Abstract
Multi-substituted pyrroles are synthesized from regiospecific aziridine ring-opening and subsequent intramolecular cyclization with a carbonyl group at the γ-position in the presence of Lewis acid or protic acid. This method is highly atom economical where all the atoms of the reactants are incorporated into the final product with the removal of water. This new protocol is applied to the synthesis of various pyrroles, including natural products.
1. Introduction
Pyrroles are molecules of great interest as key structural elements of various compounds, including pharmaceuticals and natural products [1,2]. For example, inonotus obliquus [3,4,5]. The white rot fungus that belongs to the family Hymenochaetaceae (Basidiomycetes) and is mainly distributed in Europe, Asia, and North America has been used for the treatment of gastrointestinal cancer, cardiovascular disease, and diabetes since the sixteenth century in Russia, Poland, and the Baltic countries. Moreover, the fungus has been reported to have anti-inflammatory [6], antioxidant [7,8,9,10], immunomodulatory [11], and hepatoprotective effects [12]. Some representative examples of 5-hydroxymethyl pyrrole-2-carbaldehydes found in the inonotus obliquus, sometimes referred to as 2-formylpyrroles or pyrralines, are displayed in Figure 1.
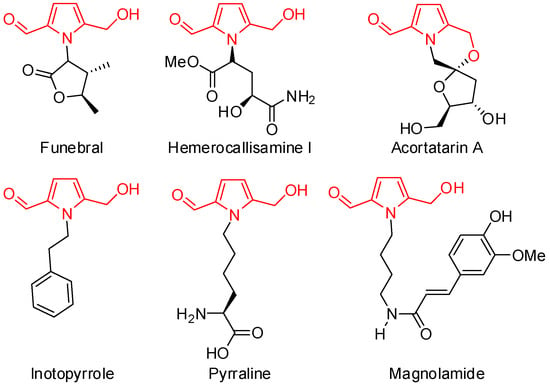
Figure 1.
Structures of 2-formyl pyrrole-containing bioactive natural products.
The synthesis of highly functionalized pyrroles has drawn considerable attention from organic and medicinal chemists. In general, the classical synthesis routes for multi-substituted pyrroles, including the Knorr condensation [13], the Paal–Knorr reaction [14], the Hantzsch reaction [15], transition metal-catalyzed reactions [16,17], and multicomponent coupling reactions [18,19,20], have been in existence for many years. However, most of them are limited by the inefficient synthesis of highly functionalized pyrroles; it is challenging to introduce various substituents to the pyrrole ring due to its harsh reaction conditions and the instability of widely used keto functionality. The construction of the pyrrole ring allows regioselective functionalization and subsequent diversification of the pyrrole ring with various substituents.
Many synthetic methods have commenced from aziridine and its derivatives by expanding the ring whose nitrogen ends at the pyrrole ring. Specifically, pyrroles are synthesized from propargyl aziridines through intramolecular cyclization and breaking of the aziridine ring with the assistance of various metal catalysts (“M”) including “Au(I)” followed by rearrangement for aromatization (Scheme 1, (1)) [16,17]. Our group developed a similar pyrrole synthesis method with 3-(aziridine-2-yl)-3-hydroxypropyne taking an advantage of nucleophilic aziridine ring-opening prior to cyclization [18,19,20]. Vinyl aziridines also served as starting materials for pyrrole after 1,3-sigmatropic shift and oxidation or 2+3 cycloaddition reaction with olefin via the cleavage of the C-N bond (Scheme 1, (2)). Similar [3+2]-cycloadditions were used to generate five-membered rings from 2-methyleneaziridine as a 1,3-dipole with an olefin (Scheme 1, (3)). However, most of these reported methods have two critical drawbacks. First, most of the methods require a metal (“M”) catalyst. Second, only a single substituted pyrrole is generated from one set of aziridine substituents properly decorated as a starting material with the necessary counterparts, including olefins and alkynes [21,22].
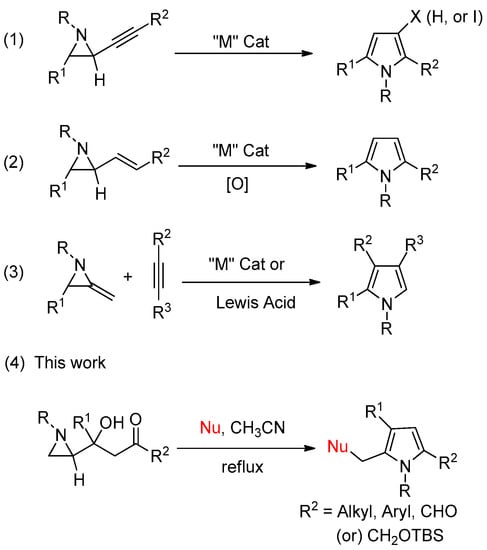
Scheme 1.
Previous works for construction of pyrroles from activated-aziridines.
In this report, we describe an atom economical synthesis of multi-substituted pyrroles from regiospecific aziridine ring-opening by various nucleophiles [23,24,25,26] and the following cyclization in Knorr-type reactions.
2. Results and Discussion
Treatment of hydroxy keto aziridine 1a [25,26] with TMSN3 in THF or dioxane under reflux did not yield the desired pyrrole product 2a (entries 1 and 2, Table 1). In dichloromethane, under reflux conditions, we obtained the expected pyrrole with a 70% yield (entry 3), whereas in CH3CN the yield increased to 85% (entry 4). In the presence of various Lewis acids such as BF3.OEt2 and FeCl3 with NaN3 nucleophile, we did not obtain the desired pyrrole product 2a (entries 5 and 6, Table 1) with all the starting materials remaining.

Table 1.
Optimization of the reaction conditions.
Next, the generality of the method was evaluated under optimized conditions that had been cyclization. This protocol provided a versatile entry for a variety of pyrroles (2) is well determined in Table 1. Then, we examined the scope and limitations of several β-(aziridin-2-yl)-β-hydroxy ketones (1) through the one-step regioselective ring-opening of aziridine followed by intramolecular to moderate yields (Scheme 2).
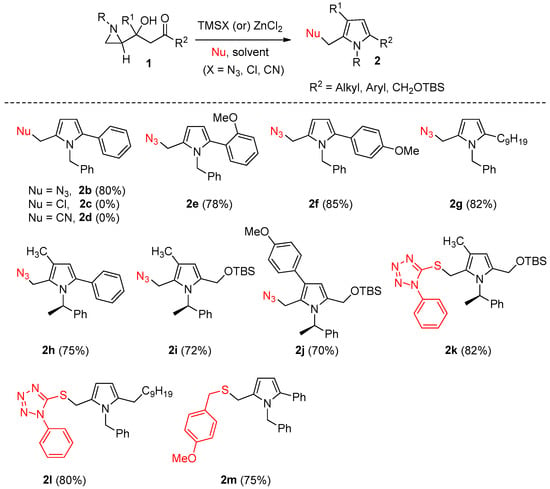
Scheme 2.
Representative scheme and various examples of multi-substituted pyrroles (2) from substituted aziridines (1).
In the successive reactions of regioselective ring opening in CH3CN under reflux and Knorr cyclization, the pyrrole compound 2b was obtained in an 80% yield from the aziridine starting compounds bearing a substituent at R2 such as phenyl (1b) using TMSN3, whereas no pyrrole product 2c or 2d was obtained using TMSCl or TMSCN (see Scheme 2). After TMSN3 screening (as mentioned in Table 1), we next screened a substrate variant using aziridines bearing a substituent at R2, such as o-methoxyphenyl (1e), p-methoxyphenyl (1f), and n-nonanyl (1g), as starting materials, which gave a pyrrole variant (2e–2g) in moderate to good yield under TMSN3 conditions. The starting substrates with an additional substituent (R2 as phenyl and t-butyldimethylsilyloxymethyl) and R1 as methyl and p-methoxyphenyl) gave pyrroles (2h, 2i, and 2j) in 75%, 72%, and 70% yields, respectively. We also applied various thiol nucleophiles under the ZnCl2 catalyst in MeOH to compounds (1k–1m) with substituents at C2 and C4, resulting in high yields of pyrroles (2k–2m) (Scheme 2).
Next, oxidation of the secondary alcohol of compound 3 at the γ-position of aziridine with Dess–Martin periodinane in CH2Cl2 yielded a complex mixture of compounds, which were directly reacted for the ring-opening with various nucleophiles such as OMe, OAc, Cl, and CN to afford 2,3-disubstituted pyrrole 5-aldehydes (4a–4d) in the one-pot procedure as shown in Scheme 3 with examples in the Scheme 4. Whereas Swern oxidation of secondary alcohol of compound 3, followed by regio and stereoselective aziridine ring-opening with incoming nucleophile, yielded OTBS-protected pyrrole 2 as shown in Scheme 3 (see compounds 2k–2l in Scheme 2).
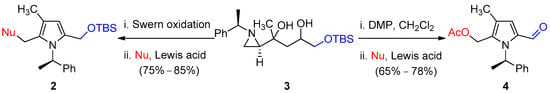
Scheme 3.
Oxidation-state controlled synthesis of pyrrole product.
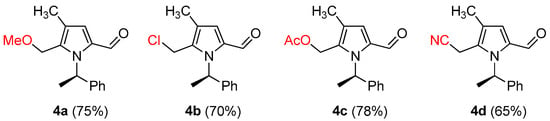
Scheme 4.
Examples of synthesis of 2-formyl pyrroles.
The difference in cyclization is raised by the substituent of R2, whether the substituent R2 is a simple alkyl or aryl, or hydroxymethyl in Scheme 2. The initial Paal–Knorr cyclization step gives either 6 or 7, regardless of the characteristics of R2, with the removal of water molecules. After the generation of the hydroxy pyrrolidine intermediate 6, generated from most substrates with alkyl or aryl substituent on R2, the reaction proceeds to aromatization to yield 2 as shown in Scheme 2. From the substrate-bearing hydroxymethyl group, the ammonium ion intermediate 8 was generated, from which the deprotonation occurs to give 9 and its resonance form as 10. One more deprotonation from 10 gives rise to the final 2-formyl pyrroles 4, as shown in Scheme 4 (Scheme 5).
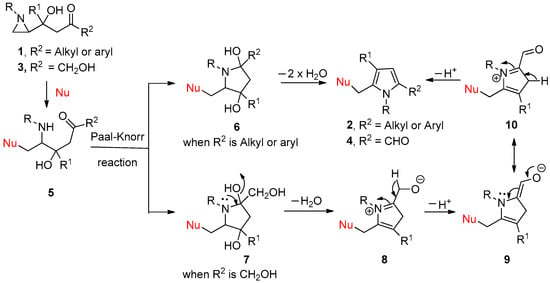
Scheme 5.
Proposed reaction mechanism for the formation of 2 and 4 from 1 and 3 in two different pathways.
This method was extended to the synthesis of the natural product inotopyrrole 19 (Scheme 5). Treatment of compound 11 with Weinreb salt and i-PrMgCl to give compound 12, followed by allyl magnesium bromide and a subsequent reduction of aziridine ketone by NaBH4 yielded the alcohol compound 13 in 68% yield for two steps. Protection of the secondary alcohol with TBSOTf and 2,6-lutidine to furnish olefin 14 at a 73% yield. Olefin 14 was subjected to simple dihydroxylation using OsO4 and NMO to give a diol compound, followed by selective protection of the primary alcohol with TBSCl to afford secondary alcohol, and subsequently, Swern oxidation of alcohol afforded key intermediate keto compound 15 in a 62% yield. Then, we applied our optimized method on compound 15 for the synthesis of pyrrole derivative 16 from a one-step regioselective ring-opening followed by cyclization of keto compound by using AcOH and CH2Cl2 at 0 °C in 82% yield. Then, deacetylation of 16 with K2CO3 to give alcohol 17, followed by Dess-martin oxidation of primary alcohol, afforded aldehyde 18 with a 74% yield. Removal of the TBS group with TBAF gave rise to the desired natural product, inotopyrrole (19), in an 84% yield. Spectral data (1H, 13C NMR) and HRMS data of our synthetic ionotopyrrole (19) were in full agreement with those reported for the natural product (Scheme 6) [3,4,5].
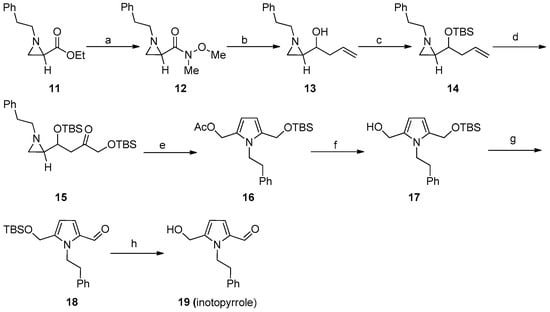
Scheme 6.
Synthesis of inotopyrrole (19) from aziridine (11). (a) NHMe(OMe), i-PrMgCl, THF, 0 °C, 2 h, 78%. (b) (i) Allylmagenisium bromide, THF, 0 °C, 1 h; (ii) NaBH4, MeOH, 0 °C, 1 h; 87% (over two steps). (c) TBSOTf, 2,6-Lutidine, CH2Cl2, 0 °C to rt, 2 h, 73%. (d) (i) OsO4, NMO, 0 °C to rt, 4 h; (ii) TBSCl, Imidazole, CH2Cl2, 0 °C to rt, 2 h; (iii) DMSO, (COCl)2, CH2Cl2, −78 °C, 2 h; 62% (over three steps). (e) AcOH, CH2Cl2, 0 °C, 12 h, 82%. (f) K2CO3, MeOH, 0 °C, 12 h, 80%. (g) DMP, CH2Cl2, 0 °C, 2 h, 74%. (h) TBAF, THF, 0 °C, 1 h, 84%.
3. Materials and Methods
3.1. General Information
Chiral aziridines are available from Sigma-Aldrich as reagents. They are also available from Imagene Co., Ltd. (http://www.imagene.co.kr/) in bulk quantities. All commercially available compounds were used as received unless stated otherwise. All reactions were carried out under an atmosphere of nitrogen in oven-dried glassware with magnetic stirrer. Dichloromethane was distilled from calcium hydride. Reactions were monitored by thin layer chromatography (TLC) with 0.25 mm E. Merck pre-coated silica gel plates (60 F254). Visualization was accomplished with either UV light, or by immersion in solutions of ninhydrin, p-anisaldehyde, or phosphomolybdic acid (PMA) followed by heating on a hot plate for about 10 sec. Purification of reaction products was carried out by flash chromatography using Kieselgel 60 Art 9385 (230–400 mesh). The 1H-NMR and 13C-NMR spectra were obtained using Varian unity lNOVA 400WB (400 MHz) or Bruker AVANCE III HD (400 MHz) spectrometer. Chemical shifts are reported relative to chloroform (δ = 7.26) for 1H NMR, chloroform (δ = 77.2) for 13C NMR, acetonitrile (δ = 1.94) for 1H NMR, and acetonitrile (δ = 1.32) for 13C NMR (see Supplementary Materials). Data are reported as br = broad, s = singlet, d = doublet, t = triplet, q = quartet, p = quintet, m = multiplet. Coupling constants are given in Hz. Ambiguous assignments were resolved using standard one-dimensional proton decoupling experiments. Optical rotations were obtained using a Rudolph Autopol III digital polarimeter and JASCO P-2000. Optical rotation data are reported as follows: [α]20 (concentration c = g/100 mL, solvent). High-resolution mass spectra were recorded on a 4.7 Tesla IonSpec ESI-TOFMS, JEOL (JMS-700), and an AB Sciex 4800 Plus MALDI TOFTM, (2,5-dihydroxybenzoic acid (DHB) matrix was used to prepare samples for MS. Data were obtained in the reflector positive mode with internal standards for calibration).
3.2. General Procedure for the Synthesis of Pyrroles
To a stirred solution of 1a (100 mg, 0.38 mmol) in CH3CN (3 mL) was added trimethylsilyl azide (0.1 mL, 0.76 mmol) at 90 °C. After being stirred for 4 h, the mixture was concentrated under reduced pressure. The crude product was purified by column chromatography (EtOAc/hexane = 1:9) to afford pyrrole compound 2a.
(R)-2-(Azidomethyl)-1-(1-phenylethyl)-5-propyl-1H-pyrrole (2a)
Yellow liquid, (80 mg) 85% yield. The 1H NMR (400 MHz, CDCl3) δ 7.32 (ddd, J = 7.6, 5.0, 2.0 Hz, 3H), 7.07–7.02 (m, 2H), 6.17 (d, J = 3.5 Hz, 1H), 5.92 (d, J = 3.5 Hz, 1H), 5.53 (q, J = 7.2 Hz, 1H), 4.17 (d, J = 14.5 Hz, 1H), 3.93 (d, J = 14.4 Hz, 1H), 2.49–2.41 (m, 1H), 2.35–2.25 (m, 1H), 1.90 (d, J = 7.2 Hz, 3H), 1.61–1.50 (m, 2H), 0.88 (t, J = 7.3 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 141.9, 136.1, 128.5, 127.1, 125.9, 124.7, 110.9, 105.6, 52.6, 47.6, 29.6, 22.1, 19.6, 14.0. HRMS-ESI (m/z): [M + H]+ calcd. for C16H21N4, 269.6121, found 269.6128. Copies of 1H and 13C NMR could be found in Supplementary Materials.
2-(Azidomethyl)-1-benzyl-5-phenyl-1H-pyrrole (2b)
Yellow liquid, (90 mg) 80% yield. The 1H NMR (400 MHz, CDCl3) δ 7.28 (ddd, J = 10.9, 6.7, 3.5 Hz, 8H), 6.89 (d, J = 7.2 Hz, 2H), 6.33 (d, J = 3.6 Hz, 1H), 6.25 (d, J = 3.6 Hz, 1H), 5.21 (s, 2H), 4.15 (s, 2H). The 13C NMR (101 MHz, CDCl3) δ 138.6, 137.3, 133.0, 128.9, 128.8, 128.5, 127.4, 127.3, 127.0, 125.5, 111.3, 108.4, 47.7, 47.2. HRMS-ESI (m/z): [M + H]+ calcd. for C18H17N4, 289.1358, found 289.1362. Copies of 1H and 13C NMR could be found in Supplementary Materials.
2-(Azidomethyl)-1-benzyl-5-(2-methoxyphenyl)-1H-pyrrole (2e)
Yellow liquid, (93 mg) 78% yield. The 1H NMR (400 MHz, CDCl3) δ 7.39–7.03 (m, 5H), 7.00–6.78 (m, 4H), 6.34 (d, J = 3.5 Hz, 1H), 6.16 (d, J = 3.5 Hz, 1H), 5.03 (s, 2H), 4.14 (s, 2H), 3.65 (s, 3H). The 13C NMR (101 MHz, CDCl3) δ 157.4, 138.6, 133.6, 132.7, 129.6, 128.4, 127.0, 126.4, 126.0, 122.1, 120.6, 111.0, 110.8, 108.6, 55.3, 48.2. HRMS-ESI (m/z): [M + H]+ calcd. for C19H19N4O, 319.0446, found 319.0449. Copies of 1H and 13C NMR could be found in Supplementary Materials.
2-(Azidomethyl)-1-benzyl-5-(4-methoxyphenyl)-1H-pyrrole (2f)
Yellow liquid, (89 mg) 85% yield. The 1H NMR (400 MHz, CDCl3) δ 7.01 (ddd, J = 6.6, 5.2, 2.7 Hz, 5H), 6.67–6.65 (m, 2H), 6.62–6.59 (m, 2H), 6.09 (d, J = 3.5 Hz, 1H), 5.96 (d, J = 3.5 Hz, 1H), 4.95 (s, 2H), 3.89 (s, 2H), 3.54 (s, 3H). The 13C NMR (101 MHz, CDCl3) δ 159.1, 138.7, 137.0, 130.3, 128.8, 127.2, 126.4, 125.5, 125.5, 113.9, 111.1, 107.8, 55.3, 47.6, 47.3. HRMS-ESI (m/z): [M + H]+ calcd. for C19H19N4O, 319.1228, found 319.1230. Copies of 1H and 13C NMR could be found in Supplementary Materials.
2-(Azidomethyl)-1-benzyl-5-nonyl-1H-pyrrole (2g)
Yellow liquid, (85 mg) 82% yield. The 1H NMR (400 MHz, CDCl3) δ 7.37–7.28 (m, 3H), 6.92 (d, J = 7.0 Hz, 2H), 6.27 (d, J = 3.5 Hz, 1H), 6.01 (d, J = 3.5 Hz, 1H), 5.18 (s, 2H), 4.20 (s, 2H), 2.51 (dd, J = 13.6, 6.0 Hz, 2H), 1.67–1.59 (m, 2H), 1.38–1.29 (m, 12H), 0.94 (t, J = 6.9 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 138.3, 136.2, 128.7, 127.2, 125.5, 125.4, 125.0, 110.2, 105.3, 47.2, 46.9, 31.8, 29.3, 28.6, 26.5, 22.5, 13.8. HRMS-ESI (m/z): [M + H]+ calcd. for C21H31N4, 339.4618, found 339.4620. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-2-(Azidomethyl)-3-methyl-5-phenyl-1-(1-phenylethyl)-1H-pyrrole (2h)
Yellow liquid, (83 mg) 75% yield. The 1H NMR (400 MHz, CDCl3) δ 7.35–7.24 (m, 8H), 7.04–7.02 (m, 2H), 6.08 (s, 1H), 5.59 (q, J = 7.3 Hz, 1H), 4.23 (d, J = 14.7 Hz, 1H), 3.75 (d, J = 14.8 Hz, 1H), 2.16 (s, 3H), 1.88 (d, J = 7.2 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 142.4, 133.8, 129.4, 128.6, 128.4, 127.4, 127.1, 125.8, 122.6, 121.7, 110.3, 53.2, 45.1, 19.9, 11.3. HRMS-ESI (m/z): [M + H]+ calcd. for C20H21N4, 317.5973, found 317.5975. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-2-(Azidomethyl)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3-methyl-1-(1-phenylethyl)-1H-pyrrole (2i)
Yellow liquid, (91 mg) 72% yield. The 1H NMR (400 MHz, CDCl3) δ 7.34–7.26 (m, 3H), 7.18–7.14 (m, 2H), 5.97 (s, 1H), 5.72 (q, J = 7.2 Hz, 1H), 4.53 (s, 2H), 4.22 (d, J = 14.7 Hz, 1H), 3.81 (d, J = 14.7 Hz, 1H), 2.12 (s, 3H), 1.94 (d, J = 7.2 Hz, 3H), 0.88 (s, 9H), 0.05 (d, J = 7.1 Hz, 6H). The 13C NMR (101 MHz, CDCl3) δ 142.0, 133.0, 128.4, 127.1, 126.2, 122.6, 119.9, 109.7, 57.8, 53.1, 44.6, 25.8, 19.6, 18.2, 11.2, −5.2. HRMS-ESI (m/z): [M + Na]+ calcd. for C21H32N4NaOSi, 407.8471, found 407.8474. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-2-(Azidomethyl)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3-(4-methoxyphenyl)-1-(1-phenylethyl)-1H-pyrrole (2j)
Yellow liquid, (87 mg) 70% yield. The 1H NMR (400 MHz, CDCl3) δ 7.40–7.34 (m, 4H), 7.32–7.29 (m, 1H), 7.22 (dd, J = 5.1, 4.2 Hz, 2H), 6.97 (d, J = 8.8 Hz, 2H), 6.25 (s, 1H), 5.82 (q, J = 7.1 Hz, 1H), 4.55 (s, 2H), 4.34 (d, J = 14.6 Hz, 1H), 4.05 (d, J = 14.6 Hz, 1H), 3.87 (s, 3H), 2.04 (d, J = 7.2 Hz, 3H), 0.91 (s, 9H), 0.08 (d, J = 3.9 Hz, 6H). The 13C NMR (101 MHz, CDCl3) δ 158.2, 141.6, 133.7, 129.6, 128.6, 128.5, 127.3, 126.4, 126.3, 122.5, 113.9, 109.1, 57.9, 55.3, 53.6, 45.4, 25.9, 19.6, 18.2, −5.2. HRMS-ESI (m/z): [M + H]+ calcd. for C27H37N4O2Si, 477.0417, found 477.0419. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-5-(((5-(((tert-Butyldimethylsilyl)oxy)methyl)-3-methyl-1-(1-phenylethyl)-1H-pyrrol-2-yl)methyl)thio)-1-phenyl-1H-tetrazole (2k)
Yellow liquid, (107 mg) 82% yield. The 1H NMR (400 MHz, CDCl3) δ 7.83–7.80 (m, 2H), 7.56–7.48 (m, 3H), 7.21 (t, J = 7.6 Hz, 2H), 7.11–7.03 (m, 3H), 6.08 (s, 1H), 5.85 (q, J = 7.1 Hz, 1H), 5.28 (s, 2H), 4.53 (s, 2H), 2.19 (s, 3H), 1.93 (d, J = 7.2 Hz, 3H), 0.88 (s, 9H), 0.06 (d, J = 2.8 Hz, 6H). The 13C NMR (101 MHz, CDCl3) δ 162.1, 141.3, 134.7, 133.6, 129.4, 129.1, 128.2, 126.8, 126.1, 123.6, 121.4, 120.3, 110.5, 58.0, 53.5, 42.6, 25.9, 19.9, 18.3, 11.5, −5.2. HRMS-ESI (m/z): [M + H]+ calcd. for C28H38N5OSSi, 520.4336, found 520.4340. Copies of 1H and 13C NMR could be found in Supplementary Materials.
5-(((1-Benzyl-5-nonyl-1H-pyrrol-2-yl)methyl)thio)-1-phenyl-1H-tetrazole (2l)
Yellow liquid, (115 mg) 81% yield. The 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 8.2, 1.5 Hz, 2H), 7.51–7.43 (m, 3H), 7.17 (t, J = 7.4 Hz, 2H), 7.11 (d, J = 7.3 Hz, 1H), 6.67 (d, J = 7.5 Hz, 2H), 6.52 (d, J = 3.5 Hz, 1H), 6.03 (d, J = 3.5 Hz, 1H), 5.44 (s, 2H), 5.32 (s, 2H), 2.44–2.39 (m, 2H), 1.57 (dd, J = 15.0, 7.4 Hz, 2H), 1.30–1.22 (m, 12H), 0.87 (t, J = 6.8 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 162.4, 137.9, 136.5, 134.5, 129.4, 128.9, 128.4, 127.0, 124.9, 123.8, 123.4, 111.9, 105.7, 46.9, 43.6, 31.8, 29.5, 29.4, 29.3, 28.5, 26.4, 22.6, 14.1. HRMS-ESI (m/z): [M + H]+ calcd. for C28H36N5S, 474.3226, found 474.3228. Copies of 1H and 13C NMR could be found in Supplementary Materials.
1-Benzyl-2-(((4-methoxybenzyl)thio)methyl)-5-phenyl-1H-pyrrole (2m)
Yellow liquid, (105 mg) 75% yield. The 1H NMR (400 MHz, CDCl3) δ 7.32–7.29 (m, 4H), 7.25–7.15 (m, 6H), 6.86–6.79 (m, 4H), 6.22 (d, J = 3.5 Hz, 1H), 6.17 (d, J = 3.5 Hz, 1H), 5.25 (s, 2H), 3.79 (s, 3H), 3.62 (s, 2H), 3.44 (s, 2H). The 13C NMR (101 MHz, CDCl3) δ 158.5, 138.9, 136.2, 133.4, 130.3, 130.0, 128.8, 128.6, 128.3, 126.9, 125.6, 113.8, 110.0, 108.0, 55.3, 47.4, 34.9, 27.5. HRMS-ESI (m/z): [M + Na]+ calcd. for C26H25NNaOS, 422.5371, found 422.5375. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-5-(methoxymethyl)-4-methyl-1-(1-phenylethyl)-1H-pyrrole-2-carbaldehyde (4a)
To a stirred solution of secondary alcohol 3 (200 mg, 0.527 mmol) was dissolved in CH2Cl2 (6 mL) under N2 at 0 °C and Dess–Martin periodinane (335 mg, 0.791 mmol) was added to the reaction mixture and allowed to stir for 2 h. Ether was added to the reaction mixture and the solid was filtered. The filtrate was washed with saturated NaHCO3 solution, dried over anhydrous Na2SO4, and solvents were removed under vacuum to obtain a crude product, which was used for the next reaction without further purification.
To a stirred solution of above crude ketone compound was dissolved in MeOH (3 mL) under N2 at 0 °C and ZnCl2 (86 mg, 0.632 mmol) was added to the reaction mixture and allowed to stir for 2 h. After 2 h, the reaction mixture was diluted with CH2Cl2 (10 mL), quenched with water, and extracted with CH2Cl2 (2 × 10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain a crude product, which was purified by silica gel column chromatography (EtOAc/hexane, 1:9) to obtain pyrrole compound 4a (102 mg, 75% yield) as a yellow liquid. The 1H NMR (400 MHz, CDCl3) δ 9.41 (s, 1H), 7.32–7.26 (m, 3H), 7.13 (d, J = 8.1 Hz, 2H), 6.80 (s, 1H), 6.56 (s, 1H), 4.20 (d, J = 12.4 Hz, 1H), 4.07 (d, J = 12.4 Hz, 1H), 3.21 (s, 3H), 2.12 (s, 3H), 1.91 (d, J = 7.1 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 179.1, 141.5, 136.3, 131.4, 128.3, 127.0, 126.1, 125.1, 121.7, 63.5, 57.7, 53.9, 19.4, 11.1. HRMS-ESI (m/z): [M + H]+ calcd. for C16H20NO2, 258.2714, found 258.2718. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-5-(Chloromethyl)-4-methyl-1-(1-phenylethyl)-1H-pyrrole-2-carbaldehyde (4b)
Yellow liquid, (92 mg) 70% yield. The 1H NMR (400 MHz, CDCl3) δ 9.50 (s, 1H), 7.37–7.29 (m, 3H), 7.16–7.13 (m, 2H), 6.82 (s, 1H), 6.77 (s, 1H), 4.43 (d, J = 12.8 Hz, 1H), 4.27 (d, J = 12.9 Hz, 1H), 2.15 (s, 3H), 2.00 (d, J = 7.2 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 179.6, 141.1, 135.3, 131.6, 128.6, 128.4, 127.3, 125.9, 125.3, 53.8, 35.4, 19.4, 10.8. HRMS-ESI (m/z): [M + H]+ calcd. for C15H17ClNO, 262.1479, found 262.1483. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-(5-Formyl-3-methyl-1-(1-phenylethyl)-1H-pyrrol-2-yl)methyl acetate (4c)
Yellow liquid, (98 mg) 78% yield. The 1H NMR (400 MHz, CDCl3) δ 9.47 (s, 1H), 7.32–7.26 (m, 3H), 7.11 (d, J = 8.0 Hz, 2H), 6.81 (s, 1H), 6.65 (s, 1H), 4.96 (d, J = 13.4 Hz, 1H), 4.69 (d, J = 13.4 Hz, 1H), 2.11 (s, 3H), 1.91 (t, J = 3.5 Hz, 6H). The 13C NMR (101 MHz, CDCl3) δ 179.5, 170.3, 141.0, 134.0, 131.8, 128.4, 127.2, 126.0, 125.4, 55.6, 53.9, 20.6, 19.4, 10.9. HRMS-ESI (m/z): [M + H]+ calcd. for C17H20NO3, 286.6442, found 286.6446. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(R)-2-(5-Formyl-3-methyl-1-(1-phenylethyl)-1H-pyrrol-2-yl)acetonitrile (4d)
Yellow liquid, (93 mg) 65% yield. The 1H NMR (400 MHz, CDCl3) δ 9.47 (s, 1H), 7.32–7.27 (m, 3H), 7.12–7.09 (m, 2H), 6.81 (s, 1H), 6.65 (s, 1H), 4.96 (d, J = 13.4 Hz, 1H), 4.69 (d, J = 13.3 Hz, 1H), 2.11 (s, 3H), 1.90 (d, J = 2.2 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 179.4, 141.0, 133.9, 131.8, 128.5, 128.4, 127.2, 126.0, 125.9, 125.3, 55.6, 53.9, 20.6, 19.4, 10.9. HRMS-ESI (m/z): [M + H]+ calcd. for C16H17N2O, 253.4441, found 253.4446. Copies of 1H and 13C NMR could be found in Supplementary Materials.
Ethyl 1-phenethylaziridine-2-carboxylate (11)
To a stirred solution of ethyl 2,3-dibromopropanoate (5.0 g, 19.30 mmol, 1.0 equiv) dissolved in acetonitrile (60 mL), were added potassium carbonate (8.0 g, 57.9 mmol, 3.0 equiv) followed by 2-phenylethanamine (2.9 mL, 23.16 mmol, 1.2 equiv) in dropwise manner at room temperature and reaction mixture were allowed to stir for 12 h. After completion, quenched with water (25 mL) and filtered out over filter paper (pore size 8–10 µm). The organic mixture was extracted with Et2O (2 × 30 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain a crude mixture of Ethyl 1-phenethylaziridine-2-carboxylate 11 as a yellow liquid (3.8 g, 89%). The 1H NMR (400 MHz, CDCl3) δ 7.27 (ddd, J = 7.4, 3.1, 1.3 Hz, 2H), 7.22–7.16 (m, 3H), 4.24–4.11 (m, 2H), 2.93 (dd, J = 15.1, 6.9 Hz, 2H), 2.65–2.49 (m, 2H), 2.14 (dd, J = 3.1, 1.2 Hz, 1H), 1.94 (dd, J = 6.5, 3.1 Hz, 1H), 1.52 (dd, J = 6.5, 1.1 Hz, 1H), 1.27 (t, J = 7.1 Hz, 3H). The 13C NMR (101 MHz, CDCl3) δ 170.7, 139.3, 128.7, 128.3, 126.1, 62.3, 61.0, 37.5, 36.0, 34.3, 14.1. HRMS-ESI (m/z): [M + H]+ calcd. for C13H18NO2, 220.6121, found 220.6128. Copies of 1H and 13C NMR could be found in Supplementary Materials.
N-Methoxy-N-methyl-1-phenethylaziridine-2-carboxamide (12)
To a stirred solution of ester 11 (3.8 g, 17.35 mmol) and N,O-dimethylhydroxylamine hydrochloride (2.53 g, 26.0 mmol) in dry THF (50 mL) at 0 °C was slowly added i-PrMgCl (26.0 mL, 2.0 M in THF, 52.05 mmol), and the reaction mixture was stirred for 1 h. The reaction mixture was quenched with saturated NH4Cl solution and extracted with EtOAc (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo to obtain the crude product, which was purified by silica gel column chromatography (EtOAc/hexanes, 1:1) to afford Weinreb amide 12 as a yellow color oil (3.2 g, 78.8%) yield. The 1H NMR (400 MHz, CDCl3) δ 7.29–7.25 (m, 2H), 7.20 (d, J = 7.2 Hz, 3H), 3.68 (s, 3H), 3.21 (s, 3H), 2.99–2.88 (m, 2H), 2.71 (ddd, J = 11.4, 8.7, 6.6 Hz, 1H), 2.56–2.42 (m, 2H), 2.17 (dd, J = 3.2, 1.3 Hz, 1H), 1.51 (dd, J = 6.5, 1.2 Hz, 1H). The 13C NMR (101 MHz, CDCl3) δ 170.3, 139.6, 128.7, 128.3, 126.1, 62.7, 61.6, 36.1, 35.3, 34.0, 32.5. HRMS-ESI (m/z): [M + H]+ calcd. for C13H19N2O2, 235.0336, found 234.0340. Copies of 1H and 13C NMR could be found in Supplementary Materials.
1-(1-Phenethylaziridin-2-yl)but-3-en-1-ol (13)
To a stirred solution of Weinreb amide 12 (3.2 g, 13.67 mmol) was slowly added allylMgBr (8.2 mL, 2.0 M in THF, 16.4 mmol) in dry THF (40 mL) at 0 °C, and the reaction mixture was stirred for 1 h. The reaction mixture was quenched with saturated NH4Cl solution and extracted with EtOAc (2 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo to obtain the crude allyl product, which was used for the next reaction without further purification.
To a stirred solution of above keto compound (3.2 g, 14.86 mmol) was slowly added NaBH4 (0.45 g, 11.88 mmol) in MeOH (40 mL) at 0 °C, and the reaction mixture was stirred for 1 h. Then, MeOH was removed under vacuum and extracted with CH2Cl2 (2 × 10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude allyl alcohol product, which was purified by column chromatography (EtOAc/hexanes, 2:8) to give pure 1-(1-phenethylaziridin-2-yl)but-3-en-1-ol (13) as a yellow liquid (2.6 g, 87%) yield. The 1H NMR (400 MHz, CDCl3) δ 7.33–7.16 (m, 5H), 5.84 (ddt, J = 17.2, 10.2, 7.1 Hz, 1H), 5.16–5.06 (m, 2H), 3.66 (td, J = 6.3, 3.8 Hz, 1H), 2.85 (t, J = 7.4 Hz, 2H), 2.67 (dt, J = 11.6, 7.3 Hz, 1H), 2.52–2.43 (m, 1H), 2.24 (t, J = 6.7 Hz, 2H), 1.80 (d, J = 3.6 Hz, 1H), 1.49 (dt, J = 7.0, 3.7 Hz, 1H), 1.23 (d, J = 6.4 Hz, 1H). The 13C NMR (101 MHz, CDCl3) δ 139.7, 134.3, 128.7, 128.3, 126.1, 117.4, 67.9, 61.7, 42.2, 39.3, 36.3, 29.3. HRMS-ESI (m/z): [M + H]+ calcd. for C14H20NO, 218.0231, found 218.0234. Copies of 1H and 13C NMR could be found in Supplementary Materials.
2-(1-((tert-Butyldimethylsilyl)oxy)but-3-en-1-yl)-1-phenethylaziridine (14)
To a stirred solution of allyl alcohol 13 (2.5 g, 11.50 mmol) in dry CH2Cl2 (30 mL) was added imidazole (1.5 g, 23.0 mmol) and TBSCl (1.9 g, 12.65 mmol), sequentially, at 0 °C under an N2 atmosphere. After 4 h of being stirred at rt, the reaction mixture was quenched with saturated aqueous NH4Cl (10 mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude product, which was purified by column chromatography (EtOAc/hexanes, 2:8) to give pure 2-(1-((tert-butyldimethylsilyl)oxy)but-3-en-1-yl)-1-phenethylaziridine 14 as a yellow liquid (2.8 g, 73%) yield. The 1H NMR (400 MHz, CDCl3) δ 7.30–7.24 (m, 2H), 7.19 (dd, J = 7.1, 5.2 Hz, 3H), 5.91 (ddt, J = 17.1, 10.2, 7.1 Hz, 1H), 5.13–5.04 (m, 2H), 3.20 (td, J = 7.0, 4.4 Hz, 1H), 2.86 (t, J = 8.0 Hz, 2H), 2.55 (dt, J = 11.5, 7.7 Hz, 1H), 2.48–2.33 (m, 3H), 1.69 (d, J = 3.4 Hz, 1H), 1.45 (ddd, J = 7.6, 6.4, 3.4 Hz, 1H), 1.29 (d, J = 6.3 Hz, 1H), 0.88 (s, 9H), 0.02 (d, J = 2.1 Hz, 6H). The 13C NMR (101 MHz, CDCl3) δ 139.8, 135.0, 128.6, 128.3, 126.0, 116.9, 74.6, 62.7, 43.6, 40.9, 36.3, 33.9, 25.8, 18.1, −4.1, −4.6. HRMS-ESI (m/z): [M + H]+ calcd. for C20H34NOSi, 332.1222, found 332.1224. Copies of 1H and 13C NMR could be found in Supplementary Materials.
Octamethyl-8-(1-phenethylaziridin-2-yl)-4,9-dioxa-3,10-disiladodecan-6-one (15)
To a stirred solution of 2-(1-((tert-butyldimethylsilyl)oxy)but-3-en-1-yl)-1-phenethylaziridine 14 (2.5 g, 7.5 mmol) and N-Methylmorpholine N-oxide (2.64 g, 22.61 mmol) in acetone: H2O (4:1) (20 mL) at room temperature was slowly added OsO4 (3.2 mL, 0.75 mmol), and the reaction mixture was stirred for 6 h. The reaction mixture was quenched with saturated NH2SO3 solution and extracted with EtOAc (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo to obtain the crude dihydroxy product, which was used for the next reaction without further purification.
To a stirred solution of dihydroxy alcohol (2.5 g, 6.8 mmol) in dry CH2Cl2 (30 mL) was added imidazole (0.93 g, 13.67 mmol) and TBSCl (1.13 g, 7.5 mmol), sequentially, at 0 °C under an N2 atmosphere. After 2 h of being stirred at rt, the reaction mixture was quenched with saturated aqueous NH4Cl (10 mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude product, which was used for the next reaction without further purification.
To a solution of oxalyl chloride (0.67 mL, 7.81 mmol) in CH2Cl2 (20 mL) at −78 °C was added dimethyl sulfoxide (1.1 mL, 15.63 mmol) over 15 min. The resulting mixture was stirred for another 45 min and then a solution of alcohol (2.5 g, 5.21 mmol) in CH2Cl2 (20 mL) was added dropwise. The resulting white suspension was stirred for 2h before adding triethylamine (2.18 mL, 15.63 mmol). The reaction mixture was stirred for 30 min at −78 °C and then warmed to 0 °C and allowed to stir for 15 min. The reaction mixture was quenched with water (20 mL) and the aqueous phase was extracted with CH2Cl2 (2 × 20 mL). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to obtain a crude, which was purified by column chromatography (EtOAc/hexanes, 2:8) to give pure Octamethyl-8-(1-phenethylaziridin-2-yl)-4,9-dioxa-3,10-disiladodecan-6-one 15 as a yellow liquid (2.1 g, 62%) yield. The 1H NMR (400 MHz, CDCl3) δ 7.27–7.25 (m, 2H), 7.18 (t, J = 7.6 Hz, 3H), 4.17 (s, 2H), 4.00–3.90 (m, 1H), 2.82 (t, J = 6.9 Hz, 2H), 2.65–2.59 (m, 2H), 2.59–2.52 (m, 1H), 2.35–2.28 (m, 1H), 1.66 (d, J = 2.5 Hz, 1H), 1.54 (dd, J = 9.0, 6.4 Hz, 1H), 1.18 (d, J = 6.0 Hz, 1H), 0.92 (s, 9H), 0.87 (s, 9H), 0.10 (s, 6H), 0.08 (s, 6H). The 13C NMR (101 MHz, CDCl3) δ 208.1, 139.9, 128.6, 128.3, 126.0, 70.1, 70.0, 62.9, 43.9, 43.8, 36.3, 31.1, 25.8, 25.8, 18.3, 18.0, −3.5, −4.3, −4.9, −5.4. HRMS-ESI (m/z): [M + H]+ calcd. for C26H47NO3Si2, 448.4378, found 448.4382. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(5-(((tert-Butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrol-2-yl)methyl acetate (16)
To a stirred solution of Octamethyl-8-(1-phenethylaziridin-2-yl)-4,9-dioxa-3,10-disiladodecan-6-one 15 (1.5 g, 3.13 mmol) in dry CH2Cl2 (30 mL) was added acetic acid (0.56 mL, 6.27 mmol) at 0 °C under an N2 atmosphere. After 6 h stirred at 0 °C, the reaction mixture was quenched with saturated aqueous NH2CO3 (10 mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude product, which was purified by column chromatography (EtOAc/hexanes, 2:8) to give pure (5-(((tert-butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrol-2-yl)methyl acetate 16 as a yellow liquid (1.0 g, 82%) yield. The 1H NMR (400 MHz, CDCl3) δ 7.30 (dd, J = 7.9, 6.4 Hz, 2H), 7.23 (d, J = 7.4 Hz, 1H), 7.14–7.11 (m, 2H), 6.15 (d, J = 3.5 Hz, 1H), 6.00 (d, J = 3.5 Hz, 1H), 4.96 (s, 2H), 4.53 (s, 2H), 4.17 (t, J = 6.5 Hz, 2H), 3.06 (t, J = 6.2 Hz, 2H), 2.06 (s, 3H), 0.89 (s, 9H), 0.05 (s, 6H). The 13C NMR (101 MHz, CDCl3) δ 170.7, 138.6, 133.5, 128.8, 128.6, 127.3, 126.6, 110.4, 108.0, 57.9, 57.6, 45.8, 38.0, 25.9, 21.1, 18.3, −5.2. HRMS-ESI (m/z): [M + Na]+ calcd. for C22H33NNaO3Si, 410.6150, found 410.6158. Copies of 1H and 13C NMR could be found in Supplementary Materials.
(5-(((tert-Butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrol-2-yl)methanol (17)
To a stirred solution of (5-(((tert-butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrol-2-yl)methyl acetate 16 (0.7 g, 1.80 mmol) in MeOH (10 mL) was added potassium carbonate (0.249 g, 1.80 mmol) at 0 °C, and the mixture was stirred for 1 h at rt. Then, MeOH was removed under vacuum and extracted with CH2Cl2 (2 × 10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude product, which was purified by column chromatography (EtOAc/hexanes, 4:6) to give pure (5-(((tert-butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrol-2-yl)methanol (17) as a yellow liquid (0.5 g, 80% yield). The 1H NMR (400 MHz, CDCl3) δ 7.32–7.20 (m, 3H), 7.15–7.10 (m, 2H), 6.01 (d, J = 3.5 Hz, 1H), 5.97 (d, J = 3.5 Hz, 1H), 4.55 (s, 2H), 4.42 (s, 2H), 4.24 (t, J = 6.5 Hz, 2H), 3.10 (t, J = 6.2 Hz, 2H), 0.90 (s, 9H), 0.06 (s, 6H). The 13C NMR (101 MHz, CDCl3) δ 138.9, 133.1, 132.7, 128.9, 128.5, 126.5, 107.8, 107.7, 57.6, 56.9, 45.7, 38.0, 25.9, 18.3, −5.2. HRMS-ESI (m/z): [M + H]+ calcd. for C20H32NO2Si, 346.5226, found 346.5231.
5-(((tert-Butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrole-2-carbaldehyde (18)
To a stirred solution of alcohol 17 (0.5 g, 1.29 mmol) in dry CH2Cl2 (4 mL) was added Dess–Martin periodinane (0.820 g, 1.93 mmol) at 0 °C, and the mixture was stirred for 1 h at rt. Then, the reaction mixture was quenched with a 1:1 mixture of saturated solution of NaHCO3 (10 mL) and extracted with CH2Cl2 (2 × 10 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude product, which was purified by column chromatography (EtOAc/hexanes, 2:8) to give pure aldehyde 18 as a yellow liquid (330 mg, 74% yield). The 1H NMR (400 MHz, CDCl3) δ 9.55 (s, 1H), 7.27 (dd, J = 5.2, 2.1 Hz, 3H), 7.16–7.13 (m, 2H), 6.90 (d, J = 4.0 Hz, 1H), 6.11 (d, J = 4.0 Hz, 1H), 4.53 (t, J = 6.5 Hz, 2H), 4.31 (s, 2H), 3.04 (t, J = 6.2 Hz, 2H), 0.89 (s, 9H), 0.04 (s, 6H). The 13C NMR (101 MHz, CDCl3) δ 179.2, 142.2, 138.6, 132.0, 129.0, 128.4, 126.5, 124.5, 109.6, 57.0, 47.6, 37.6, 25.8, 18.2, −5.3. HRMS-ESI (m/z): [M + H]+ calcd. for C20H30NO2Si, 344.2264, found 344.2269. Copies of 1H and 13C NMR could be found in Supplementary Materials.
5-(Hydroxymethyl)-1-phenethyl-1H-pyrrole-2-carbaldehyde (19)
To a stirred solution of 5-(((tert-butyldimethylsilyl)oxy)methyl)-1-phenethyl-1H-pyrrole-2-carbaldehyde (18) (0.3 g, 0.87 mmol) in dry THF (10 mL) was added TBAF (0.94 mL, 1.0 M in THF, 0.96 mmol) at 0 °C and stirred for 1 h. After completion of the reaction was quenched with saturated aqueous NH2CO3 (10 mL). The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (2 × 20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo to obtain the crude product, which was purified by column chromatography (EtOAc/hexanes, 3:7) to give pure 5-(hydroxymethyl)-1-phenethyl-1H-pyrrole-2-carbaldehyde 19 as a yellow oil (168 mg, 84% yield). The 1H NMR (400 MHz, CDCl3) δ 9.58 (s, 1H), 7.27–7.21 (m, 3H), 7.10 (d, J = 6.5 Hz, 2H), 6.93 (d, J = 4.0 Hz, 1H), 6.17 (d, J = 4.0 Hz, 1H), 4.55 (t, J = 7.2 Hz, 2H), 4.29 (s, 2H), 3.05 (t, J = 7.2 Hz, 2H). The 13C NMR (101 MHz, CDCl3) δ 179.4, 141.7, 138.5, 132.2, 129.0, 128.6, 126.7, 124.6, 110.0, 56.3, 47.6, 37.7. HRMS-ESI (m/z): [M + H]+ calcd. for C14H16NO2, 230.1178, found 230.1185. Copies of 1H and 13C NMR could be found in Supplementary Materials.
4. Conclusions
In summary, multi-substituted pyrroles were synthesized from regiospecific aziridine ring opening and subsequently intramolecular cyclization with a carbonyl group at the γ-position in the presence of Lewis acid (TMSN3 or ZnCl2) or protic acid (AcOH). This method is high atom economical in that all reactants are incorporated into the final product with the removal of water. This new protocol can be applied to the synthesis of various pyrroles, including natural products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27206869/s1; All analytical data of compounds other than the representative example are reported along the copies of 1H and 13C NMR spectra.
Author Contributions
Conceptualization, H.-J.H. and L.M.; methodology, R.J. and S.-Y.N.; investigation, H.-J.H., L.M. and R.J.; experiments and data curation, L.M. and R.J.; writing—original draft preparation, H.-J.H.; writing—review and editing, H.-J.H. and L.M.; supervision, H.-J.H.; project administration, H.-J.H.; funding acquisition, H.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF-2012M3A7B4049645 and 2021R1A5A6002803 with the Centre for New Directions in Organic Synthesis). Additional financial support from 2020R1A2C1007102 and HUFS Grant 2022 (for the preparation of this manuscript) are also appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF-2012M3A7B4049645 and 2014R1A5A1011165 with the Centre for New Directions in Organic Synthesis). Additional financial support from HUFS Grant 2018 is also appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Gholap, S.S. Pyrrole: An emerging scaffold for construction of valuable therapeutic agents. Eur. J. Med. Chem. 2016, 110, 13–31. [Google Scholar] [CrossRef]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Multicomponent reactions for the synthesis of pyrroles. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Bai, H.-B.; Shan, W.-G.; Zhan, Z.-J. A new Alkaloid from the Mycelium of Inonotus Obliquus. J. Chem. Res. 2014, 38, 245–246. [Google Scholar] [CrossRef]
- Wood, J.M.; Furkert, D.P.; Brimble, M.A. 2-Formylpyrrole natural products: Origin, structural diversity, bioactivity and synthesis. Nat. Prod. Rep. 2019, 36, 289–306. [Google Scholar] [CrossRef]
- Mal, D.; Shome, B.; Dinda, B.K. Pyrrole and Its Derivatives. In Heterocycles in Natural Product Synthesis; Majumdar, K.C., Chattopadhyay, S.K., Eds.; Wiley: Hoboken, NJ, USA, 2011; Chapter 6; p. 187. [Google Scholar]
- Park, Y.M.; Won, J.H.; Kim, Y.H.; Choi, J.W.; Park, H.J.; Lee, K.L. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J. Ethnopharmacol. 2005, 101, 120–128. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, Y.S.; Jang, Y.W.; Jung, J.Y.; Yun, B.S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg Med Chem Lett. 2007, 17, 6678–6681. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skora, B.; Pomianek, T.; Gminski, J. Inonotus obliquus - from folk medicine to clinical use. J. Tradit. Complement. Med. 2021, 11, 293–302. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Wang, Y.; Miao, K.; Wei, Z. Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Bioresour. Technol. 2009, 100, 1327–1335. [Google Scholar] [CrossRef]
- Cui, Y.; Kim, D.-S.; Park, K.-C. Antioxidant effect of Inonotus obliquus. J. Ethnopharmacol. 2005, 96, 79–85. [Google Scholar] [CrossRef]
- Kim, Y.O.; Han, S.B.; Lee, H.W.; Ahn, H.J.; Yoon, Y.D.; Jung, J.K.; Shin, C.S. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005, 77, 2438–2456. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Jun, B.-S.; Yoo, K.-S.; Hahm, J.-R.; Cho, Y.-S. Fermented Chaga Mushroom (Inonotus obliquus) Effects on Hypolipidemia and Hepatoprotection in Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Food Sci. Biotechnol. 2006, 15, 122–127. [Google Scholar]
- Paal, C. Synthese von Thiophen-und Pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1885, 18, 367. [Google Scholar] [CrossRef]
- Knorr, L. Synthesis of pyrroline-derivatives II. Chem. Ber. 1884, 17, 1635. [Google Scholar] [CrossRef]
- Hantzsch, A. Neue Bildungsweise von Pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1890, 23, 1474–1476. [Google Scholar] [CrossRef]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Dong, K.; Humeidi, A.; Griffith, W.; Arman, H.; Xu, X. AgI-Catalyzed Reaction of Enol Diazoacetates and Imino Ethers: Synthesis of Highly Functionalized Pyrroles. Angew. Chem., Int. Ed. 2021, 60, 13394–13400. [Google Scholar]
- Stankovic, S.; D’hooghe, M.; Catak, S.; Eum, H.; Waroquier, M.; Van Speybroeck, V.; De Kimpe, N.; Ha, H.-J. Regioselectivity in the ring-opening of non-activated aziridines. Chem. Soc. Rev. 2012, 41, 643–665. [Google Scholar] [CrossRef]
- Choi, J.; Yu, T.; Ha, H.-J. Alkylative Aziridine Ring-Opening Reactions. Molecules 2021, 26, 1703. [Google Scholar] [CrossRef]
- Ranjith, J.; Ha, H.-J. Synthetic Applications of Aziridinium Ions. Molecules 2021, 26, 1774. [Google Scholar] [CrossRef]
- Chen, J.R.; Hu, X.Q.; Lu, L.Q.; Xiao, W.J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef]
- Estevez, V.; Villacampa, M.; Menendez, J.C. Recent advances in the synthesis of pyrroles by multicomponent reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.N.; Jeong, H.; Ha, H.-J. Atom-Economical and Metal-Free Synthesis of Multisubstituted Furans from Intramolecular Aziridine Ring Opening. ACS Omega 2017, 2, 7525–7531. [Google Scholar] [CrossRef] [PubMed]
- Macha, L.; D’hooghe, M.; Ha, H.J. Deployment of Aziridines for the Synthesis of Alkaloids and Their Derivatives. Synthesis 2019, 51, 1491–1515. [Google Scholar]
- Kim, J.H.; Lee, S.B.; Lee, W.K.; Yoo, D.-H.; Ha, H.-J. Synthesis of 1,2,5- and 1,2,3,5-substituted pyrroles from substituted aziridines via Ag(I)-catalyzed intramolecular cyclization. Tetrahedron 2011, 67, 3553–3558. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.H.; Lee, W.K.; Jung, J.-H.; Ha, H.-J. Synthesis of 2,5-Disubstituted 6-Azaindoles from Substituted Aziridines via Intramolecular Cyclization. Org. Lett. 2012, 14, 3120–3122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
