Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L
Abstract
1. Introduction
2. Results and Discussion
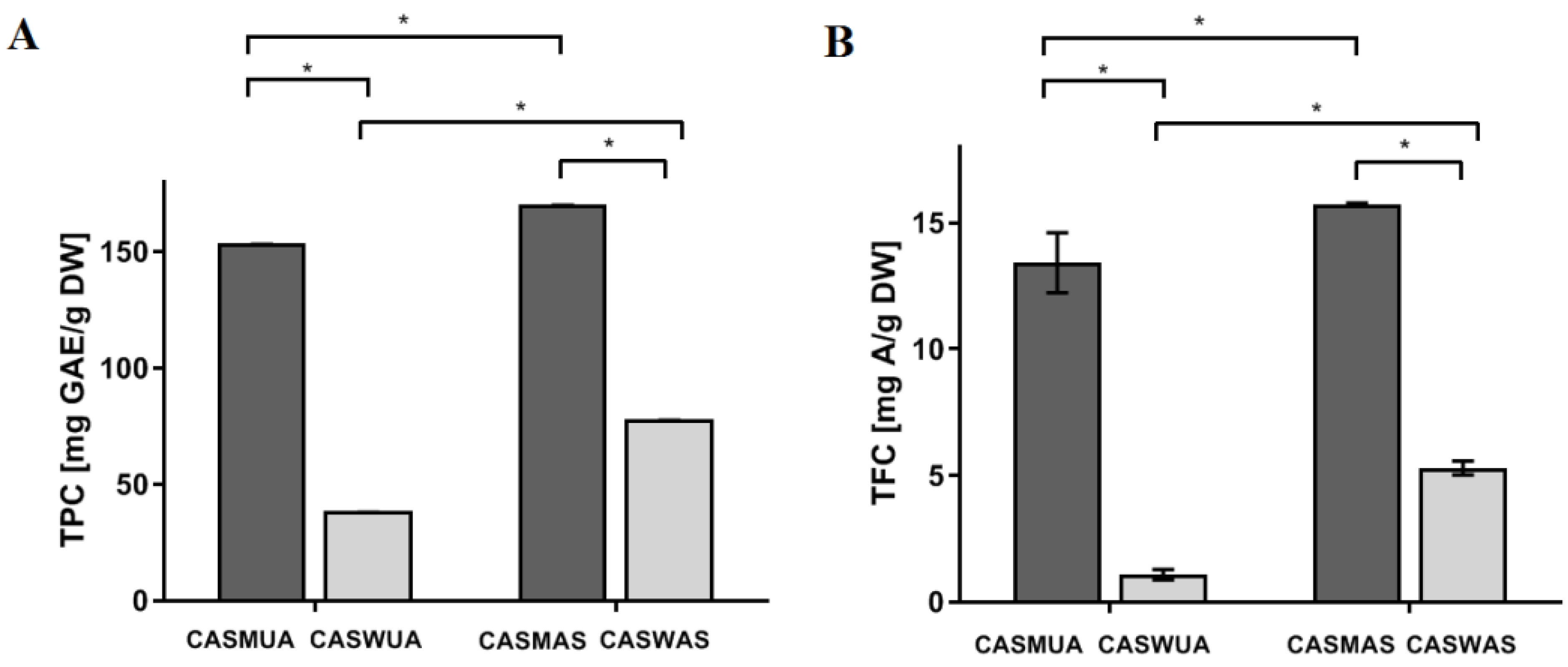
2.1. Determination of Total Phenolic Content and Total Flavonoid Content
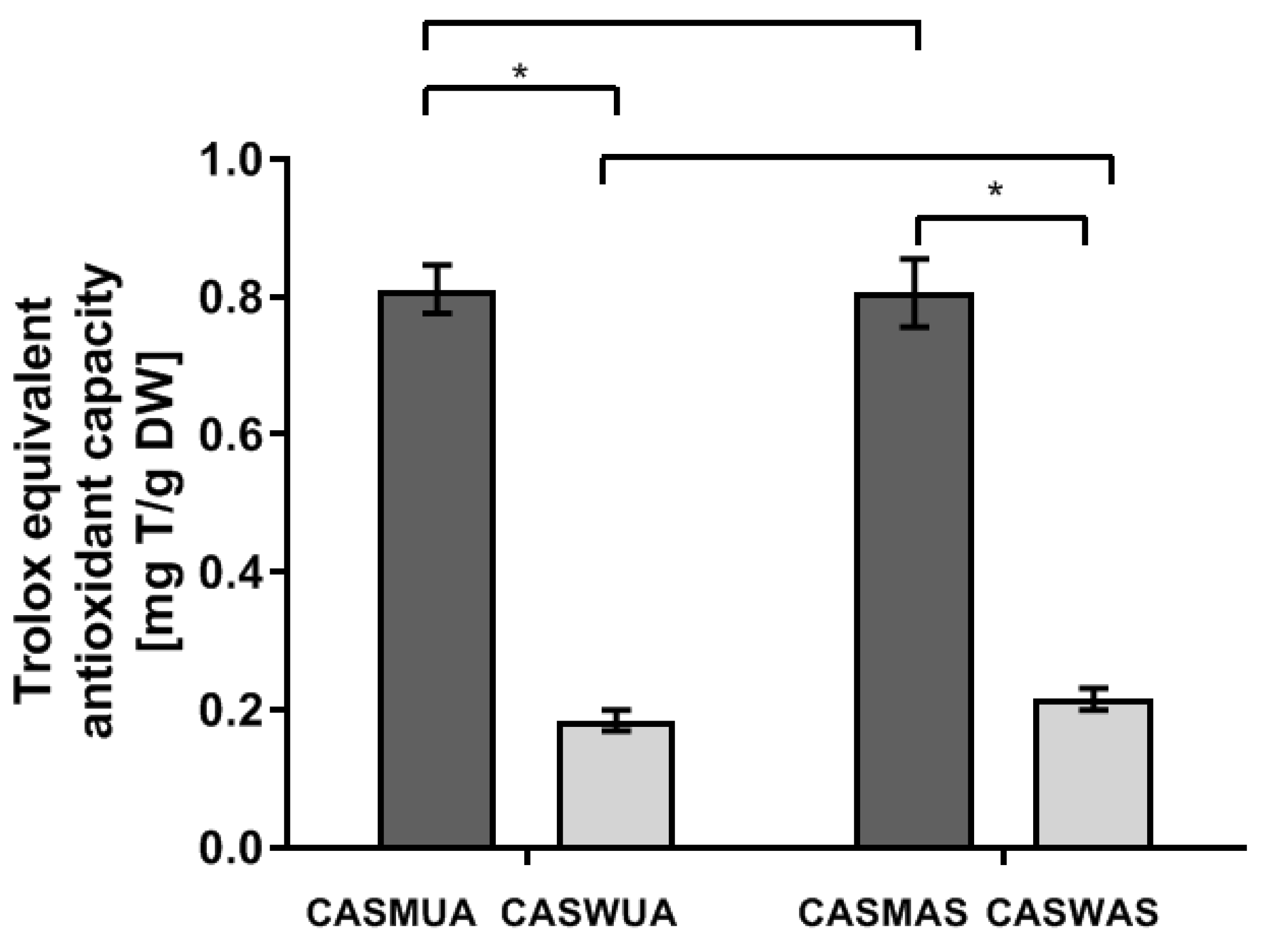
2.2. Determination of the Ability to Reduce Copper Ions (CUPRAC)
2.3. Qualitative and Quantitative Analysis
2.3.1. RP-HPLC/DAD Analysis
2.3.2. Qualitative Analysis—LC/ESI-QTOF-MS
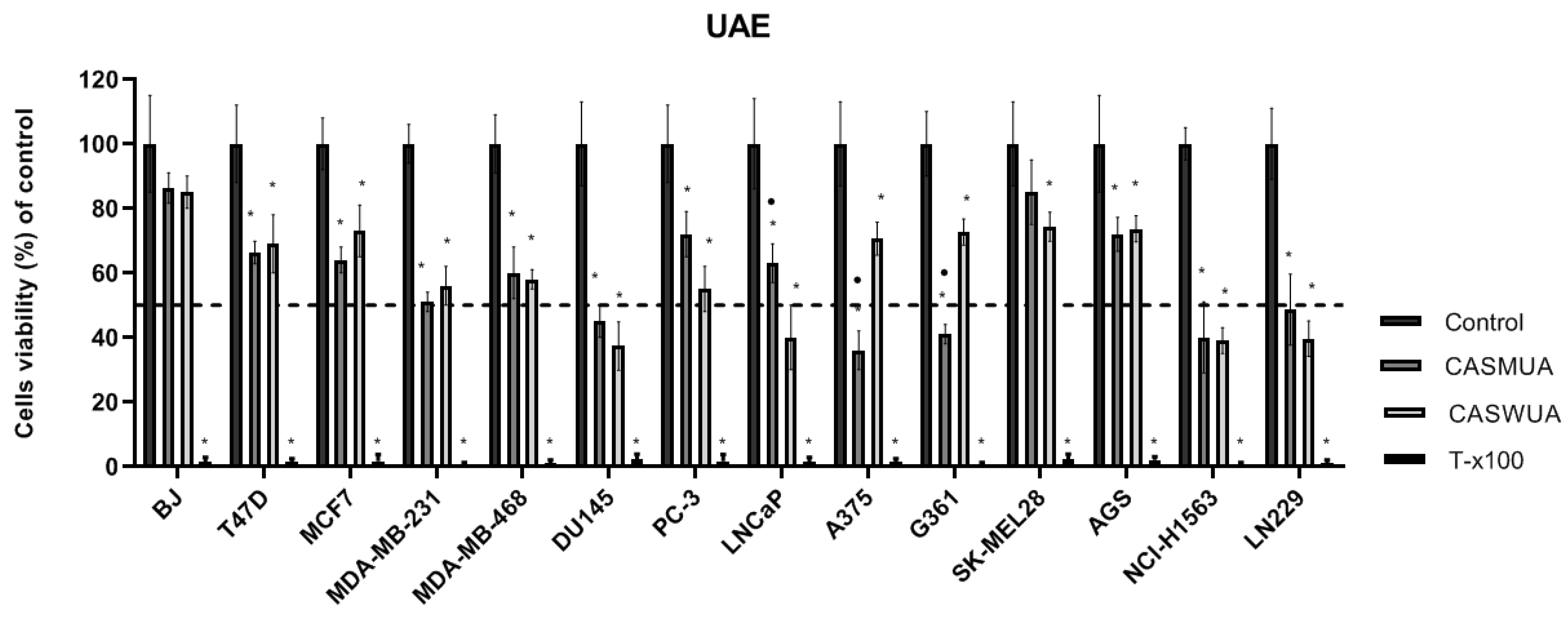
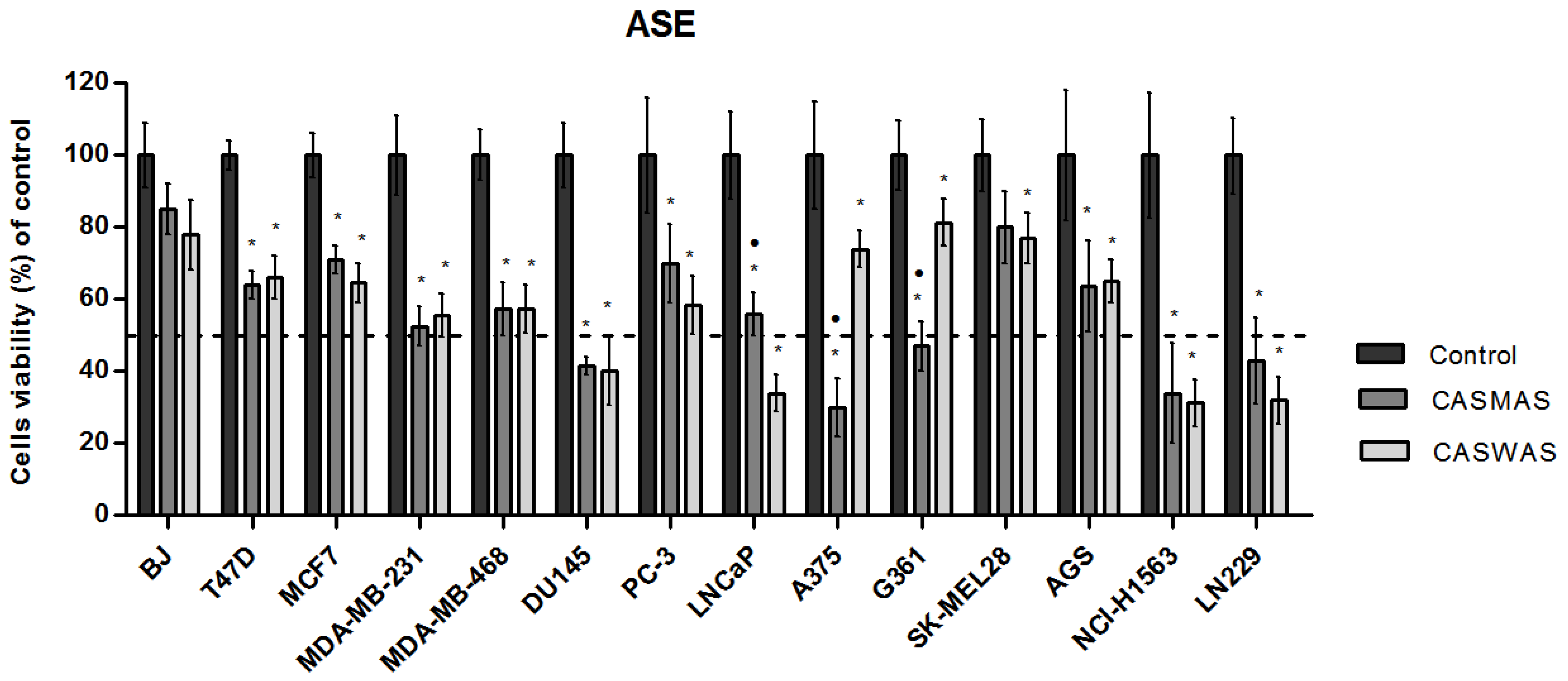
2.4. Cytotoxicity of Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Plant Material
3.2.1. Ultrasound-Assisted Extraction (UAE)
3.2.2. Accelerated Solvent Extraction (ASE)
3.2.3. Solid Phase Extraction (SPE)
3.3. Chemical Analysis
3.3.1. Determination of Flavonoid Compounds (TFC)
3.3.2. Determination of Total Polyphenols (TPC)
3.3.3. RP-HPLC/DAD Analysis
3.3.4. HPLC/ESI-QTOF-MS
3.3.5. Copper Reduction Assay (CUPRAC)
3.4. Biological Activity
3.4.1. Cell Culturing
3.4.2. The Cytotoxicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- El-Najjar, N.; Dakdouki, S.; Darwiche, N.; El-Sabban, M.; Saliba, N.; Gali-Muhtasib, H. Anti-Colon Cancer Effects of Salograviolide A Isolated from Centaurea Ainetensis. Oncol. Rep. 2008, 19, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Ozbas Turan, S.; Bitis, L. Bioactivity-Guided Isolation of Anti-Proliferative Compounds from Endemic Centaurea Kilaea. Pharm. Biol. 2017, 55, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Asadi, M.; Mosaddegh, M.; Motamed, S.M.; Hamzeloo-Moghadam, M. Cytotoxic activity of Centaurea albonitens Turrill aerial parts in colon and breast cancer cell lines. J. Med. Plants 2021, 20, 59–67. [Google Scholar] [CrossRef]
- Alper, M.; Güneş, H. The Anticancer and Anti-Inflammatory Effects of Centaurea Solstitialis Extract on Human Cancer Cell Lines. Turk. J. Pharm. Sci. 2019, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Korga, A.; Józefczyk, A.; Zgórka, G.; Homa, M.; Ostrowska, M.; Burdan, F.; Dudka, J. Evaluation of the Phytochemical Composition and Protective Activities of Methanolic Extracts of Centaurea Borysthenica and Centaurea Daghestanica (Lipsky) Wagenitz on Cardiomyocytes Treated with Doxorubicin. Food Nutr. Res. 2017, 61, 1344077. [Google Scholar] [CrossRef]
- Baharfar, R.; Khalilzadeh, M.; Gheibi, S.; Jazayeri, O.; Azimi, R.; Tajbakhsh, M. Antioxidant and Antibacterial Activities of the Methanolic Extract of Centaurea Zuvandica Sosn. Iran. J. Org. Chem. 2009, 1, 172–177. [Google Scholar]
- Radan, M.; Carev, I.; Tešević, V.; Politeo, O.; Čulić, V.Č. Qualitative HPLC-DAD/ESI-TOF-MS Analysis, Cytotoxic, and Apoptotic Effects of Croatian Endemic Centaurea Ragusina L. Aqueous Extracts. Chem. Biodivers. 2017, 14, e1700099. [Google Scholar] [CrossRef]
- Bancheva, S.; Kaya, Z.; Binzet, R. Morphological, Cytological And Palynological Features Of Three Closely Related Centaurea Species (Asteraceae) From Turkey. Mod. Phytomorphol. 2014, 5, 79–84. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Kamel, E.M. Cytotoxic Activities of Flavonoids from Centaurea Scoparia. Sci. World J. 2014, 2014, 274207. [Google Scholar] [CrossRef] [PubMed]
- Dalar, A.; Uzun, Y.; Mukemre, M.; Turker, M.; Konczak, I. Centaurea Karduchorum Boiss. from Eastern Anatolia: Phenolic Composition, Antioxidant and Enzyme Inhibitory Activities. J. Herb. Med. 2015, 5, 211–216. [Google Scholar] [CrossRef]
- Ozsoy, N.; Kultur, S.; Yilmaz-Ozden, T.; Ozbek Celik, B.; Can, A.; Melikoglu, G. Antioxidant, Anti-Inflammatory, Acetylcholinesterase Inhibitory and Antimicrobial Activities of Turkish Endemic C Entaurea antiochia Var P raealta: Biological Activities of Centaurea antiochia Var. Praealta. J. Food Biochem. 2015, 39, 771–776. [Google Scholar] [CrossRef]
- Tesevic, V.; Aljancic, I.; Milosavljevic, S.; Vajs, V.; Djordjevic, I.; Jadranin, M.; Menkovic, N.; Matevski, V. Secondary Metabolites of Three Endemic Centaurea L. Species. J. Serb. Chem. Soc. 2014, 79, 1355–1362. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea. Volume 4. Plantaginaceae to Compositae (and Rubiaceae); Cambridge University Press: Cambridge, UK, 1976; ISBN 978-0-521-08717-9. [Google Scholar]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive Oxygen Species and Cancer: A Complex Interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
- Ramundo, V.; Giribaldi, G.; Aldieri, E. Transforming Growth Factor-β and Oxidative Stress in Cancer: A Crosstalk in Driving Tumor Transformation. Cancers 2021, 13, 3093. [Google Scholar] [CrossRef]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxidative Med. Cell. Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, C.; Zhu, W.; Li, X.; Chen, T.; Liu, Q.; Zhou, S.; Zhang, T.-C.; Ma, W. Chemotherapeutic Drugs Induce Oxidative Stress Associated with DNA Repair and Metabolism Modulation. Life Sci. 2022, 289, 120242. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in Cancer Therapy: Anti-Cancer Effects and Mechanisms of Action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, L.; Seghiri, R.; Benayache, S.; Mosset, P.; Lobstein, A.; Chaabi, M.; León, F.; Brouard, I.; Bermejo, J.; Benayache, F. A New Flavonoid and Other Constituents from Centaurea nicaeensis All. Var. Walliana M. Nat. Prod. Res. 2012, 26, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Alnsour, L.; Segun, P.; Servi, H.; Celik, S.; Gokturk, S.; Al-Groshi, A.; Al-Majmaie, S.; Guetchueng, S.T.; Nahar, L.; et al. Flavonoids from Two Turkish Centaurea Species and Their Chemotaxonomic Implications. Trends Phytochem. Res. (TPR) 2017, 1, 243–248. [Google Scholar]
- Gülcemal, D.; Alankuş-Çalışkan, Ö.; Karaalp, C.; Örs, A.U.; Ballar, P.; Bedir, E. Phenolic Glycosides with Antiproteasomal Activity from Centaurea urvillei DC. Subsp. Urvillei. Carbohydr. Res. 2010, 345, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative Assessment of the Phytochemical Composition and Biological Activity of Extracts of Flowering Plants of Centaurea cyanus L., Centaurea jacea L. and Centaurea scabiosa L. Plants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Huang, C.; Wei, Y.-X.; Shen, M.-C.; Tu, Y.-H.; Wang, C.-C.; Huang, H.-C. Chrysin, Abundant in Morinda citrifolia Fruit Water–EtOAc Extracts, Combined with Apigenin Synergistically Induced Apoptosis and Inhibited Migration in Human Breast and Liver Cancer Cells. J. Agric. Food Chem. 2016, 64, 4235–4245. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin Inhibits Proliferation and Invasion, and Induces Apoptosis and Cell Cycle Arrest in Human Melanoma Cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of Nuclear Factor-Kappa B, Bax and Bcl-2 in Induction of Cell Cycle Arrest and Apoptosis by Apigenin in Human Prostate Carcinoma Cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Song, Y.U.; Yao, J.; Huang, K.; Zhu, X. Apigenin Suppresses Colorectal Cancer Cell Proliferation, Migration and Invasion via Inhibition of the Wnt/β-Catenin Signaling Pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Orta, M.L.; Pastor, N.; Pérez-Guerrero, C.; Austin, C.; Mateos, S.; López-Lázaro, M. The Coffee Constituent Chlorogenic Acid Induces Cellular DNA Damage and Formation of Topoisomerase I- and II-DNA Complexes in Cells. J. Agric. Food Chem. 2012, 60, 7384–7391. [Google Scholar] [CrossRef]
- El-Garawani, I.M.; El-Nabi, S.H.; El-Shafey, S.; Elfiky, M.; Nafie, E. Coffea Arabica Bean Extracts and Vitamin C: A Novel Combination Unleashes MCF-7 Cell Death. Curr. Pharm. Biotechnol. 2020, 21, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ekbatan, S.; Li, X.-Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Nieborowska-Skorska, M.; Kolasa, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. A Preliminary Study of Apoptosis Induction in Glioma Cells via Alteration of the Bax/Bcl-2-P53 Axis by Transformed and Non-Transformed Root Extracts of Leonurus sibiricus L. Tumour Biol. 2016, 37, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Xie, Z.; Rao, J.; Xu, G.; Huang, K.; Li, W.; Yin, Z. Chlorogenic Acid Inhibits Proliferation and Induces Apoptosis in A498 Human Kidney Cancer Cells via Inactivating PI3K/Akt/MTOR Signalling Pathway. J. Pharm. Pharm. 2019, 71, 1100–1109. [Google Scholar] [CrossRef]
- Chang, S.-L.; Chiang, Y.-M.; Chang, C.L.-T.; Yeh, H.-H.; Shyur, L.-F.; Kuo, Y.-H.; Wu, T.-K.; Yang, W.-C. Flavonoids, Centaurein and Centaureidin, from Bidens Pilosa, Stimulate IFN-γ Expression. J. Ethnopharmacol. 2007, 112, 232–236. [Google Scholar] [CrossRef]
- Orallo, F.; Lamela, M.; Camiña, M.; Uriate, E.; Calleja, J. Preliminary Study of the Potential Vasodilator Effects on Rat Aorta of Centaurein and Centaureidin, Two Flavonoids from Centaurea corcubionensis. Planta Med. 1998, 64, 116–119. [Google Scholar] [CrossRef]
- Jachak, S.M.; Gautam, R.; Selvam, C.; Madhan, H.; Srivastava, A.; Khan, T. Anti-Inflammatory, Cyclooxygenase Inhibitory and Antioxidant Activities of Standardized Extracts of Tridax procumbens L. Fitoterapia 2011, 82, 173–177. [Google Scholar] [CrossRef]
- Kim, D.E.; Min, K.-J.; Kim, M.-J.; Kim, S.-H.; Kwon, T.K. Hispidulin Inhibits Mast Cell-Mediated Allergic Inflammation through Down-Regulation of Histamine Release and Inflammatory Cytokines. Molecules 2019, 24, 2131. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hung, C.-M.; Tsai, J.-C.; Lee, J.-C.; Chen, Y.-L.S.; Wei, C.-W.; Kao, J.-Y.; Way, T.-D. Hispidulin Potently Inhibits Human Glioblastoma Multiforme Cells through Activation of AMP-Activated Protein Kinase (AMPK). J. Agric. Food Chem. 2010, 58, 9511–9517. [Google Scholar] [CrossRef]
- Gao, H.; Wang, H.; Peng, J. Hispidulin Induces Apoptosis Through Mitochondrial Dysfunction and Inhibition of P13k/Akt Signalling Pathway in HepG2 Cancer Cells. Cell Biochem. Biophys. 2014, 69, 27–34. [Google Scholar] [CrossRef]
- Yu, C.Y.; Su, K.-Y.; Lee, P.-L.; Jhan, J.-Y.; Tsao, P.-H.; Chan, D.-C.; Chen, Y.-L.S. Potential Therapeutic Role of Hispidulin in Gastric Cancer through Induction of Apoptosis via NAG-1 Signaling. Evid. Based Complement. Altern. Med. 2013, 2013, 518301. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, F.; Yan, J.; Xia, Z.; Jiang, D.; Ma, P. Hispidulin: A Promising Flavonoid with Diverse Anti-Cancer Properties. Life Sci. 2020, 259, 118395. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, S.; Atasagun, B.; Aksoy, A. Comparison of Phenolic Components and Biological Activities of Two Centaurea sp. Obtained by Three Extraction Techniques. Asian Pac. J. Trop. Med. 2017, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic Extraction of Flavonoids, Phenolics and Antioxidants from Vernonia Amygdalina Leaf Using Two-Level Factorial Design. J. King Saud Univ.-Sci. 2020, 32, 7–16. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.; Bektaşoğlu, B.; Berker, K.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
| Species | Ultrasound Assisted Extraction (UAE) | Accelerated Solvent Extraction (ASE) | ||
|---|---|---|---|---|
| Methanol-Water (7:3 v/v) | Water | Methanol-Water (7:3 v/v) | Water | |
| C. castriferrei Borbás & Waisb. | CASMUA | CASWUA | CASMAS | CASWAS |
| Extract | Polyphenol Content from the Standard Curve (μg/mL) | Polyphenol Content/ GAE Equivalent per g Plant Substance (mg GAE/g) | Mean Content (mg GAE/g) | ±SD; ±RSD |
|---|---|---|---|---|
| CASMUA | 14.63 7.31 3.66 | 153.56 153.53 153.73 | 153.60 | SD ± 0.10 RSD ± 0.08 |
| CASWUA | 3.65 1.82 0.92 | 38.27 38.21 38.41 | 38.30 | SD ± 0.11 RSD ± 0.08 |
| CASMAS | 16.19 8.12 4.05 | 170.04 170.55 170.04 | 170.21 | SD ± 0.29 RSD ± 0.23 |
| CASWAS | 7.40 3.70 1.86 | 77.74 77.68 78.05 | 77.82 | SD ± 0.20 RSD ± 0.15 |
| Extract | Flavonoid Content from the Standard Curve (μg/mL) | Flavonoid Content/ A (Apigenin) Equivalent per g Plant Substance (mg A/g) | Mean Content (mg A/g) | ±SD; ±RSD |
|---|---|---|---|---|
| CASMUA | 72.06 34.36 15.12 | 14.41 13.75 12.10 | 13.42 | SD ± 1.19 RSD ± 0.88 |
| CASWUA | 6.49 2.21 1.32 | 1.30 0.88 1.06 | 1.09 | SD ± 0.21 RSD ± 0.15 |
| CASMAS | 78.63 39.34 19.73 | 15.73 15.74 15.78 | 15.75 | SD ± 0.03 RSD ± 0.02 |
| CASWAS | 25.59 14.02 6.45 | 5.12 5.61 5.16 | 5.30 | SD ± 0.27 RSD ± 0.21 |
| Extract | Trolox Concentration from the Standard Curve (mg/mL) | Trolox Equivalent Antioxidant Capacity (mg T/g .d.wt.) | Mean Content (mg T/g .d.wt.) | ±SD; ±RSD |
|---|---|---|---|---|
| CASMUA | 0.39 0.21 0.10 0.05 0.03 | 0.78 0.83 0.81 0.78 0.86 | 0.81 | SD ± 0.03 RSD ± 0.03 |
| CASWUA | 0.35 0.19 0.10 0.05 0.02 | 0.17 0.19 0.21 0.19 0.17 | 0.18 | SD ± 0.01 RSD ± 0.01 |
| CASMAS | 0.38 0.20 0.11 0.05 0.10 | 0.75 0.79 0.86 0.86 0.77 | 0.81 | SD ± 0.05 RSD ± 0.04 |
| CASWAS | 0.42 0.23 0.12 0.05 0.03 | 0.21 0.23 0.24 0.21 0.20 | 0.22 | SD ± 0.02 RSD ± 0.01 |
| No. | Name of Compound | CASMUA | CASWUA | CASMAS | CASWAS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/g) | Content (mg/g) | Content (mg/g) | Content (mg/g) | ||||||||||
| ±SD | ±RSD | ±SD | ±RSD | ±SD | ±RSD | ±SD | ±RSD | ||||||
| 1 | Neochlorogenic acid | 0.90 | 0.01 | 0.6 | 0.51 | 0.00 | 0.2 | 1.02 | 0.00 | 0.4 | 0.14 | 0.00 | 2.5 |
| 2 | Chlorogenic acid | 4.14 | 0.03 | 0.7 | 1.30 | 0.01 | 0.5 | 4.06 | 0.01 | 0.3 | 0.17 | 0.00 | 0.2 |
| 3 | Crypto-chlorogenic acid | 0.11 | 0.00 | 1.7 | 0.04 | 0.00 | 0.0 | 0.11 | 0.00 | 0.0 | - | - | - |
| 4 | Caffeic acid | 0.09 | 0.00 | 0.7 | 0.09 | 0.00 | 1.0 | 0.06 | 0.00 | 1.0 | - | - | - |
| 5 | Protocatechuic acid | 0.26 | 0.01 | 3.5 | 0.17 | 0.00 | 2.2 | 0.31 | 0.01 | 3.2 | 0.07 | 0.00 | 0.0 |
| 6 | 4-Hydroxybenzoic acid | 0.15 | 0.00 | 0.8 | - | - | - | 0.17 | 0.00 | 0.4 | 0.20 | 0.00 | 1.4 |
| 7 | Isoferulic acid | 0.02 | 0.00 | 0.8 | - | - | - | 0.02 | 0.00 | 1.7 | - | - | - |
| 8 | p-Coumaric acid | - | - | - | - | - | - | - | - | - | 0.04 | 0.00 | 0.0 |
| 9 | Cynarin 1.3 | - | - | - | 0.38 | 0.00 | 0.6 | - | - | - | - | - | - |
| 10 | Chlorogenic acid glucoside | 1.70 | 0.00 | 0.1 | 0.14 | 0.00 | 0.9 | 1.91 | 0.02 | 1.2 | 0.04 | 0.00 | 0.0 |
| 11 | Caffeic acid derivative 1 | 0.16 | 0.00 | 1.4 | 0.08 | 0.00 | 0.4 | 0.17 | 0.00 | 1.3 | 0.07 | 0.00 | 0.9 |
| 12 | Caffeic acid derivative 2 | 0.08 | 0.00 | 0.6 | - | - | - | 0.08 | 0.00 | 0.0 | 0.04 | 0.00 | 0.7 |
| 13 | Caffeic acid derivative 3 | 0.11 | 0.00 | 0.5 | - | - | - | 0.14 | 0.00 | 0.4 | - | - | - |
| 14 | Apigenin derivative | 0.40 | 0.01 | 1.3 | 0.21 | 0.00 | 0.2 | 0.41 | 0.01 | 1.5 | 0.37 | 0.00 | 0.0 |
| 15 | Isoquercetin | 0.05 | 0.00 | 0.9 | - | - | - | 0.05 | 0.00 | 1.1 | - | - | - |
| 16 | Luteolin 7-O-glucoside | 0.85 | 0.01 | 0.6 | 0.51 | 0.01 | 2.4 | 0.62 | 0.02 | 2.6 | 0.39 | 0.00 | 0.3 |
| 17 | Apigenin 7-O-glucoside | 0.17 | 0.00 | 0.4 | - | - | - | 0.17 | 0.00 | 0.4 | - | - | - |
| 18 | Apigenin 7-O-glucuronide | 5.26 | 0.02 | 0.3 | 1.76 | 0.01 | 0.3 | 5.26 | 0.02 | 0.3 | 2.62 | 0.01 | 0.2 |
| 19 | Dimethyl apigenin | 0.56 | 0.00 | 0.3 | 0.11 | 0.00 | 0.6 | 0.60 | 0.00 | 0.7 | 0.14 | 0.00 | 0.4 |
| 20 | Dihydrokaempferol | 0.82 | 0.02 | 0.0 | 0.23 | 0.00 | 0.0 | 0.92 | 0.03 | 2.8 | 0.36 | 0.00 | 0.0 |
| 21 | Kaempferol dihydro- glucoside | 0.09 | 0.00 | 0.0 | - | - | - | 0.09 | 0.00 | 0.0 | - | - | - |
| 22 | Kaempferol glucoside | 0.08 | 0.00 | 0.0 | - | - | - | 0.11 | 0.00 | 0.0 | - | - | - |
| 23 | Centaurein | 3.97 | 0.02 | 0.4 | 0.16 | 0.00 | 0.6 | 4.11 | 0.01 | 0.2 | 0.76 | 0.00 | 0.4 |
| 24 | Jacein | 1.21 | 0.00 | 0.2 | - | 0 | 0 | 0.97 | 0.00 | 0.3 | 0.43 | 0.00 | 1.1 |
| 25 | Apigenin | 7.32 | 0.03 | 0.4 | 0.38 | 0.01 | 2.6 | 7.98 | 0.02 | 0.3 | 11.09 | 0.01 | 0.1 |
| 26 | Luteolin | 0.13 | 0.00 | 0.0 | - | - | - | 0.14 | 0.00 | 0.0 | 0.03 | 0.00 | 0.0 |
| 27 | Centaureidin | - | - | - | - | - | - | - | - | - | 0.51 | 0.1 | 0.0 |
| Total (identified compounds) | 28.63 | 6.07 | 29.48 | 17.33 | |||||||||
| No. | Name of Compound | Rt (min) | Chemical Formula | Molecular Ion (m/z) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 15.735 | C16H18O9 | 353.0846 | 191.0542 |
| 2 | Feruloylquinic acid | 20.158 | C17H20O9 | 367.0989 | 191.0531; 134.0258; 93.0413 |
| 3 | Apigenin glucuronide-glucoside | 21.429 | C27H28O16 | 607.1286 | 431.0946; 269.0409; 175.0196; 113.0224 |
| 4 | Kaempferide glucoside | 23.376 | C22H22O11 | 463.0861 | 301.0397; 151.0012; 97.3310 |
| 5 | Isorhamnetin glucoside | 24.507 | C22H22O12 | 477.1000 | 315.0631 |
| 6 | Chlorogenic acid glucoside | 25.911 | C21H28O24 | 515.1150 | 353.0852; 191.0536 |
| 7 | Isorhamnetin glucuronide | 26.546 | C22H20O13 | 491.1154 | 315.0631 |
| 8 | Apigenin 7-O glucuronide | 27.153 | C21H18O11 | 445.0736 | 269.0441; 175.0241; 113.0202 |
| 9 | Centaurein | 27.455 | C24H26O13 | 521.1231 | 506.1033; 343.0375 |
| 10 | Jacein | 27.960 | C24H26O13 | 521.1240 | 506.1076; 359.0687; 343.0444 |
| 11 | Hispidulin glucuronide | 28.282 | C22H20O12 | 475.0842 | 299.0494; 284.0258; 255.0054; 227.0327; 85.0249 |
| 12 | Isorhamnetin | 30.863 | C16H12O7 | 315.0470 | 300.0327; 199.0447; 65.0458 |
| 13 | Luteolin | 31.090 | C15H10O6 | 285.0475 | 133.0242; 107.0099 |
| 14 | Apigenin | 34.074 | C15H10O5 | 269.0310 | 117.0348 |
| 15 | Hispidulin | 35.317 | C16H12O6 | 299.0524 | 283.0272; 255.0443; 227.0484; |
| No. | Name of Compound | Rt (min) | Chemical Formula | Molecular Ion (m/z) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | Quinic acid | 1.865 | C7H12O6 | 191.0525 | 111.0543 |
| 2 | Protocatechuic acid glucoside | 7.799 | C13H16O7 | 153.0165 | 153.0154; 109.0277 |
| 3 | Chlorogenic acid | 10.087 | C16H18O9 | 353.0863 | 191.0532; 179.0320 |
| 4 | Neochlorogenic acid | 15.646 | C16H18O9 | 353.4838 | 191.0520; 135.0389; 85.0277 |
| 5 | Feruloylquinic acid | 20.186 | C17H20O9 | 367.0987 | 191.0571; 134.0351; 93.0317 |
| 6 | Ferulic acid | 25.478 | C10H10O4 | 193.0472 | 133.0283 |
| 7 | Apigenin 7-O glucuronide | 27.153 | C21H18O11 | 445.0736 | 269.0440; 175.0241; 113.0202 |
| 8 | Apigenin | 34.138 | C15H10O5 | 269.0439 | 117.0334 |
| No. | Name of Compound | Rt (min) | Chemical Formula | Molecular Ion (m/z) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 15.829 | C16H18O9 | 353.0827 | 191.0530 |
| 2 | Feruloylquinic acid | 20.198 | C17H20O9 | 367.0987 | 191.0571; 134.0351; 93.0317 |
| 3 | Isorhamnetin glucoside | 24.512 | C21H18O13 | 477.0994 | 315.0458; 299.0158 |
| 4 | Chlorogenic acid glucoside | 25.899 | C21H28O24 | 515.1150 | 353.0836; 191.0506 |
| 5 | Isorhamnetin glucuronide | 26.569 | C22H20O13 | 491.1184 | 315.7631 |
| 6 | Apigenin 7-O glucuronide | 27.199 | C21H18O11 | 445.0756 | 269.0440; 175.0241; 113.0222 |
| 7 | Hispidulin glucuronide | 28.306 | C22H20O12 | 475.0867 | 299.0544; 284.0308; 255.0254; 227.0327; 85.0289 |
| 8 | Eriodictyol | 29.846 | C15H12O6 | 287.0517 | 151.0010; 135.0440 |
| 9 | Isorhamnetin | 30.893 | C16H12O7 | 315.0490 | 300.0227; 199.0465; 65.0051 |
| 10 | Luteolin | 31.104 | C15H10O6 | 285.0365 | 133.0269; 107.0092 |
| 11 | Apigenin | 34.109 | C15H10O5 | 269.0439 | 117.0334 |
| 12 | Hispidulin | 35.304 | C16H12O6 | 299.0545 | 283.0282; 255.0513; 227.0544; |
| No. | Name of Compound | Rt (min) | Chemical Formula | Molecular Ion (m/z) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | Coumaroylquinic acid | 19.089 | C16H18O8 | 337.0911 | 191.0517; 93.0339 |
| 2 | Feruloylquinic acid | 20.173 | C17H20O9 | 367.1024 | 191.0533; 134.0361; 93.0341 |
| 3 | Isorhamnetin glucuronide | 26.544 | C22H20O13 | 491.1184 | 315.7631 |
| 4 | Apigenin 7-O glucuronide | 27.116 | C21H18O11 | 445.0756 | 269.0440; 175.0241; 113.0222 |
| 5 | Hispidulin glucuronide | 28.272 | C22H20O12 | 475.0867 | 299.0544; 284.0308; 255.0254; 227.0327; 85.0289 |
| 6 | Isorhamnetin | 31.142 | C16H12O7 | 315.0490 | 300.0227; 199.0465; 65.0051 |
| 7 | Apigenin | 34.055 | C15H10O5 | 269.0439 | 269.0442; 117.0334 |
| 8 | Hispidulin | 35.264 | C16H12O6 | 299.0545 | 283.0542; 255.0513; 227.0544; |
| Cell Lines | Extraction Method: ASE | Extraction Method: UAE | |||
|---|---|---|---|---|---|
| CASMAS | CASWAS | CASMUA | CASWUA | ||
| IC50 (µg/mL) | IC50 (µg/mL) | ||||
| Breast cancer | T47D | >125 | >125 | >125 | >125 |
| MCF7 | >125 | >125 | >125 | >125 | |
| MDA-MB231 | >125 | >125 | >125 | >125 | |
| MDA-MB468 | >125 | >125 | >125 | >125 | |
| Prostate cancer | PC-3 | >125 | >125 | >125 | >125 |
| DU145 | 120.01 | 115.86 | 124.87 | 120.97 | |
| LNCaP | >125 | 69.13 | >125 | 62.35 | |
| Melanoma | A375 | 64.53 | >125 | 76.13 | >125 |
| G361 | 123.42 | >125 | 110.27 | >125 | |
| SK-MEL28 | >125 | >125 | >125 | >125 | |
| Glioma | LN229 | 120.68 | 104.25 | 123.21 | 112.69 |
| Gastric cancer | AGS | >125 | >125 | >125 | >125 |
| Lung cancer | NCI-H1563 | 102.06 | 116.98 | 117.79 | 121.95 |
| Fibroblast | BJ | >125 | >125 | >125 | >125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubik, J.; Waszak, Ł.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Adamczuk, K.; Michalczuk, M.; Korga-Plewko, A.; Józefczyk, A. Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L. Molecules 2022, 27, 7537. https://doi.org/10.3390/molecules27217537
Kubik J, Waszak Ł, Adamczuk G, Humeniuk E, Iwan M, Adamczuk K, Michalczuk M, Korga-Plewko A, Józefczyk A. Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L. Molecules. 2022; 27(21):7537. https://doi.org/10.3390/molecules27217537
Chicago/Turabian StyleKubik, Joanna, Łukasz Waszak, Grzegorz Adamczuk, Ewelina Humeniuk, Magdalena Iwan, Kamila Adamczuk, Mariola Michalczuk, Agnieszka Korga-Plewko, and Aleksandra Józefczyk. 2022. "Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L" Molecules 27, no. 21: 7537. https://doi.org/10.3390/molecules27217537
APA StyleKubik, J., Waszak, Ł., Adamczuk, G., Humeniuk, E., Iwan, M., Adamczuk, K., Michalczuk, M., Korga-Plewko, A., & Józefczyk, A. (2022). Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L. Molecules, 27(21), 7537. https://doi.org/10.3390/molecules27217537





