Abstract
In this report, new, easily accessible reagents for highly Z-selective HWE reactions are presented. Alkyl di-(1,1,1,3,3,3-hexafluoroisopropyl)phosphonoacetates, structurally similar to Still–Gennari type reagents, were tested in HWE reactions with a series of various aldehydes. Very good Z-selectivity (up to a 98:2 Z:E ratio) was achieved in most cases along with high yields. Application of the new reagents may be a valuable, practical alternative to the well-established Still–Gennari or Ando Z-selective carbonyl group olefination protocols.
1. Introduction
Stereoselective alkene synthesis is one of the major challenges in organic synthesis [1]. The configuration of carbon–carbon double bonds affects all properties of molecules, therefore, highly selective methods for the synthesis of E or Z olefins are of great value. However, Z-selective reactions are considerably more difficult and less developed, mainly because of thermodynamic factors, which usually favor formation of the more stable E-products [2]. One of the well-established, typically highly E-selective alkene formation methods is the Horner–Wadsworth–Emmons (HWE) reaction, which is based on the olefination of carbonyl groups using dialkyl phosphonate reagents (Scheme 1) [3,4,5,6,7]. Its high E-selectivity results from the thermodynamic stabilization of E-products and intermediates leading to its formation. The selectivity of the HWE reaction is one of its important advantages, but in its classical form, it is restricted to the synthesis of E-alkenes. Nevertheless, the selectivity of the HWE reaction is highly dependent on the structure of the phosphonate reagents and it can be modified [8,9]. Attempts to develop Z-selective HWE reagents were made already in the late 1970s [10,11,12], but the first reliable and highly Z-selective modification of HWE reaction was reported in 1983 by Still and Gennari (Figure 1) [13]. In the standard HWE reaction, diethyl or dimethyl phosphonate reagents are usually applied. The Still–Gennari modification of the HWE reaction, fairly called “Still–Gennari olefination” due to its broad applicability and inverted selectivity, is based on the application of bis(2,2,2-trifluoroethyl) phosphonate reagents for the olefination of carbonyl compounds, usually in the presence of a strong base system—potassium bis(trimethylsilyl)amide (KHMDS) with 18-crown-6 crown ether. Along with the modification developed in the mid-1990s by Ando [14,15,16,17,18], the Still–Gennari olefination is one of the most widely applied Z-selective modifications of the HWE reaction. Its scope of applications was recently discussed in our review article [19].
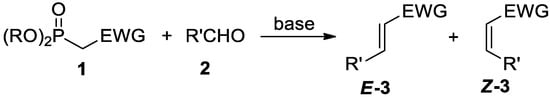
Scheme 1.
General scheme of the Horner–Wadsworth–Emmons reaction.
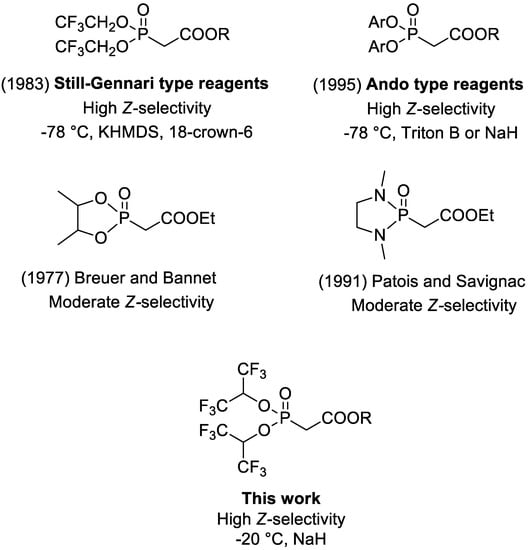
Figure 1.
Comparison of Z-selective reagents for the HWE reaction [11,12,13,14].
Still–Gennari and Ando-type reagents constitute important tools for the Z-selective alkene formation. However, examples from a total synthesis of biologically active, complex molecules show that achieving high Z-selectivity using these reagents is not always easy and the outcome of the olefination reactions is highly dependent on the reaction conditions and the type of reagent used [19]. Therefore, it would be desirable to broaden the scope of reliable Z-selective carbonyl olefination reagents in order to improve our synthetic toolbox.
The Z-selectivity of Still–Gennari olefination is a result of the kinetic control of the reaction. An electron-withdrawing effect of R groups (Scheme 1), such as 2,2,2-trifluoroethyl or phenyl, favors the Z-selective course of the reaction in contrast to standard E-selective HWE reaction where R is usually the ethyl group (pKa of 2,2,2-trifluoroethanol is 12.4 and pKa of phenol is 10, while pKa of ethanol is 16). The correlation between the electron-withdrawing effect of the R group and the stereoselectivity of the reaction was investigated in more detail by Motoyoshiya and coworkers [9]. Moreover, steric hindrance of R groups may further affect Z-selectivity as in the case of Ando-type reagents bearing aryl substituents.
In our previous study, we reported a very simple protocol for the synthesis of Still–Gennari and Ando-type phosphonates [20]. We also reported the synthesis of new phosphonate reagents bearing 1,1,1,3,3,3-hexafluoroisopropyl R groups. These compounds are expected to be highly Z-selective olefination reagents because of a stronger electron-withdrawing effect of 1,1,1,3,3,3-hexafluoroisopropyl R groups (pKa of 1,1,1,3,3,3-hexafluoroisopropanol is 9.4). In the present research, we decided to test the performance of our new reagents and evaluate their applicability on the basis of a series of model reactions with various aldehydes.
2. Results and Discussion
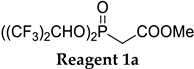
The reagents being subjects of this study, methyl, and ethyl bis(1,1,1,3,3,3-hexafluoroisopropyl) phosphonates 1a and 1b, were synthesized according to our previously reported procedure [20]. Because of a structural resemblance and similar reactivity we may consider 1a and 1b as “Still–Gennari-type” reagents. We decided to test these reagents for the synthesis of disubstituted alkenes by Z-selective HWE reaction. In order to maximize the yield and the stereoselectivity of the reaction, optimization of the reaction conditions was necessary (Table 1, Scheme 2).

Table 1.
Reaction of 1b with benzaldehyde 2a—optimization [a].
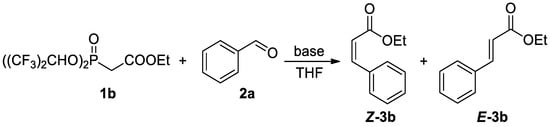
Scheme 2.
Optimization of conditions based on the reaction of 1b with benzaldehyde 2a.
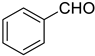
During the optimization study, several base systems were evaluated in the reaction of 1b with benzaldehyde at various temperatures (Scheme 2). All the reactions were run in THF for 1 h. When using NaH as a base at −78 °C, the reaction was very slow, only traces of Z-product could be detected after 1 h (Table 1, entry 1). This is most probably due to the slow deprotonation of the phosphonate reagent at this low temperature because when the reaction was heated from −78 °C to room temperature, hydrogen gas evolved, and the reaction proceeded. Based on this observation, higher temperatures were evaluated (Table 1, entries 2–4). Quite unexpectedly, the best results regarding yield (94%) and selectivity (97:3 Z:E) were obtained using NaH at −20 °C (Table 1, entry 3), while Still–Gennari olefination is usually conducted at lower temperatures (typically −78 °C). This result is very promising because it shows that by using our new reagents high stereoselectivity may be achieved at higher temperatures. Only a slight decrease in stereoselectivity was observed at 0 °C (Table 1, entry 4). It is noteworthy that using an excess of a base resulted in a significant decrease in the yield (Table 1, entry 5). The possibility of increasing the stereoselectivity of the reaction by providing additional sodium ions to the reaction mixture according to Pihko et al. was also investigated. However, no influence of the additive on the reaction course was observed (Table 1, entry 6) [21].
In contrast to the classic Still–Gennari olefination protocol, application of KHMDS or KHMDS with 18-crown-6 additive appears to be an inferior option (we made a similar observation in our previous work concerning the synthesis of Z-α,β-unsaturated phosphonates) [22]. The yields of the reactions were moderate (34–61%) and the selectivity was lower in comparison to the results obtained with NaH (up to a 91:9 Z:E ratio—Table 1, entries 7–10). Running the reaction at a lower temperature somewhat favored Z-selectivity, however, it decreased the yield (Table 1, entries 8 and 10). The addition of crown ether surprisingly decreased the selectivity of the reaction. Moreover, in our hands, the reaction with KHMDS tends to be a little capricious, sensitive to the reaction conditions, and difficult to reproduce, since we have previously reported better results which we were unable to repeat now.
Other bases which were tested include t-BuOK, K2CO3, triton-B (benzyltrimethylammonium hydroxide), and (CF3)2CHONa. Reaction with t-BuOK at −20 °C gave 62% yield of the product in only an 81:19 Z:E ratio (Table 1, entry 11), however, conducting the reaction at a lower temperature may improve the yield and Z-selectivity to 80% and 92:8 Z:E, as presented earlier [20]. Unfortunately, the reaction with a mild base K2CO3 was unsuccessful, and only traces of Z-product were detected (Table 1, entry 12). Interestingly, the application of triton-B (according to Ando) [14] inverted the stereoselectivity of the reaction (14:86 Z:E ratio) proving the high influence of the reaction conditions on the observed results (Table 1, entry 13). Unexpectedly, very good results were obtained using (CF3)2CHONa—93% yield and 96:4 Z:E product ratio. Based on this observation we decided to investigate the possibility of using this base with other HWE reagents (Table 2, Scheme 3).

Table 2.
HWE reaction of phosphonate reagents 1 and benzaldehyde 2a with (CF3)2CHONa as a base [a].
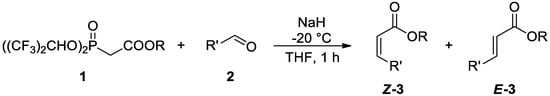
Scheme 3.
Reaction of reagents 1 with various aldehydes 2 under optimized conditions.
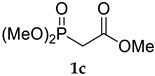
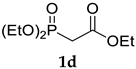
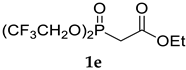
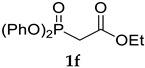
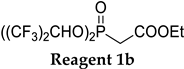
The application of (CF3)2CHONa as a base in the standard HWE reaction of methyl dimethylphosphonoacetate 1c or ethyl diethylphosphonoacetate 1d with benzaldehyde resulted in excellent E-selectivity and very good yields (Table 2, entries 1 and 2). This observation indicates that (CF3)2CHONa may be successfully used in HWE reactions. Despite excellent results with 1b and standard HWE reagents 1c and 1d, the reaction using (CF3)2CHONa with Still–Gennari and Ando-type phosphonates (1e and 1f, respectively) was only moderately stereoselective, although very high yielding (Table 2, entries 3–6). It is noteworthy that lowering the temperature to −78 °C resulted in little increased Z-selectivity compared to the reaction at −20 °C, without significant loss of yield.
The time course of the reaction of 1b with benzaldehyde in the presence of NaH at −20 °C was also investigated (Figure 2). Measurements were taken after 5, 10, 15, 30, 60, and 120 min. The reaction proceeded very fast, after 5 min the yield reached 72% and the reaction was complete within 1 h (94%).
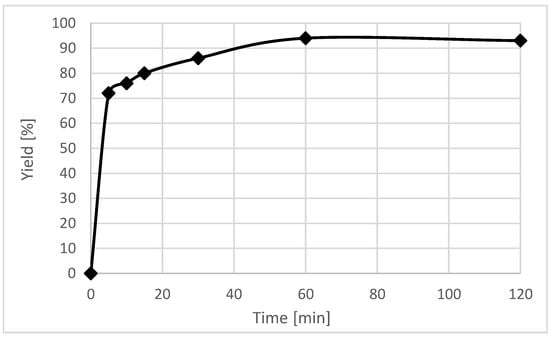
Figure 2.
Time course of the reaction of 1b and benzaldehyde with NaH at −20 °C.
Based on the above observations, all further reactions of 1a and 1b with a series of various aldehydes 2a–2m were carried out in THF at −20 °C for 1 h, using NaH as a base (Scheme 3, Table 3). Generally, similar reaction yields were observed for both reagents 1a and 1b; however, slightly better stereoselectivities were observed for ethyl bis-(1,1,1,3,3,3-hexafluoroisopropyl)phosphonoacetate 1b than for methyl bis-(1,1,1,3,3,3-hexafluoroisopropyl)phosphonoacetate 1a.

Table 3.
Reactions of 1a and 1b with aldehydes 2a–2m [a].
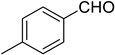
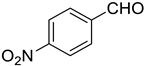
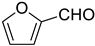
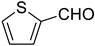
Very good results were obtained with most of the aromatic aldehydes tested (Table 3, entries 1–9). The standard reaction of 1a or 1b with benzaldehyde 2a gave very high yields of products 3aa and 3ba respectively along with excellent Z-selectivity with a 97:3 Z:E ratio. Similarly, reactions with para, meta, and ortho tolualdehydes 2b–2d resulted in high yields of products 3ab–3ad and 3bb–3bd (81–87%) and a very high Z-selectivity. Besides minimally better stereoselectivity with o-tolualdehyde 2d, no significant differences in reactivity of 2b–2d with 1a or 1b were observed. Olefination of para chloro, bromo, and nitro benzaldehydes 2e, 2f, and 2g, respectively, proceeds in a nearly quantitative manner (93–99% yield) with very high Z-selectivity as well (94:6–96:4 Z:E ratio). Olefination of heterocyclic furfural 2h and 2-thiophenecarboxaldehyde 2i resulted in slightly lower yields, however, the reactions were still highly stereoselective.
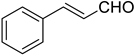
Olefination of α,β-unsaturated cinnamaldehyde 2j and aliphatic aldehydes 2k–2m using reagents 1a and 1b gave the corresponding products 3aj–3am and 3bj–3bm in good yields (69–90%) with high Z-selectivity (86:14–91:9 Z:E ratio, Table 3, entries 10–13). Similar to that reported for Ando and Still–Gennari Z-selective HWE reaction, olefination of aliphatic aldehydes resulted in a bit inferior selectivity compared to reactions with aromatic aldehydes. Nevertheless, the results obtained with our new reagents 1a and 1b are comparable with those previously reported [13,14,15,16,17,18].
In order to compare the performance of the newly developed reagent 1b under the reported conditions (using NaH in dry THF at −20 °C) with standard Still–Gennari reagent 1e, two model reactions with benzaldehyde and octanal were performed (Table 3, entries 1 and 12 in brackets). The Z:E selectivity using Still–Gennari reagent 1e with NaH at −20 °C was found to be inferior both in the olefination of aromatic and aliphatic aldehydes. The reaction of 1e with benzaldehyde resulted in a quantitative yield but only moderate Z:E selectivity 74:26, while the application of 1b resulted in an excellent 97:3 Z:E ratio. Similarly, the reaction of 1e with octanal resulted in poorer Z:E selectivity (78:22) than the reaction using reagent 1b (88:12 Z:E ratio). These observations (along with data from Table 1—entries 2–4) suggest that a very good stereochemical outcome of the reactions with 1b may be achieved using an easily accessible base, at higher temperatures than in the case of Still–Gennari reagent 1e, as typically −78 °C and KHMDS with 18-crown-6 additive is required in order to achieve high Z-selectivity in standard Still–Gennari olefination.
3. Materials and Methods
3.1. General Information
The NMR spectra were recorded using a Bruker Avance Neo 400 spectrometer. Dimethyl terephthalate was used as an internal standard in all NMR experiments [23]. All solvents were dried and distilled prior to use. All the starting materials were purchased from Merck, Sigma-Aldrich, TCI Chemicals, or Fluorochem. Reagents 1a and 1b were prepared according to the procedure reported earlier [20]. All the reactions were run in duplicate. The spectra of all the products obtained were in agreement with the data reported in the literature (see Supplementary Materials) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
3.2. General Procedure for the Reaction of 1a or 1b with Aldehydes 2a–2m
In a round bottom flask with a magnetic stirrer, under an argon atmosphere, 1.2 mmol of base (typically sodium hydride—48 mg of 60% dispersion in mineral oil) was placed and 3 mL of THF was added. The solution was cooled to −20 °C and 1.3 mmol of reagent 1a (590 mg) or 1b (608 mg) in 2 mL of dry THF was added. The reaction mixture was stirred for 15 min and 1 mmol of appropriate aldehyde in 5 mL of THF was added. After 1 h, 0.5 mL samples were collected by a syringe and quenched with saturated NH4Cl solution. The aqueous layer was extracted two times with 0.5 mL of CH2Cl2. Combined organic fractions were dried using anhydrous Na2SO4, filtered, and next condensed under reduced pressure. To a thus obtained crude product, a specific amount of dimethyl terephthalate was added (as an internal standard for the 1H-NMR measurements), and the mixture was dissolved in CDCl3 to take 1H-NMR spectra. Yields and Z:E product ratios of the reactions were calculated based on 1H-NMR with an internal standard [12].
4. Conclusions
In conclusion, we have developed a successful application of new reagents, methyl, and ethyl bis(1,1,1,3,3,3-hexafluoroisopropyl)phosphonates, 1a and 1b in a highly Z-selective HWE reaction. The reagents are easily accessible via previously reported synthetic protocol [20]. In contrast to previous Z-selective HWE reagents, the application of 1a or 1b does not require very low temperatures (−78 °C) to achieve high stereoselectivity. Moreover, readily accessible sodium hydride was found to be a very good base for the presented reaction.
Olefination of aromatic aldehydes using reagents 1a and 1b gives excellent results—up to a 98:2 Z:E product ratio, and up to quantitative yield. Slightly lower, however, very high Z-selectivity can also be achieved in the olefination of aliphatic aldehydes. The presented reagents may constitute a valuable alternative to well-established Ando and Still–Gennari-type reagents for highly Z-selective olefination of carbonyl compounds, especially in the total synthesis of complex biologically active products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27207138/s1, Section S1: Time study; Section S2: NMR spectra.
Author Contributions
Conceptualization, I.J.; methodology, I.J.; software, I.J.; validation, I.J. and P.K.; formal analysis, I.J.; investigation, I.J.; resources, I.J. and P.K.; data curation, I.J.; writing—original draft preparation, I.J.; writing—review and editing, I.J. and P.K.; visualization, I.J.; supervision, I.J. and P.K.; project administration, I.J.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences (500-02).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Negishi, E.-I.; Huang, Z.; Wang, G.; Mohan, S.; Wang, C.; Hattori, H. Recent Advances in Efficient and Selective Synthesis of Di-, Tri-, and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation−Carbonyl Olefination Synergy. Acc. Chem. Res. 2008, 41, 1474–1485. [Google Scholar] [CrossRef]
- Siau, W.-Y.; Zhang, Y.; Zhao, Y. Stereoselective Synthesis of Z-Alkenes. Top. Curr. Chem. 2012, 327, 33–58. [Google Scholar] [PubMed]
- Maryanoff, B.E.; Reitz, A.B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Bisceglia, J.Á.; Orelli, L.R. Recent applications of the Horner-Wadsworth-Emmons reaction to the synthesis of natural products. Curr. Org. Chem. 2012, 16, 2206–2230. [Google Scholar] [CrossRef]
- Bisceglia, J.Á.; Orelli, L.R. Recent progress in the Horner-Wadsworth-Emmons reaction. Curr. Org. Chem. 2015, 19, 744–775. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanaka, K., III; Kogen, H. Recent topics of the natural product synthesis by Horner–Wadsworth–Emmons reaction. Tetrahedron Lett. 2018, 59, 568–582. [Google Scholar] [CrossRef]
- Roman, D.; Sauer, M.; Beemelmanns, C. Applications of the Horner–Wadsworth–Emmons Olefination in Modern Natural Product Synthesis. Synthesis 2021, 53, 2713–2739. [Google Scholar]
- Nagaoka, H.; Kishi, Y. Further synthetic studies on rifamycin S. Tetrahedron 1981, 37, 3873–3888. [Google Scholar] [CrossRef]
- Motoyoshiya, J.; Kusaura, T.; Kokin, K.; Yokoya, S.; Takaguchi, Y.; Narita, S.; Aoyama, H. The Horner–Wadsworth–Emmons reaction of mixed phosphonoacetates and aromatic aldehydes: Geometrical selectivity and computational investigation. Tetrahedron 2001, 57, 1715–1721. [Google Scholar] [CrossRef]
- Deschamps, B.; Lampin, J.P.; Mathey, F.; Seyden-Penne, J. Stereoselectivity of wittig type olefin synthesis using 5 membered cyclic phosphine oxides or phosphonous acid dimethylamides. Tetrahedron Lett. 1977, 18, 1137–1140. [Google Scholar] [CrossRef]
- Breuer, E.; Bannet, D.M. Stereoselectivity of wittig type olefin synthesis using five-membered cyclic phosphonates. preferential formation of cis olefins. Tetrahedron Lett. 1977, 18, 1141–1144. [Google Scholar] [CrossRef]
- Patois, C.; Savignac, P. 1,3-Dimethyl 2-oxo 1,3,2-diazaphospholidine precursor of (Z) α,β-unsaturated esters. Tetrahedron Lett. 1991, 32, 1317–1320. [Google Scholar] [CrossRef]
- Still, W.C.; Gennari, C. Direct synthesis of Z-unsaturated esters. A useful modification of the horner-emmons olefination. Tetrahedron Lett. 1983, 24, 4405–4408. [Google Scholar] [CrossRef]
- Ando, K. Practical synthesis of Z-unsaturated esters by using a new Horner-Emmons reagent, ethyl diphenylphosphonoacetate. Tetrahedron Lett. 1995, 36, 4105–4108. [Google Scholar] [CrossRef]
- Ando, K. Highly Selective Synthesis of Z-Unsaturated Esters by Using New Horner−Emmons Reagents, Ethyl (Diarylphosphono)acetates. J. Org. Chem. 1997, 62, 1934–1939. [Google Scholar] [CrossRef]
- Ando, K. Z-Selective Horner−Wadsworth−Emmons Reaction of α-Substituted Ethyl (Diarylphosphono)acetates with Aldehydes. J. Org. Chem. 1998, 63, 8411–8416. [Google Scholar] [CrossRef]
- Touchard, F.P.; Capelle, N.; Mercier, M. Efficient and Scalable Protocol for the Z-Selective Synthesis of Unsaturated Esters by Horner—Wadsworth—Emmons Olefination. Adv. Synth. Catal. 2005, 347, 707–711. [Google Scholar] [CrossRef]
- Touchard, F.P. Phosphonate Modification for a Highly (Z)-Selective Synthesis of Unsaturated Esters by Horner–Wadsworth–Emmons Olefination. Eur. J. Org. Chem. 2005, 2005, 1790–1794. [Google Scholar] [CrossRef]
- Janicki, I.; Kiełbasiński, P. Still–Gennari Olefination and its Applications in Organic Synthesis. Adv. Synth. Catal. 2020, 362, 2552–2596. [Google Scholar] [CrossRef]
- Janicki, I.; Kiełbasiński, P. A Straightforward, Purification-Free Procedure for the Synthesis of Ando and Still–Gennari Type Phosphonates. Synthesis 2022, 54, 378–382. [Google Scholar] [CrossRef]
- Pihko, P.M.; Salo, T.M. Excess sodium ions improve Z selectivity in Horner–Wadsworth–Emmons olefinations with the Ando phosphonate. Tetrahedron Lett. 2003, 44, 4361–4364. [Google Scholar] [CrossRef]
- Janicki, I.; Kiełbasiński, P. Application of the Z-Selective Still–Gennari Olefination Protocol for the Synthesis of Z-α,β-Unsaturated Phosphonates. Synthesis 2018, 50, 4140–4144. [Google Scholar] [CrossRef]
- Mahajan, S.M.; Singh, I.P. Determining and reporting purity of organic molecules: Why qNMR. Magn. Reson. Chem. 2013, 51, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Akkarasamiyo, S.; Chitsomkhuan, S.; Buakaew, S.; Samec, J.S.M.; Chuawong, P.; Kuntiyong, P. Synthesis of (Z)-Cinnamate Esters by Nickel-Catalyzed Stereoinvertive Deoxygenation of trans-3-Arylglycidates. Synlett 2022, 33, 1353–1356. [Google Scholar] [CrossRef]
- Shu, P.; Xu, H.; Zhang, L.; Li, J.; Liu, H.; Luo, Y.; Yang, X.; Ju, Z.; Xu, Z. Synthesis of (Z)-Cinnamate Derivatives via Visible-Light-Driven E-to-Z Isomerization. SynOpen 2019, 3, 103–107. [Google Scholar] [CrossRef]
- Gilchrist, T.L.; Rees, C.W.; Tuddenham, D. Generation of 3-methoxy-3a-methyl-3aH-indene and study of its cycloaddition reactions. J. Chem. Soc. Perkin Trans. I 1981, 3214–3220. [Google Scholar] [CrossRef]
- Lewis, F.D.; Howard, D.K.; Oxman, J.D.; Upthagrove, A.L.; Quillen, S.L. Lewis-acid catalysis of photochemical reactions. 6. Selective isomerization of .beta.-furylacrylic and urocanic esters. J. Am. Chem. Soc. 1986, 108, 5964–5968. [Google Scholar] [CrossRef] [PubMed]
- Longwitz, L.; Spannenberg, A.; Werner, T. Phosphetane Oxides as Redox Cycling Catalysts in the Catalytic Wittig Reaction at Room Temperature. ACS Catal. 2019, 9, 9237–9244. [Google Scholar] [CrossRef]
- Schabel, T.; Plietker, B. Microwave-Accelerated Ru-Catalyzed Hydrovinylation of Alkynes and Enynes: A Straightforward Approach toward 1,3-Dienes and 1,3,5-Trienes. Chem. Eur. J. 2013, 19, 6938–6941. [Google Scholar] [CrossRef]
- Claridge, T.D.W.; Davies, S.G.; Lee, J.A.; Nicholson, R.L.; Roberts, P.M.; Russell, A.J.; Smith, A.D.; Toms, S.M. Highly (E)-Selective Wadsworth−Emmons Reactions Promoted by Methylmagnesium Bromide. Org. Lett. 2008, 10, 5437–5440. [Google Scholar] [CrossRef] [PubMed]
- Ando, K. Convenient Preparations of (Diphenylphosphono)acetic Acid Esters and the Comparison of the Z-Selectivities of Their Horner–Wadsworth–Emmons Reaction with Aldehydes Depending on the Ester Moiety. J. Org. Chem. 1999, 64, 8406–8408. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hammond, G.B.; Xu, B. Supported gold nanoparticles catalyzed cis-selective semihydrogenation of alkynes using ammonium formate as the reductant. Chem. Commun. 2016, 52, 6013–6016. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Babu, M.H.; Reddy, M.S. BF3·OEt2-mediated syn-selective Meyer–Schuster rearrangement of phenoxy propargyl alcohols for Z-β-aryl-α,β-unsaturated esters. Org. Biomol. Chem. 2016, 14, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Seifert, F.; Drikermann, D.; Steinmetzer, J.; Zi, Y.; Kupfer, S.; Vilotijevic, I. Z-Selective phosphine promoted 1,4-reduction of ynoates and propynoic amides in the presence of water. Org. Biomol. Chem. 2021, 19, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Duan, D.; Liu, Y.; Lv, J. Carbocation Lewis Acid TrBF4-Catalyzed 1,2-Hydride Migration: Approaches to (Z)-α,β-Unsaturated Esters and α-Branched β-Ketocarbonyls. Org. Lett. 2019, 21, 8013–8017. [Google Scholar] [CrossRef] [PubMed]
- Murai, Y.; Nakagawa, A.; Kojima, S. Highly syn-Selective Elimination of Peterson anti-Adducts to Give Z-α,β-Unsaturated Esters. Chem. Lett. 2017, 46, 228–231. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wei, S. Wittig Reactions of Stabilized Phosphorus Ylides with Aldehydes in Water. Synth. Commun. 2005, 35, 1213–1222. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kawaguchi, S.-I.; Nishimura, M.; Sato, Y.; Shimada, Y.; Tabuchi, A.; Nomoto, A.; Ogawa, A. Phosphorus-Recycling Wittig Reaction: Design and Facile Synthesis of a Fluorous Phosphine and Its Reusable Process in the Wittig Reaction. J. Org. Chem. 2020, 85, 14684–14696. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).