Phytochemical Analysis and Understanding the Antioxidant and Anticancer Properties of Methanol Extract from Litsea glutinosa: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Results
2.1. Phytochemical Components of LGBME
2.2. Chemical Compounds of LGBME
2.3. In Vitro Antioxidant Activity of LGBME
2.4. Cytotoxic-Activity Study of LGBME
2.5. Acute-Toxicity Study of LGBME
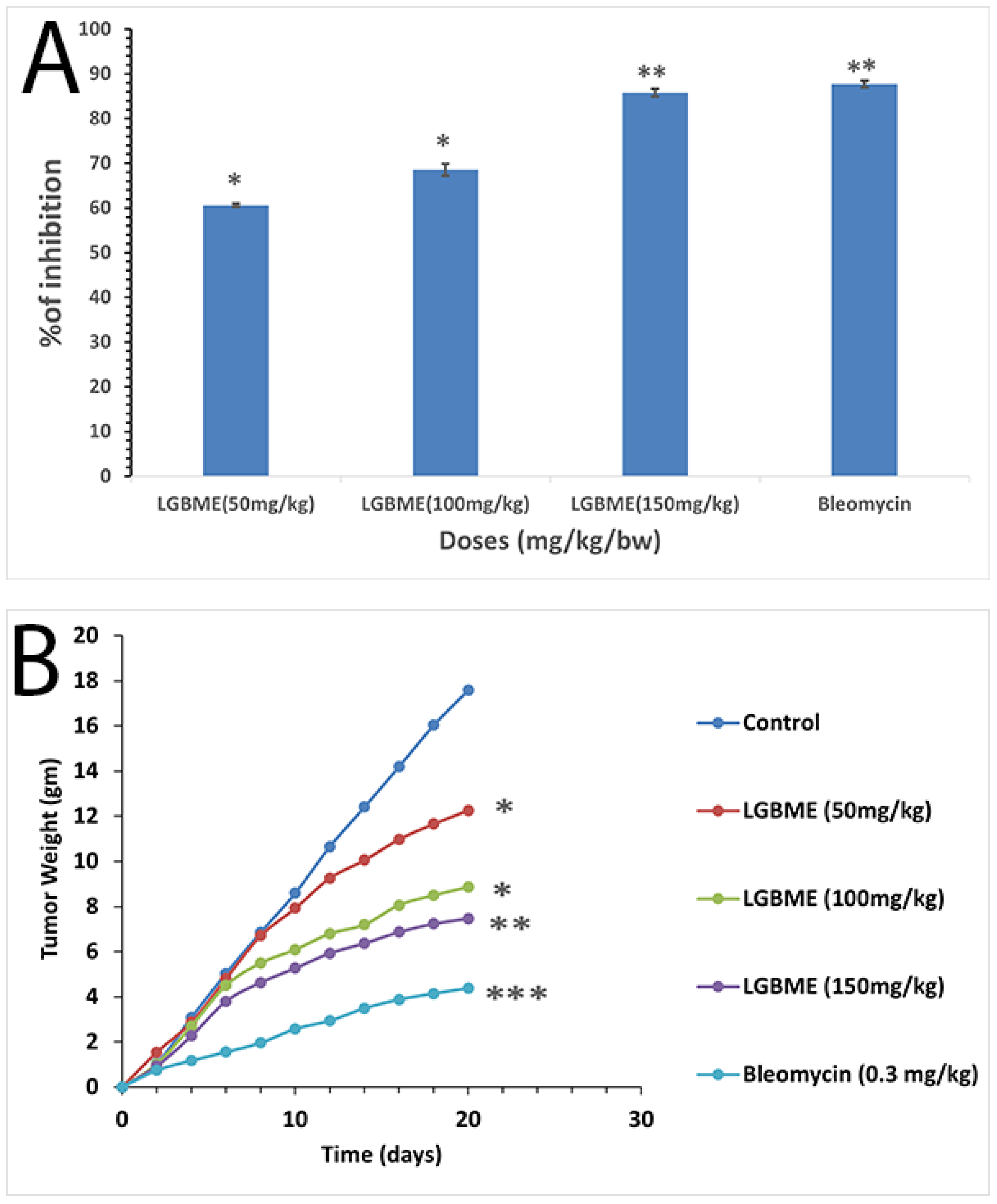
2.6. Anticancer Activity of LGBME
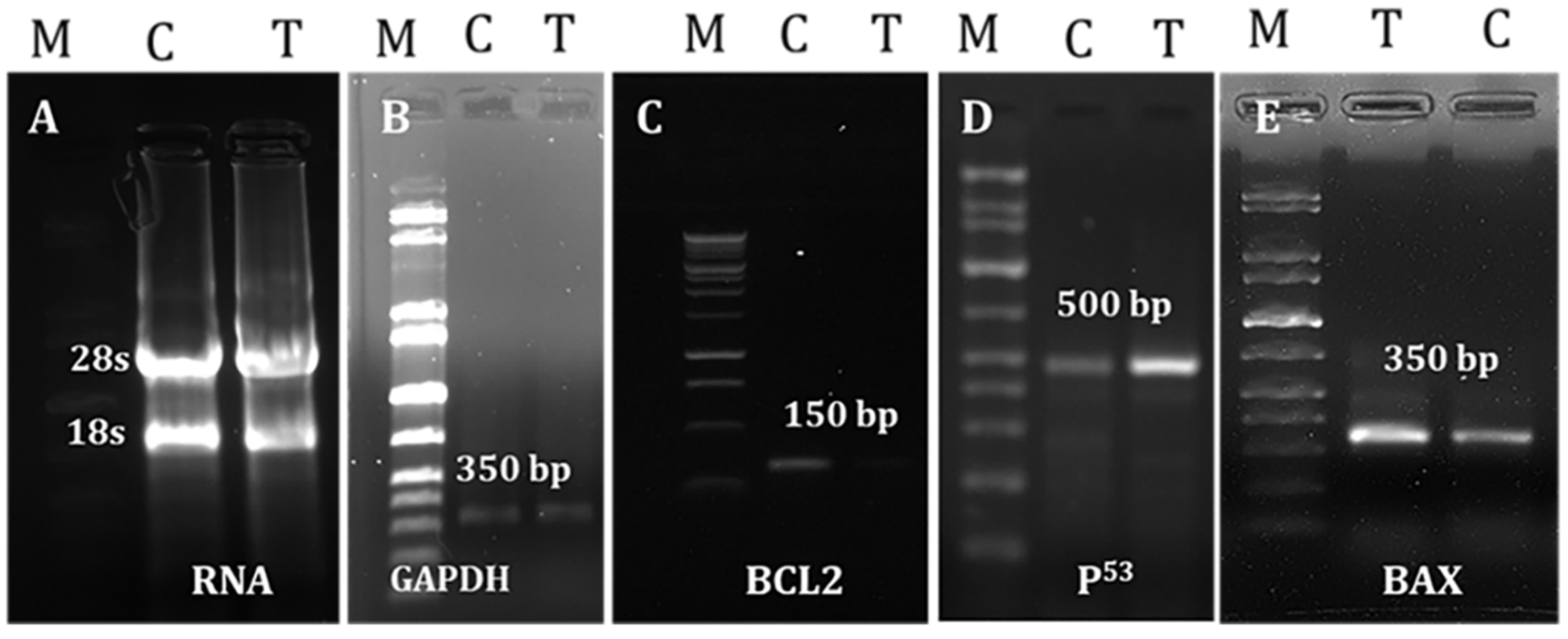
2.7. Effects of LGBME on Apoptotic Genes
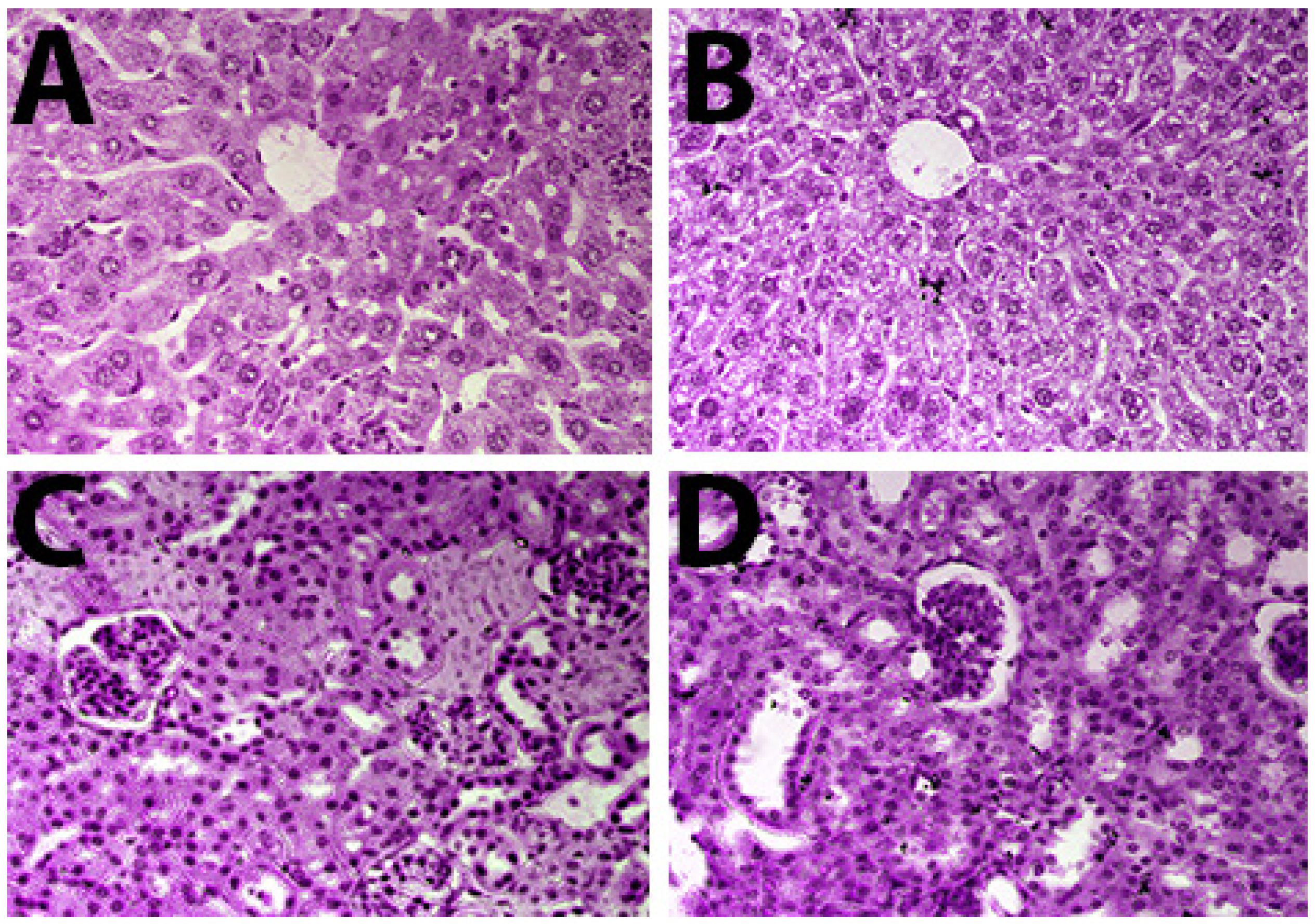
2.8. Histopathological Findings of Liver and Kidney Tissues
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Extract
4.3. Determination of Total Phenol and Flavonoid Content
4.4. Antioxidant Activity Study of LGBME
4.4.1. DPPH-Free-Radical-Scavenging-Activity Assay
4.4.2. Total-Antioxidant-Activity Assay
4.4.3. Ferric Reducing Antioxidant Power Assay
4.5. Gas-Chromatography–Mass-Spectrometry (GC–MS) Analysis of LGBME
4.6. Determination of Cytotoxic Activity
4.7. Experimental Animal and Ethical Clearance
4.7.1. Acute Oral-Toxicity Study
4.7.2. In Vivo Anticancer-Activity Study
4.7.3. Experimental Tumor Model and Preparation of Test Samples
4.7.4. Determination of EAC-Cell-Growth Inhibition
4.7.5. Determination of Hematological Parameters
4.7.6. Evaluation of Apoptotic Effectiveness of LGBME
RNA Separation and cDNA Preparation
PCR Amplification of Apoptosis-Related Genes
4.7.7. Histopathologic Evaluation
4.7.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meybodi, N.M.; Mortazavian, A.M.; Monfared, A.B.; Sohrabvandi, S.; Meybodi, F.A. Phytochemicals in cancer prevention: A review of the evidence. Iran. J. Cancer Prev. 2017, 10, e7219. [Google Scholar]
- World Health Organization. Global Health Observatory: World Health Organization. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 1 December 2020).
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The global burden of cancer: Priorities for prevention. Carcinogenesis 2010, 31, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S. Natural products and their analogues as efficient anticancer drugs. Mini Rev. Med. Chem. 2009, 9, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, T.J.; Uhrig, L.K.; Pearl, D.K.; Casto, B.C.; Warner, B.M.; Clinton, S.K.; Sardo-Molmenti, C.L.; Ferguson, J.M.; Daly, B.T.; Riedl, K. Suppression of proinflammatory and prosurvival biomarkers in oral cancer patients consuming a black raspberry phytochemical-rich trocheblack raspberries suppress biomarkers of oral carcinogenesis. Cancer Prev. Res. 2016, 9, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Mostafa-Hedeab, G.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Jeandet, P.; Saad, H.M.; Batiha, G.E.-S. A raising dawn of pentoxifylline in management of inflammatory disorders in COVID-19. Inflammopharmacology 2022, 30, 799–809. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Alorabi, M.; Cavalu, S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Mostafa-Hedeab, G.; Negm, W.A.; Youssef, A.; El-Kadem, A.H.; Saad, H.M.; Batiha, G.E.-S. Pentoxifylline and berberine mitigate diclofenac-induced acute nephrotoxicity in male rats via modulation of inflammation and oxidative stress. Biomed. Pharmacother. 2022, 152, 113225. [Google Scholar] [CrossRef]
- Saklani, A.; Kutty, S.K. Plant-derived compounds in clinical trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Huang, R.; Lu, H.; Li, F.-Y.; Yang, J.-H. A new 2′-oxygenated flavone glycoside from Litsea glutinosa (Lour.) CB Rob. Biosci. Biotechnol. Biochem. 2010, 74, 652–654. [Google Scholar] [CrossRef]
- Ghani, A. Medicinal Plants of Bangladesh: Chemical Constituents and Uses, 2nd ed.; Asiatic Society of Bangladesh: Dhaka, Bangladesh, 2003. [Google Scholar]
- Ingkachotivanich, P.; Chanbang, Y.; Chuttong, B.; Page, P.; Sommano, S. Phytochemical composition and larvicidal activity of Litsea glutinosa crude leaf extract against the virus-transmitting mosquito, Aedes aegypti (L.). Med. Plants—Int. J. Phytomed. Relat. Ind. 2017, 9, 88–94. [Google Scholar] [CrossRef]
- Kshirsagar, R.; Upadhyay, S. Free radical scavenging activity screening of medicinal plants from Tripura, Northeast India. Nat. Prod. Radiance 2009, 8, 117–122. [Google Scholar]
- Arunodaya, H.; Krishna, V.; Shashikumar, R.; Girish Kumar, K. Antibacterial and antioxidant activities of stem bark essential oil constituents of Litsea glutinosa CB Rob. Int. J. Pharm. Pharm. Sci. 2016, 8, 258–264. [Google Scholar]
- Uddin, S.N. Traditional Uses of Ethnomedicinal Plants of the Chittagong Hill Tracts; Bangladesh National Herbarium: Dhaka, Bangladesh, 2006. [Google Scholar]
- Yang, J.H.; Li, L.; Wang, Y.S.; Zhao, J.F.; Zhang, H.B.; Luo, S.D. Two new aporphine alkaloids from Litsea glutinosa. Helv. Chim. Acta 2005, 88, 2523–2526. [Google Scholar] [CrossRef]
- Ndi, C.P.; Sykes, M.J.; Claudie, D.J.; McKinnon, R.A.; Semple, S.J.; Simpson, B.S. Antiproliferative aporphine alkaloids from Litsea glutinosa and ethnopharmacological relevance to kuuku i’yu traditional medicine. Aust. J. Chem. 2015, 69, 145–151. [Google Scholar] [CrossRef]
- Thao, T.T.P.; Ninh, P.T.; Van Loc, T.; Thanh, N.T.; Van Sung, T. Chemical Constituents, Cytotoxic and α-Glucosidase Inhibitory Activity of a Compound Isolated from Litsea glutinosa. Chem. Nat. Compd. 2019, 55, 186–188. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.-T.; Luo, Y.-P.; Zhao, J.-F.; Yang, X.-D.; Zhang, H.-B. Novel cytotoxic chalcones from Litsea rubescens and Litsea pedunculata. Bioorg. Med. Chem. Lett. 2011, 21, 7431–7433. [Google Scholar] [CrossRef]
- Khalipha, A.B.R.; Hossain, M.S.; Prottay, A.A.S.; Sakib, M.R. Mini-review of Litsea glutinosa and molecular docking studies of boldine (derived from Litsea glutinosa) and its derivatives for anticancer activity against TNFRSF6B. Int. J. Evergr. Sci. Res. 2019, 3, 1–20. [Google Scholar]
- Siddika, A.; Das, P.K.; Asha, S.Y.; Aktar, S.; Tareq, A.R.; Rakib, A.; Islam, F.; Khanam, J.A. Antiproliferative activity and apoptotic efficiency of Syzygium cumini bark methanolic extract against EAC Cells in vivo. Anti-Cancer Agents Med. Chem. 2021, 21, 782–792. [Google Scholar] [CrossRef]
- Thu, H.E.; Hussain, Z.; Mohamed, I.N.; Shuid, A.N. Eurycoma longifolia, a potential phytomedicine for the treatment of cancer: Evidence of p53-mediated apoptosis in cancerous cells. Curr. Drug Targets 2018, 19, 1109–1126. [Google Scholar] [CrossRef]
- Miah, M.; Shimu, A.S.; Mahmud, S.; Omar, F.-B.; Khatun, R.; Mohanto, S.C.; Hoque, K.M.; Reza, M. Methanolic Bark extract of Abroma augusta (L.) induces apoptosis in EAC cells through altered expression of apoptosis regulatory genes. Evid. Based Complement. Altern. Med. 2020, 2020, 9145626. [Google Scholar] [CrossRef]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar] [PubMed]
- Hadi, S.; Asad, S.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin–Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Al-Thomali, A.W.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Buhadiliy, A.K.; De Waard, M.; Sabatier, J.-M.; Khan Khalil, A.A.; Saad, H.M.; Batiha, G.E.-S. Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke. Biomedicines 2022, 10, 2032. [Google Scholar] [CrossRef]
- Dozio, E.; Ruscica, M.; Passafaro, L.; Dogliotti, G.; Steffani, L.; Pagani, A.; Demartini, G.; Esposti, D.; Fraschini, F.; Magni, P. The natural antioxidant alpha-lipoic acid induces p27Kip1-dependent cell cycle arrest and apoptosis in MCF-7 human breast cancer cells. Eur. J. Pharmacol. 2010, 641, 29–34. [Google Scholar] [CrossRef]
- Aihara, J.-i. Correlation found between the HOMO–LUMO energy separation and the chemical reactivity at the most reactive site for isolated-pentagon isomers of fullerenes. Phys. Chem. Chem. Phys. 2000, 2, 3121–3125. [Google Scholar] [CrossRef]
- Kalsoom, R.; Haider, M.; Chohan, C. Phytochemical analysis and antifungal activity of some medicinal plants against Alternaria specie isolated from onion. J. Anim. Plant. Sci. 2020, 30, 454–460. [Google Scholar]
- Belakhdar, G.; Benjouad, A.; Abdennebi, E. Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci. 2015, 6, 2778–2783. [Google Scholar]
- Enema, O.; Adesina, S.; Umoh, U.; Eseyin, O. Gas chromatography-mass spectroscopy (GC-MS) studies of fixed oil of leaf of Tetrapleura tetraptera Taub.(Mimosaceae). J. Pharmacogn. Phytochem. 2019, 8, 1237–1241. [Google Scholar]
- Sushma, V.; Pal, S.M.; Viney, C. GC-MS Analysis of Phytocomponents in the Various Extracts of Shorea robusta Gaertn F. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 783–788. [Google Scholar] [CrossRef][Green Version]
- Hui, L.-M.; Zhao, G.-D.; Zhao, J.-J. δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. Int. J. Clin. Exp. Pathol. 2015, 8, 6046–6056. [Google Scholar] [PubMed]
- Tonapa, Z.G. Toxicity assay of Baccaurea motleyana mull. arg. wood extracts (rambai) and chemical compounds evaluation for the most active fraction. Res. J. Pharm. Technol. 2020, 13, 5215–5218. [Google Scholar]
- Debnath, S.; Karan, S.; Debnath, M.; Dash, J.; Chatterjee, T.K. Poly-L-Lysine Inhibits Tumor Angiogenesis and Induces Apoptosis in Ehrlich Ascites Carcinoma and in Sarcoma S-180 Tumor. Asian Pac. J. Cancer Prev. 2017, 18, 2255–2268. [Google Scholar] [CrossRef]
- Wyllie, A.H. Apoptosis: An overview. Br. Med. Bull. 1997, 53, 451–465. [Google Scholar] [CrossRef]
- Aydos, O.S.; Avci, A.; Özkan, T.; Karadağ, A.; Gürleyik, E.; Altinok, B.; Sunguroğlu, A. Antiproliferative, apoptotic and antioxidant activities of wheatgrass (Triticum aestivum L.) extract on CML (K562) cell line. Turk. J. Med. Sci. 2011, 41, 657–663. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Al-Gareeb, A.I.; Saad, H.M.; Al-Kuraishy, H.M. COVID-19 and corticosteroids: A narrative review. Inflammopharmacology 2022, 30, 1189–1205. [Google Scholar] [CrossRef]
- Zazali, K.E.; Abdullah, H.; Jamil, N.I.N. Methanol extract of Oroxylum indicum leaves induces G1/S cell cycle arrest in HeLa cells via p53-mediated pathway. Int. J. Med. Plant Res. 2013, 2, 225–237. [Google Scholar]
- Babalghith, A.O.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; De Waard, M.; Sabatier, J.-M.; Saad, H.M.; Batiha, G.E.-S. The Potential Role of Growth Differentiation Factor 15 in COVID-19: A Corollary Subjective Effect or Not? Diagnostics 2022, 12, 2051. [Google Scholar] [CrossRef]
- Xiang, J.; Chao, D.T.; Korsmeyer, S.J. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 1996, 93, 14559–14563. [Google Scholar] [CrossRef]
- Yang, E.; Korsmeyer, S.J. Molecular thanatopsis: A discourse on the BCL2 family and cell death. Blood 1996, 88, 386–401. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jung, W.-K.; Jeong, M.H.; Yoon, T.R.; Kim, H.K. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int. J. Toxicol. 2012, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Zhou, H.; Xia, J.; Jin, H.; Wang, K.; Yan, J.; Zuo, J.; Zhu, X.; Shan, T. Ziyuglycoside II-induced apoptosis in human gastric carcinoma BGC-823 cells by regulating Bax/Bcl-2 expression and activating caspase-3 pathway. Braz. J. Med. Biol. Res. 2013, 46, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Al Bashera, M.; Mosaddik, A.; El-Saber Batiha, G.; Alqarni, M.; Islam, M.A.; Zouganelis, G.D.; Alexiou, A.; Zahan, R. In Vivo and In Vitro Evaluation of Preventive Activity of Inflammation and Free Radical Scavenging Potential of Plant Extracts from Oldenlandia corymbosa L. Appl. Sci. 2021, 11, 9073. [Google Scholar] [CrossRef]
- Sabiha, S.; Ali, H.; Hasan, K.; Rahman, A.; Islam, N. Bioactive potentials of Melia azedarach L. with special reference to insecticidal, larvicidal and insect repellent activities. J. Entomol. Zool. 2017, 5, 1799–1802. [Google Scholar]
- Zahan, R.; Ahmed, S.; Sharmin, T.; Halim, M.A.; Rahi, M.S.; Sheikh, M.C.; Miyatake, R.; Zangrando, E.; Naz, T.; Islam, M.A.A.A.A.; et al. Synthesis of bis [benzyl-N′-hydrazinecarbodithioato-κ2 N′, S] nickel (II) complex as a novel lead molecule for cancer treatmennt. Appl. Organomet. Chem. 2021, 35, e6036. [Google Scholar] [CrossRef]
- Zahan, R.; Rahi, M.S.; Sheikh, M.C.; Miyatake, R.; Zangrando, E.; Naz, T.; Islam, M.A.A.A.A.; Abu Reza, M. Design, synthesis and X-ray structural studies of novel [acetonitrile-benzyl-3-N-(2,4 dihydroxyphenylmethylene) hydrazinecarbodithioato-κ3-N′, S, O] nickel(II) complex that potently inhibit cell proliferation through regulation of apoptosis related genes. Appl. Organomet. Chem. 2018, 33, e4601. [Google Scholar] [CrossRef]
- Islam, F.; Khanam, J.A.; Khatun, M.; Zuberi, N.; Khatun, L.; Kabir, S.R.; Reza, M.A.; Ali, M.; Rabbi, M.A.; Gopalan, V.; et al. A p-Menth-1-ene-4,7-diol (EC-1) from Eucalyptus camaldulensis Dhnh. Triggers Apoptosis and Cell Cycle Changes in Ehrlich Ascites Carcinoma Cells. Phytother. Res. 2015, 29, 573. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]


| Sample | Total Phenolic Content | Total Flavonoid Content |
|---|---|---|
| LGBME | 126.34 ± 0.79 a | 297.93 ± 4.304 b |
| SN | Phytoconstituents | RT (min) | Area | Height | Peak Area (%) |
|---|---|---|---|---|---|
| 1. | p-Cresol | 5.362 | 17,591 | 4248 | 9.330 |
| 2. | Bicyclo (7.2.0)undec-4-ene, 4,11,11- trimethyl-8-methylene-,(1R-(1R*,4Z,9S*))- | 14.013 | 2967 | 790 | 1.573 |
| 3. | δ-Cadinene or Naphthalene, 1,2,3,5,6,8a- hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 16.522 | 3208 | 920 | 1.701 |
| 4. | Methyl tetradecanoate | 23.018 | 12,181 | 3890 | 6.460 |
| 5. | Hexadecanoic acid, methyl ester | 28.187 | 66,349 | 23,598 | 35.192 |
| 6. | 9,12-Octadecadienoic acid, methyl ester, (E,E)- | 31.761 | 27,755 | 9934 | 14.721 |
| 7. | Oleic acid methyl ester or 9-Octadecenoic acid (Z)-, methyl ester | 31.906 | 15,498 | 4490 | 8.220 |
| 8. | 15-Octadecenoic acid, methyl ester | 32.023 | 689 | 313 | 0.365 |
| 9. | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 32.100 | 1693 | 585 | 0.897 |
| 10. | Methyl stearate | 32.462 | 4960 | 1855 | 2.630 |
| 11. | Caryophyllene | 33.523 | 2200 | 715 | 1.166 |
| 12. | Di-n-octyl phthalate | 39.616 | 3119 | 1127 | 1.654 |
| 13. | Reticuline, 6’-methyl | 41.660 | 6002 | 1990 | 3.183 |
| 14. | 22,23-Dibromostigmasterol acetate | 42.243 | 3277 | 665 | 1.738 |
| 15. | benzenemethanol,3-(ethyl(2-hydroxyethyl)amino)-.alpha.-methyl- | 43.364 | 1725 | 456 | 0.914 |
| 16. | 13-Docosenamide, (Z)- | 43.436 | 2059 | 679 | 1.092 |
| 17. | 7-Isoquinolinol, 1,2,3,4-tetrahydro-1-[(3-hydroxy-4-methoxyphenyl)methyl]-6-methoxy-2-methyl-, (S)- | 43.497 | 7145 | 2472 | 3.789 |
| 18. | 6,9,10-Trimethoxy-12H-benz(6,7)oxepino(2,3,4-i,j)isoquinoline | 45.468 | 1925 | 632 | 1.021 |
| 19. | Cyclotrisiloxane, hexaethyl- | 46.024 | 8190 | 2520 | 4.344 |
| Test Sample | LD50 (μg/mL) | 95% Confidence Limits (μg/mL) | Regression Equation | χ2 Value (Degrees of Freedom-df) |
|---|---|---|---|---|
| Gallic acid | 7.23 ± 0.44 | 4.05 to 13.02 | y =3.117 + 2.213x | 0.049 (1) |
| LGBME | 24.93 ± 1.04 | 16.95 to 36.66 | y = 2.237 + 1.977x | 0.325 (1) |
| Name of the Group | MST (in Days) | % of ILS |
|---|---|---|
| Control (EAC-cell-bearing mice) | 21.67 ± 0.58 | - |
| EAC + Bleomycin (0.3 mg/kg.bw/day) | 40.0 ± 2.08 *** | 82.08 |
| EAC + LGBME (50 mg/kg. bw/day) | 29.67 ± 2.65 * | 36.92 |
| EAC + LGBME (100 mg/kg.bw/day) | 36.33 ± 1.53 * | 67.65 |
| EAC + LGBME (150 mg/kg bw/day | 37.5 ± 1.29 * | 73.05 |
| Name of the Group | Hb (gm/dL) | RBC (Cells/mL) | WBC (Cells/mL) |
|---|---|---|---|
| Normal mice | 14.13 ± 0.72 | (7.03 ± 0.11) × 109 | (11.6 ± 0.36) × 106 |
| Control (EAC-cell-bearing mice) | 6.67 ± 0.55 | (4.32 ± 0.21) × 109 | (26.8 ± 0.72) × 106 |
| EAC + Bleomycin (0.3 mg/kg, bw) | 13.20± 0.94 ** | (6.30± 0.26) × 109 ** | (14.8 ± 0.95) × 106 ** |
| EAC + LGBME (50 mg/kg, bw) | 9.73 ± 0.56 * | (5.39 ± 0.14) × 109 * | (21.0 ± 1.00) × 106 * |
| EAC + LGBME (100 mg/kg, bw) | 11.83 ± 0.68 ** | (6.05 ± 0.19) × 109 ** | (15.8 ± 0.92) × 106 ** |
| EAC + LGBME +(150 mg/kg, bw) | 12.68 ± 0.39 ** | (6.21 ± 0.45) × 109 * | (14.7 ± 0.46) × 106 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiq, S.; Zahan, R.; Yesmin, S.; Khan, A.; Mahmud, M.S.; Reza, M.A.; Albogami, S.M.; Alorabi, M.; De Waard, M.; Saad, H.M.; et al. Phytochemical Analysis and Understanding the Antioxidant and Anticancer Properties of Methanol Extract from Litsea glutinosa: In Vitro and In Vivo Studies. Molecules 2022, 27, 6964. https://doi.org/10.3390/molecules27206964
Shafiq S, Zahan R, Yesmin S, Khan A, Mahmud MS, Reza MA, Albogami SM, Alorabi M, De Waard M, Saad HM, et al. Phytochemical Analysis and Understanding the Antioxidant and Anticancer Properties of Methanol Extract from Litsea glutinosa: In Vitro and In Vivo Studies. Molecules. 2022; 27(20):6964. https://doi.org/10.3390/molecules27206964
Chicago/Turabian StyleShafiq, Shafia, Ronok Zahan, Samina Yesmin, Alam Khan, Md. Sabbir Mahmud, Md Abu Reza, Sarah M. Albogami, Mohammed Alorabi, Michel De Waard, Hebatallah M. Saad, and et al. 2022. "Phytochemical Analysis and Understanding the Antioxidant and Anticancer Properties of Methanol Extract from Litsea glutinosa: In Vitro and In Vivo Studies" Molecules 27, no. 20: 6964. https://doi.org/10.3390/molecules27206964
APA StyleShafiq, S., Zahan, R., Yesmin, S., Khan, A., Mahmud, M. S., Reza, M. A., Albogami, S. M., Alorabi, M., De Waard, M., Saad, H. M., Sabatier, J.-M., Naz, T., & Batiha, G. E.-S. (2022). Phytochemical Analysis and Understanding the Antioxidant and Anticancer Properties of Methanol Extract from Litsea glutinosa: In Vitro and In Vivo Studies. Molecules, 27(20), 6964. https://doi.org/10.3390/molecules27206964









