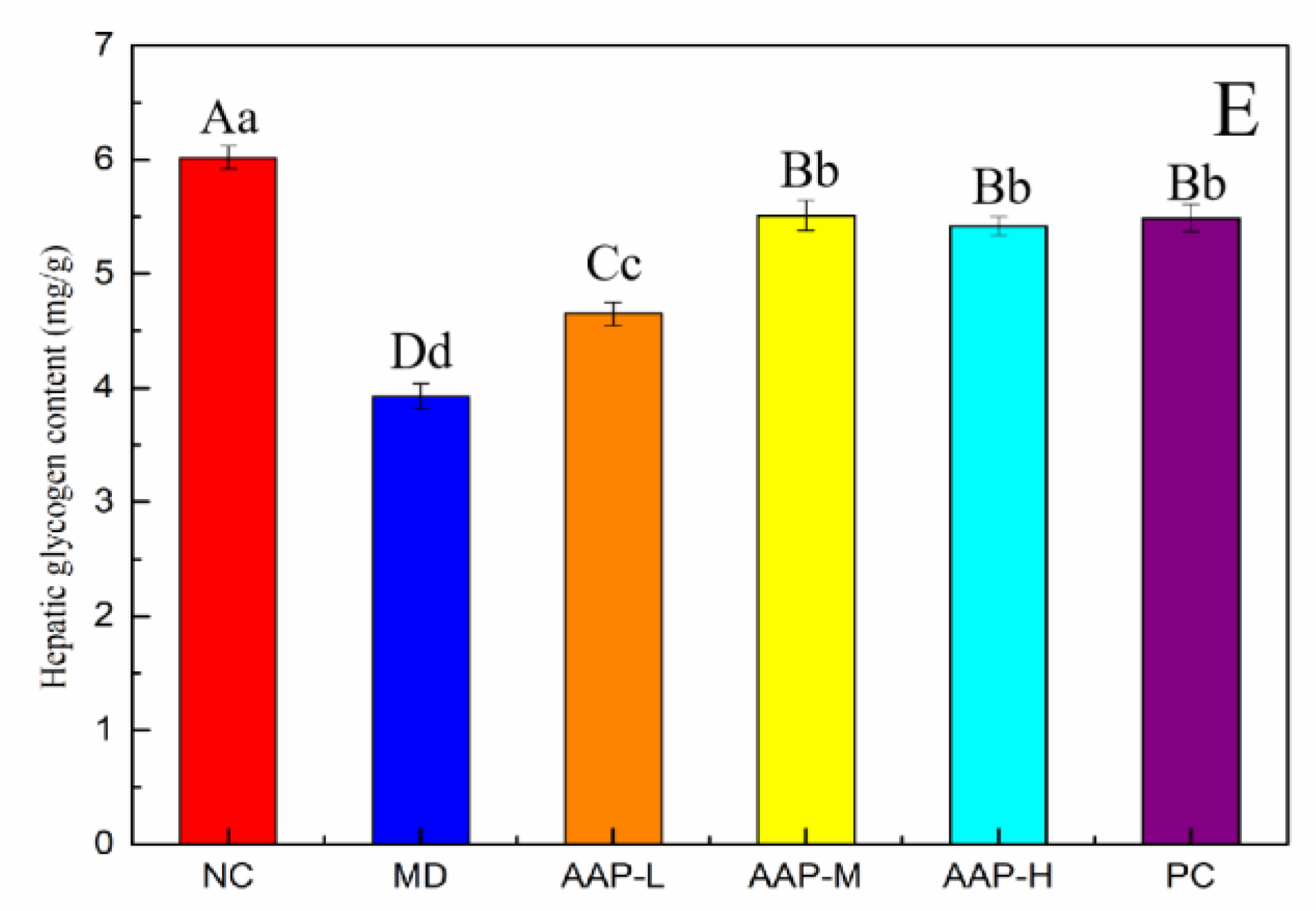
2.4. Effects of AAP on Antioxidant Enzyme Activities and Liver Glycogen in Liver Tissue
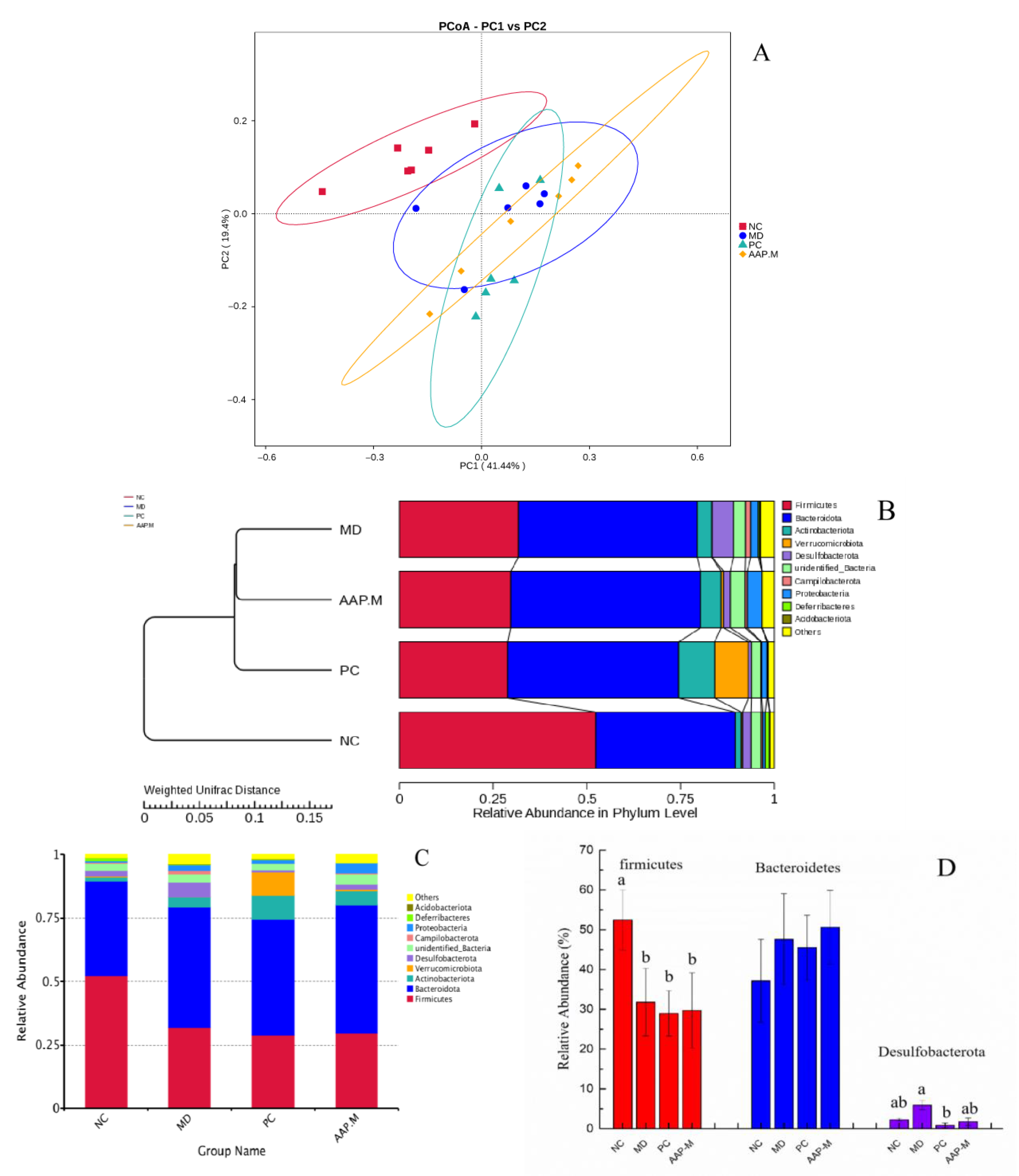
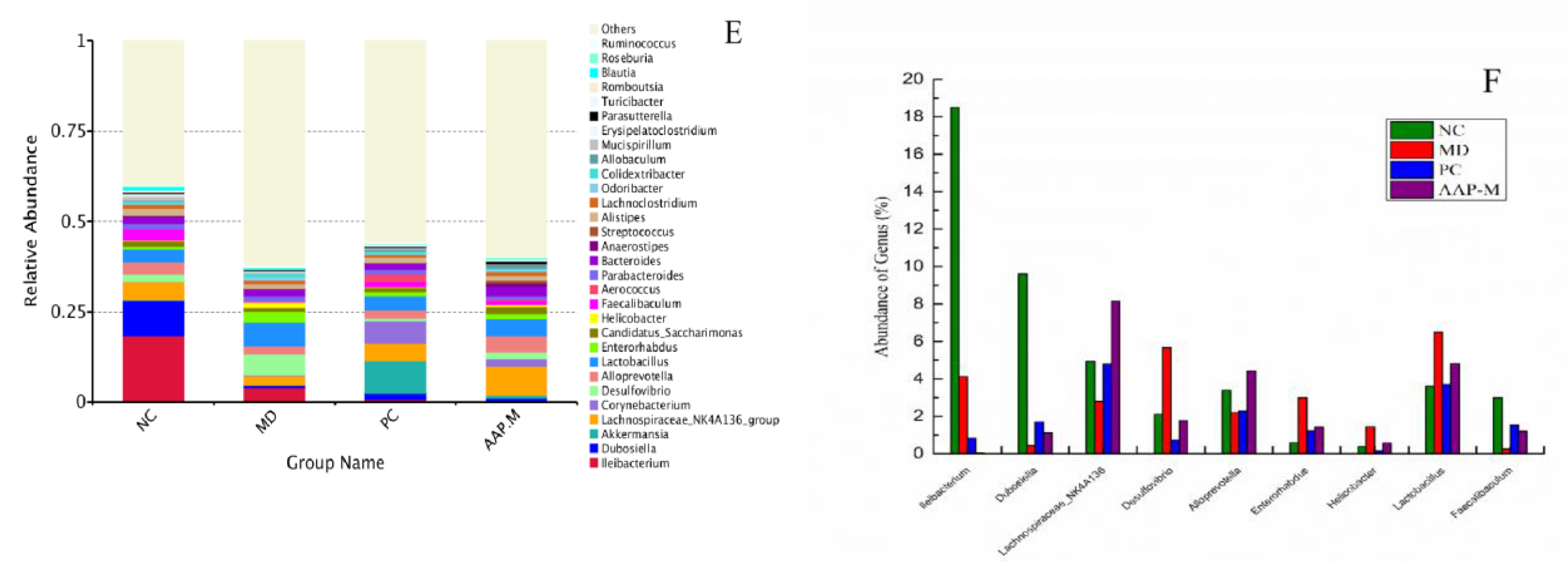
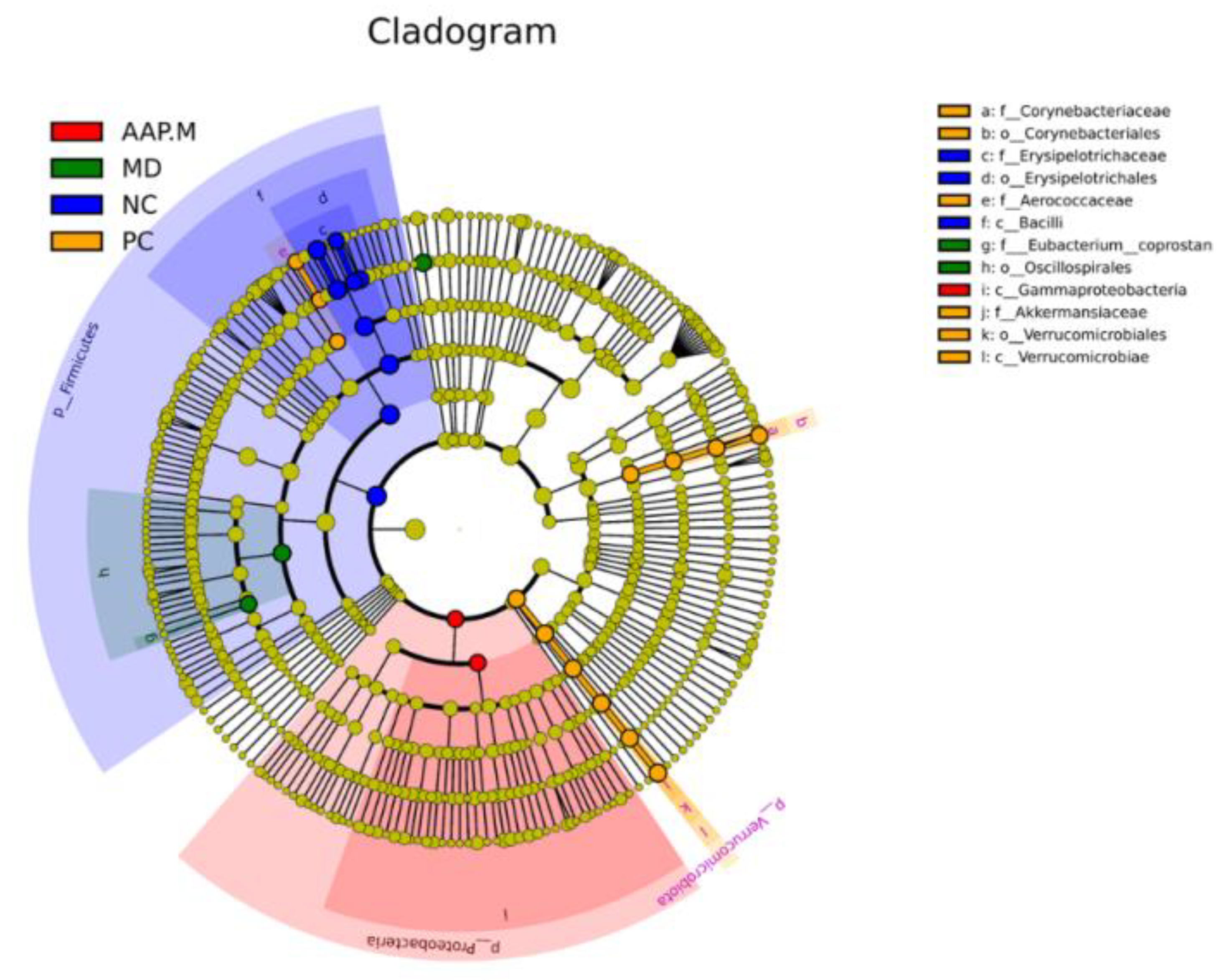
2.5. Effects of AAP on the Composition of Gut Microbiota in T2DM Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Preparation of Auricularia auricula Polysaccharide
4.3. Monosaccharide Composition and Molecular Weight of AAP
4.4. Antioxidant Activity of AAP In Vitro
4.4.1. DPPH Radical Scavenging Assay
4.4.2. Hydroxyl Radical Scavenging Ability
4.4.3. Determination of Total Reducing Capacity [49]
4.5. Animal Test
4.6. Weight, FBG, and OTGG of Mice
4.7. Measurement of Hepatic Glycogen and Anti-Oxidase Activities in Liver Tissues
4.8. Gut Microbiota Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, L.; Liu, H.; Zhang, J.; Hu, C.; Jia, L. Antioxidation, anti-hyperglycaemia and renoprotective effects of extracellular polysaccharides from Pleurotus eryngii SI-04. Int. J. Biol. Macromol. 2018, 111, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hou, X.; Li, X.; Qu, M.; Tong, C.; Li, W. Metabolomics analysis of alloxan-induced diabetes in mice using UPLC-Q-TOF-MS after Crassostrea gigas polysaccharide treatment. Int. J. Biol. Macromol. 2018, 108, 550–557. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 2015, 349, 1101–1106. [Google Scholar] [CrossRef]
- Si, J.; Lee, G.; You, H.J.; Joo, S.K.; Lee, D.H.; Ku, B.J.; Park, S.; Kim, W.; Ko, G. Gut microbiome signatures distinguish type 2 diabetes mellitus from non-alcoholic fatty liver disease. Comput. Struct. Biotechnol. J. 2021, 19, 5920–5930. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z.L. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Chai, Y.; Luo, J.; Bao, Y. Effects of Polygonatum sibiricum saponin on hyperglycemia, gut microbiota composition and metabolic profiles in type 2 diabetes mice. Biomed. Pharmacother. 2021, 143, 112155. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic mice. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a soluble polysaccharide from Auricularia polytricha and evaluation of its anti-hypercholesterolemic effect in mice. Carbohydr. Polym. 2015, 122, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, T.; Liu, J. Structural characterization and hydroxyl radicals scavenging capacity of a polysaccharide from the fruiting bodies of Auricularia polytricha. Carbohydr. Polym. 2010, 80, 377–380. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Zhang, Y.; Xing, L.; Li, S.; Wang, X. Chemical composition and antioxidant properties of five edible Hymenomycetes mushrooms. Int. J. Food Sci. Technol. 2015, 50, 465–471. [Google Scholar] [CrossRef]
- Chen, G.; Luo, Y.C.; Li, B.P.; Li, B.; Guo, Y.; Li, Y.; Su, W.; Xiao, Z.L. Effect of polysaccharide from Auricularia auricula on blood lipid metabolism and lipoprotein lipase activity of ICR mice fed a cholesterol-enriched diet. J. Food Sci. 2008, 73, H103–H108. [Google Scholar] [CrossRef]
- Wang, X.; Lan, Y.; Zhu, Y.; Li, S.; Liu, M.; Song, X.; Zhao, H.; Liu, W.; Zhang, J.; Wang, S.; et al. Hepatoprotective effects of Auricularia cornea var. Li. polysaccharides against the alcoholic liver diseases through different metabolic pathways. Sci. Rep. 2018, 8, 7574. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Song, J.; Chen, M.; Gong, P.; Jia, W.; Li, G. Hypoglycemic effects of Auricularia auricula polysaccharides on high fat diet and streptozotocin-induced diabetic mice using metabolomics analysis. Food Funct. 2021, 12, 9994–10007. [Google Scholar] [CrossRef]
- Qian, L.; Liu, H.; Li, T.; Liu, Y.; Zhang, Z.; Zhang, Y. Purification, characterization and in vitro antioxidant activity of a polysaccharide AAP-3-1 from Auricularia auricula. Int. J. Biol. Macromol. 2020, 162, 1453–1464. [Google Scholar] [CrossRef]
- Khaskheli, S.G.; Zheng, W.; Sheikh, S.A.; Khaskheli, A.A.; Liu, Y.; Soomro, A.H.; Feng, X.; Sauer, M.B.; Wang, Y.F.; Huang, W. Characterization of Auricularia auricula polysaccharides and its antioxidant properties in fresh and pickled product. Int. J. Biol. Macromol. 2015, 81, 387–395. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, Q.; Yin, J.; Yao, Y.; Zhang, J. Anti-diabetic effects of polysaccharides from Talinum triangulare in streptozotocin (STZ)-induced type 2 diabetic male mice. Int. J. Biol. Macromol. 2015, 72, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, Y.; Zhang, Z.; Yao, W.; Gao, X. Hypoglycemic, hypolipidemic and antioxidant effects of Shagan polysaccharide on type 2 diabetes mice. Food Function. 2014, 5, 2850–2860. [Google Scholar] [CrossRef]
- Wu, G.; Liu, D.; Wan, Y.; Huang, X.; Nie, S. Comparison of hypoglycemic effects of polysaccharides from four legume species. Food Hydrocoll. 2019, 90, 299–304. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Cui, C. Hypoglycemic polysaccharides from Auricularia auricula and Auricularia polytricha inhibit oxidative stress, NF-κB signaling and proinflammatory cytokine production in streptozotocin-induced diabetic mice. Food Sci. Hum. Wellness 2021, 10, 87–93. [Google Scholar] [CrossRef]
- Wang, H.; Huang, R.; Li, H.; Jiao, L.; Liu, S.; Wu, W. Serum metabolomic analysis of the anti-diabetic effect of Ginseng berry in type II diabetic mice based on ultra high-performance liquid chromatography-high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2021, 196, 113897. [Google Scholar] [CrossRef]
- Yao, Y.; Yan, L.; Chen, H.; Wu, N.; Wang, W.; Wang, D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Guo, S.; Lu, Y.Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.X.; Wang, H.Q.; Zhang, W.H.; Duan, J.A. Lycium barbarum L. leaves ameliorate type 2 diabetes in mice by modulating metabolic profiles and gut microbiota composition. Biomed Pharm. 2020, 121, 109559. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and Genomic Variation between Human-Derived Isolates of Lachnospiraceae Reveals Inter- and Intra-Species Diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Zhang, Q.; Zhang, J.; Liu, S.; Li, C.; Wang, L. Glycolipid Metabolism and Metagenomic Analysis of the Therapeutic Effect of a Phenolics-Rich Extract from Noni Fruit on Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 2876–2888. [Google Scholar] [CrossRef]
- Li, Q.; Zha, X.; Zhang, W.; Liu, J.; Pan, L.; Luo, J. Laminaria japonica polysaccharide prevents high-fat-diet-induced insulin resistance in mice via regulating gut microbiota. Food Funct. 2021, 12, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Liu, X.; Liang, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; Ai, L.; Xia, Y. Antrodin A from Antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar] [CrossRef]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T.; et al. Naringin Attenuates High Fat Diet Induced Non-alcoholic Fatty Liver Disease and Gut Bacterial Dysbiosis in Mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef]
- Ma, Y. Hypoglycemic Effect of Wuyi Rock Tea and Its Regulation on Gut Microbiota; Fujian Agriculture and Forestry University: Fuzhou, China, 2018. [Google Scholar]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Xie, B.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia mice by modulating gut microbiota. J. Funct. Foods 2020, 72, 104038. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Zhang, Y.; Li, X. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine 2019, 66, 526–537. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Zheng, H.; Joglekar, P.; Higginbottom, S.K.; Firbank, S.J.; Bolam, D.N.; Sonnenburg, J.L. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 2010, 141, 1241–1252. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, Q.; Wu, R. Structural difference of fecal flora between colon cancer mice and normal mice. World Chin. J. Gastroenterol. 2014, 22, 661–667. [Google Scholar]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y.; Zhang, S.; Feng, Y.; Wang, H.; Cheng, X.; Ma, Y.; Zhang, R.; Wang, C. Regulatory Effect of Mung Bean Peptide on Prediabetic Mice Induced by High-Fat Diet. Front. Nutr. 2022, 9, 913016. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, B.; Zheng, W.; Chen, X.; Zhang, J.; Yan, R.; Zhang, T.; Yu, L.; Dong, Y.; Ma, B. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in mice induced by streptozotocin and high-fat, high-sugar diet. Biomed Pharm. 2019, 118, 109262. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Bi, J.; Chen, Q.; Cui, H.; Bao, Y.; Tian, J.; Shu, C.; Wang, Y.; Tan, H.; Zhang, W.; et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021, 69, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Qu, L. Lactobacillus Casei Fermented Milk Remoulds Gut Microbiota Structure of Mice and Improves Type Ii Diabetes; Harbin Institute of Technology: Harbin, China, 2018. [Google Scholar]
- Huang, Y.; Wang, Z.; Ma, H.; Ji, S.; Chen, Z.; Cui, Z.; Chen, J.; Tang, S. Dysbiosis and Implication of the Gut Microbiota in Diabetic Retinopathy. Front. Cell Infect. Microbiol. 2021, 11, 646348. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Yang, Z. Carboxymethylation modification process and antioxidant activity of polysaccharides from Pleurotus eryngii. Food Ferment. Ind. 2016, 42, 119–125. [Google Scholar]
- Liu, Y.; Luo, Y.; Wei, D.-Y.; Guo, Z.-P.; Wu, Z.-Q. Effect of Solid-State Fermentation with Monascus anka on the Compositions, in Vitro Antioxidation and Amylase-inhibitory Activity of Oat Polysaccharides. Mod. Food Sci. Technol. 2019, 35, 95–101. [Google Scholar]
- Zhao, Y.; Chen, X.; Jia, W.; Gong, G.; Zhao, Y.; Li, G.; Zhou, J.; Li, X.; Zhao, Y.; Ma, W. Extraction, isolation, characterisation, antioxidant and anti-fatigue activities of Pleurotus eryngii polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 2492–2508. [Google Scholar] [CrossRef]
- Cai, W.; Ding, Z.; Wang, Y.; Yang, Y.; Zhang, H.; Yan, J. Hypoglycemic benefit and potential mechanism of a polysaccharide from Hericium erinaceus in streptozotoxin-induced diabetic mice. Process Biochem. 2020, 88, 180–188. [Google Scholar] [CrossRef]




| Animal Groups | Intervention Dose | Feed |
|---|---|---|
| Normal control (NC) | Distilled water | Standard feed |
| T2DM model (MD) | Distilled water | High-fat feed |
| AAP-low (AAP-L) | 100 mg/kg | High-fat feed |
| AAP-medium (AAP-M) | 200 mg/kg | High-fat feed |
| AAP-high (AAP-H) | 400 mg/kg | High-fat feed |
| Metformin positive control (PC) | 200 mg/kg | High-fat feed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Chen, M.; Song, J.; Zhao, Y.; Gong, P.; Chen, X. Effects of Auricularia auricula Polysaccharides on Gut Microbiota Composition in Type 2 Diabetic Mice. Molecules 2022, 27, 6061. https://doi.org/10.3390/molecules27186061
Liu N, Chen M, Song J, Zhao Y, Gong P, Chen X. Effects of Auricularia auricula Polysaccharides on Gut Microbiota Composition in Type 2 Diabetic Mice. Molecules. 2022; 27(18):6061. https://doi.org/10.3390/molecules27186061
Chicago/Turabian StyleLiu, Nannan, Mengyin Chen, Juanna Song, Yuanyuan Zhao, Pin Gong, and Xuefeng Chen. 2022. "Effects of Auricularia auricula Polysaccharides on Gut Microbiota Composition in Type 2 Diabetic Mice" Molecules 27, no. 18: 6061. https://doi.org/10.3390/molecules27186061
APA StyleLiu, N., Chen, M., Song, J., Zhao, Y., Gong, P., & Chen, X. (2022). Effects of Auricularia auricula Polysaccharides on Gut Microbiota Composition in Type 2 Diabetic Mice. Molecules, 27(18), 6061. https://doi.org/10.3390/molecules27186061





