Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast
Abstract
1. Introduction
2. Results and Discussion
2.1. Process Optimization of Extracting Protein
2.1.1. The Content of Crude Protein in WBY
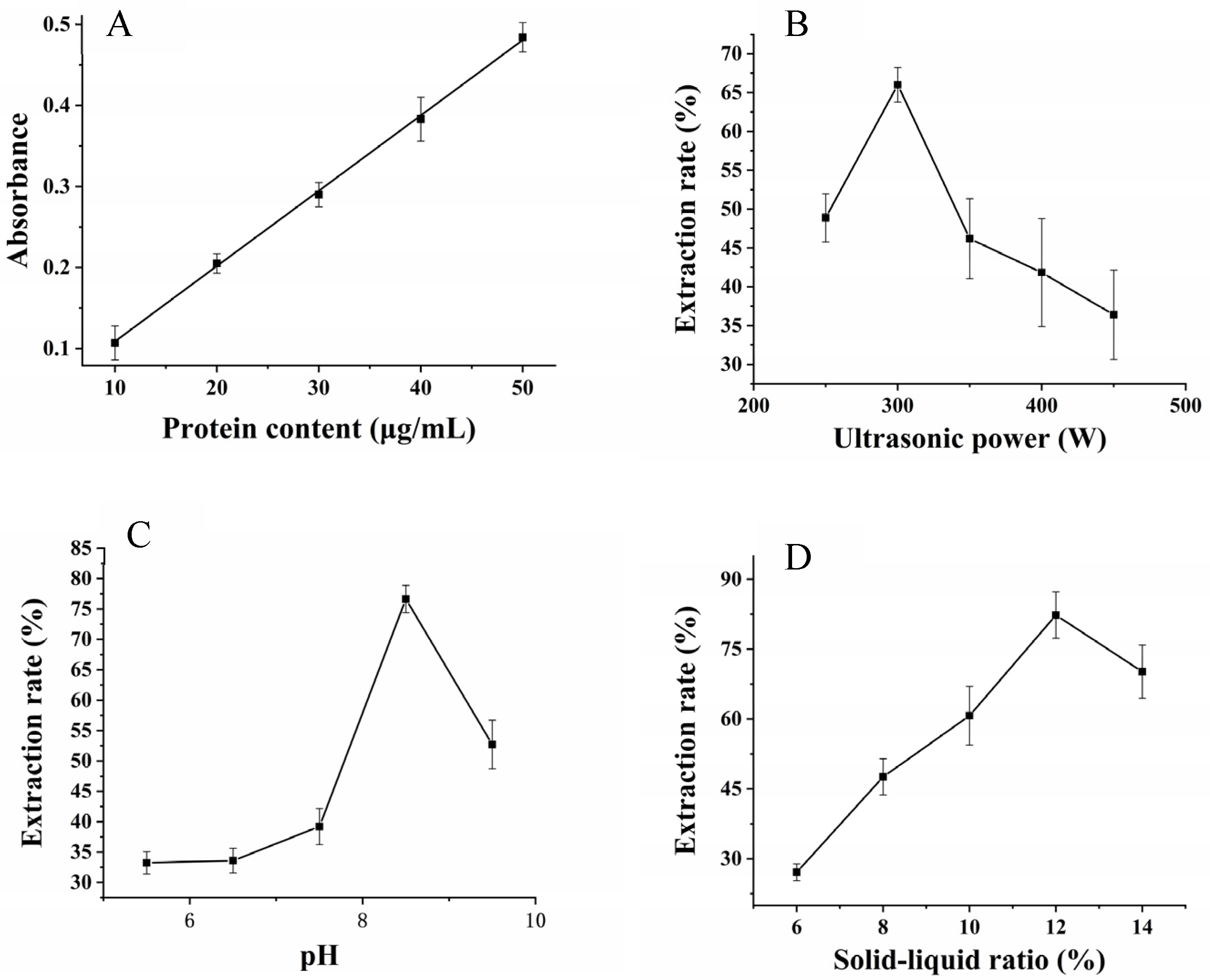
2.1.2. Single-Factor Experiments
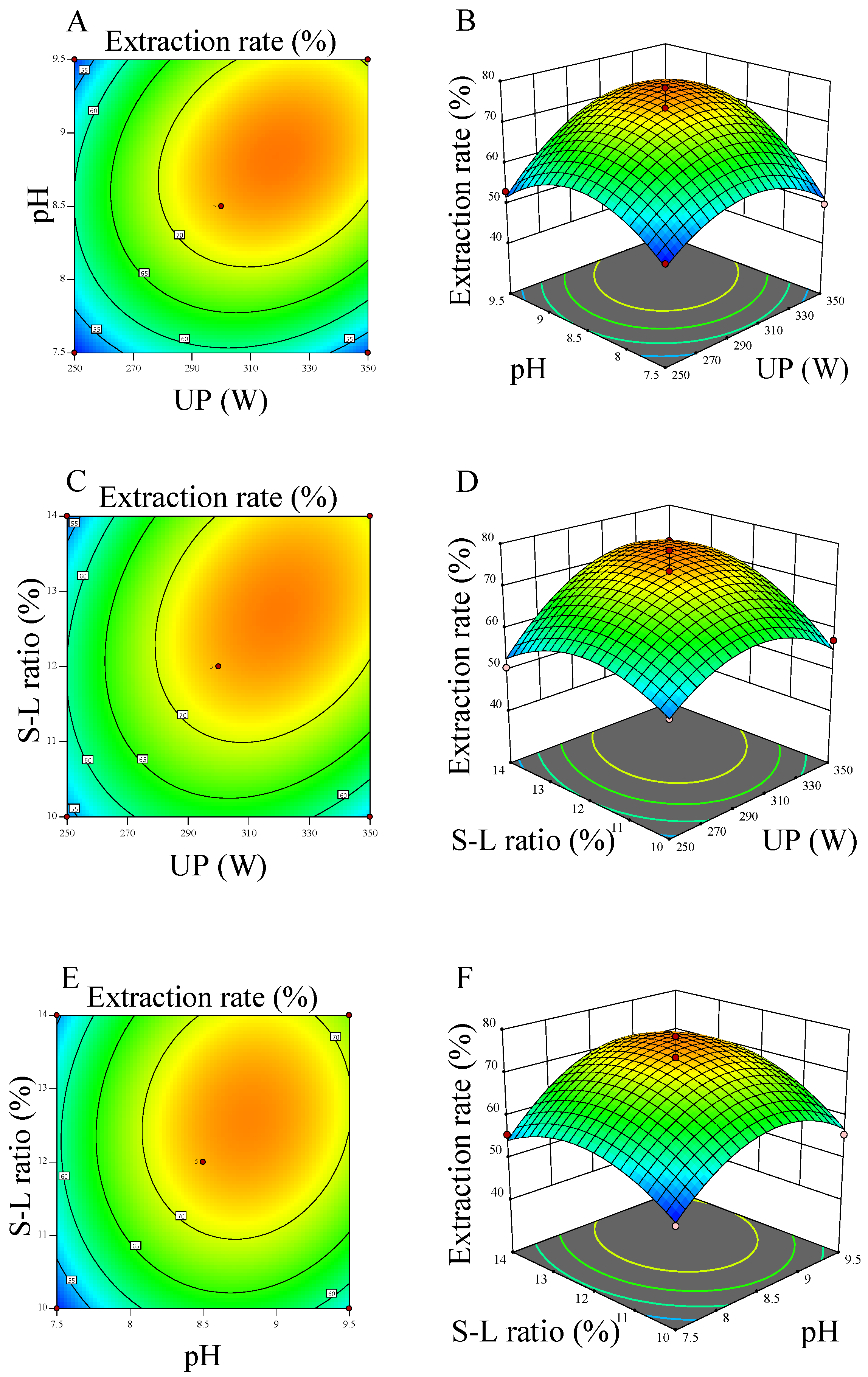
2.1.3. RSM Experiments
2.2. Process Optimization of Preparing Polypeptide
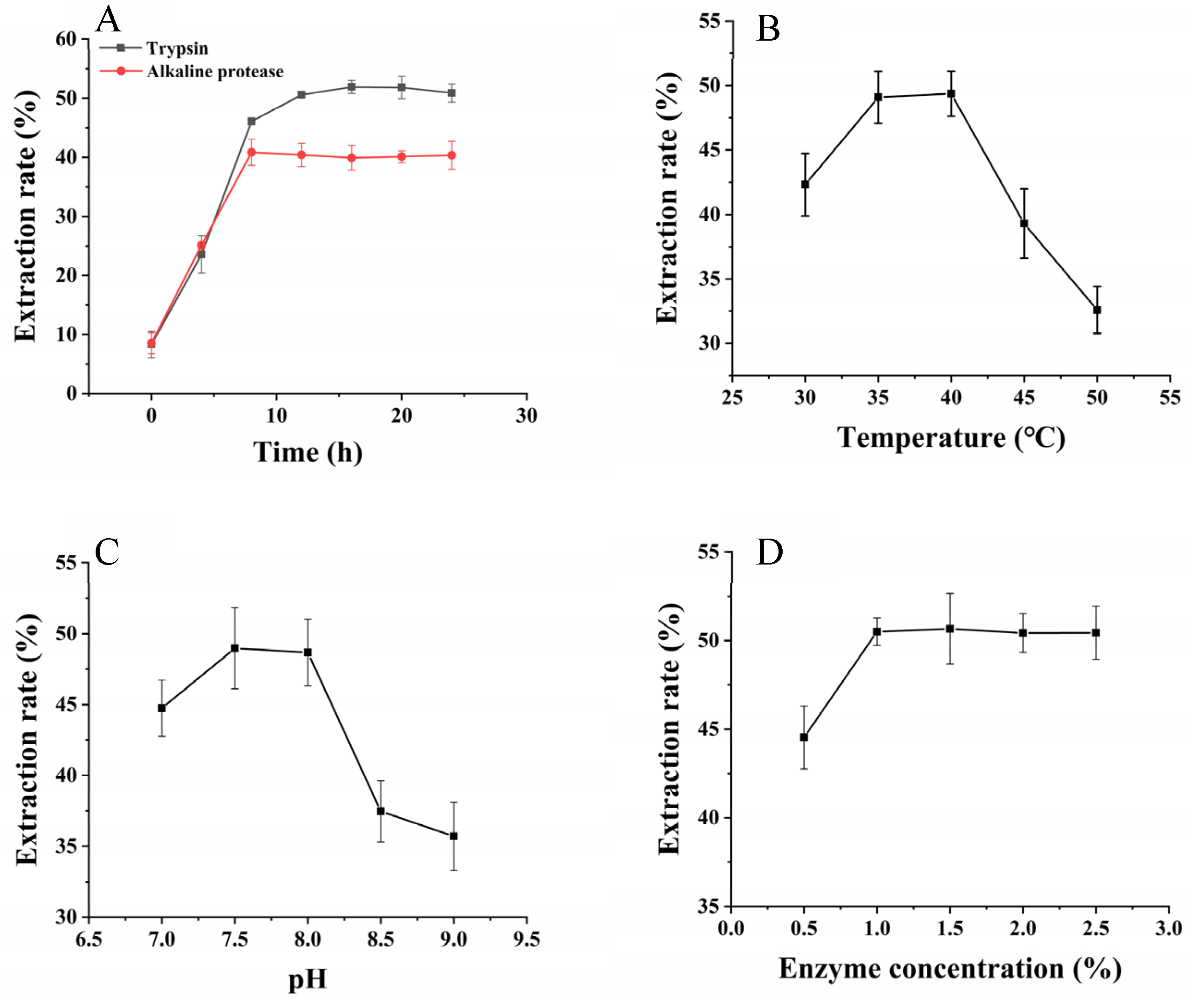
2.2.1. Comparison of Two Types of Enzymes
2.2.2. Single-Factor Experiments
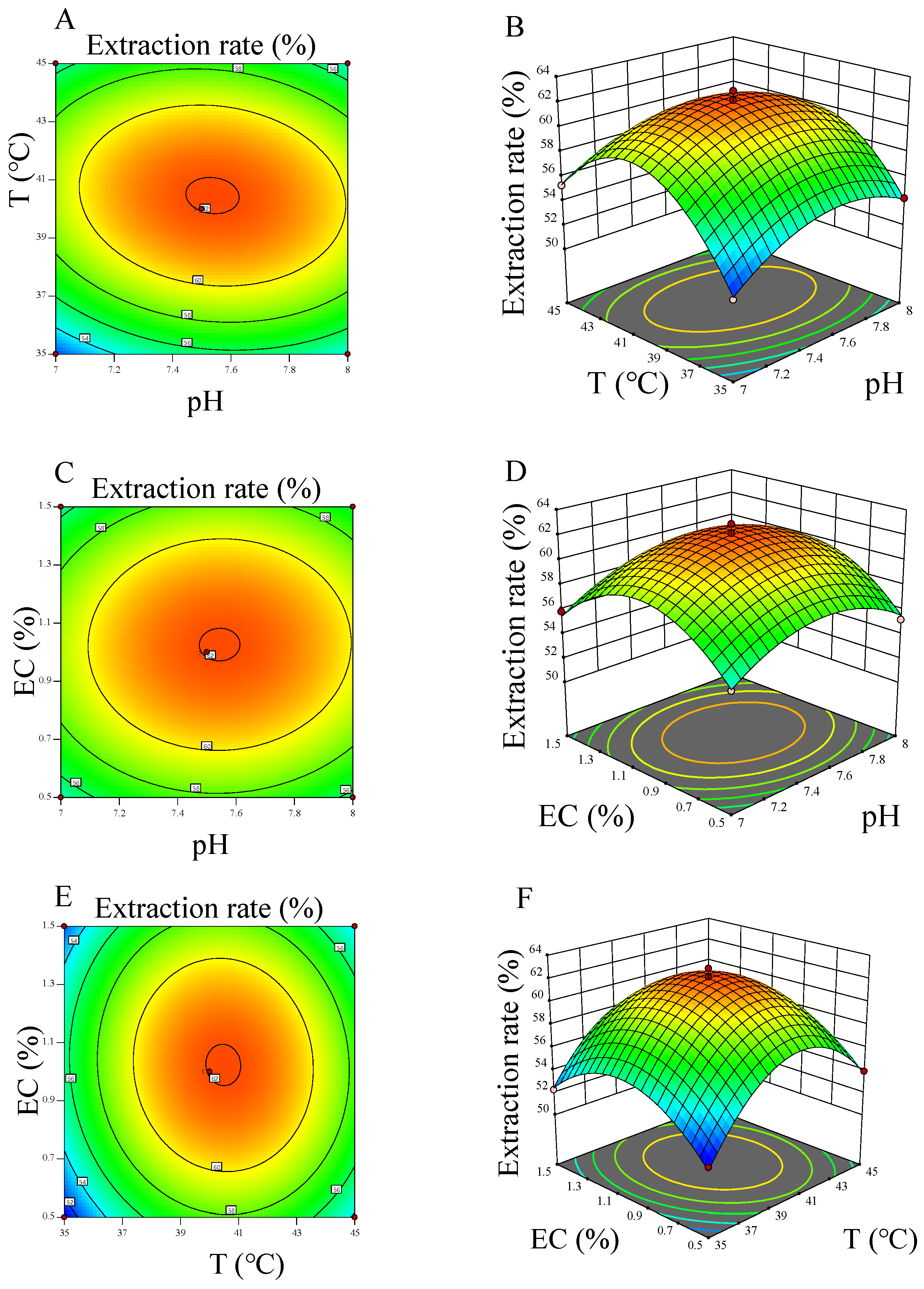
2.2.3. RSM Experiments
2.3. Molecular Weight Distribution
2.4. Amino Acid Composition
2.5. Antioxidant Activity
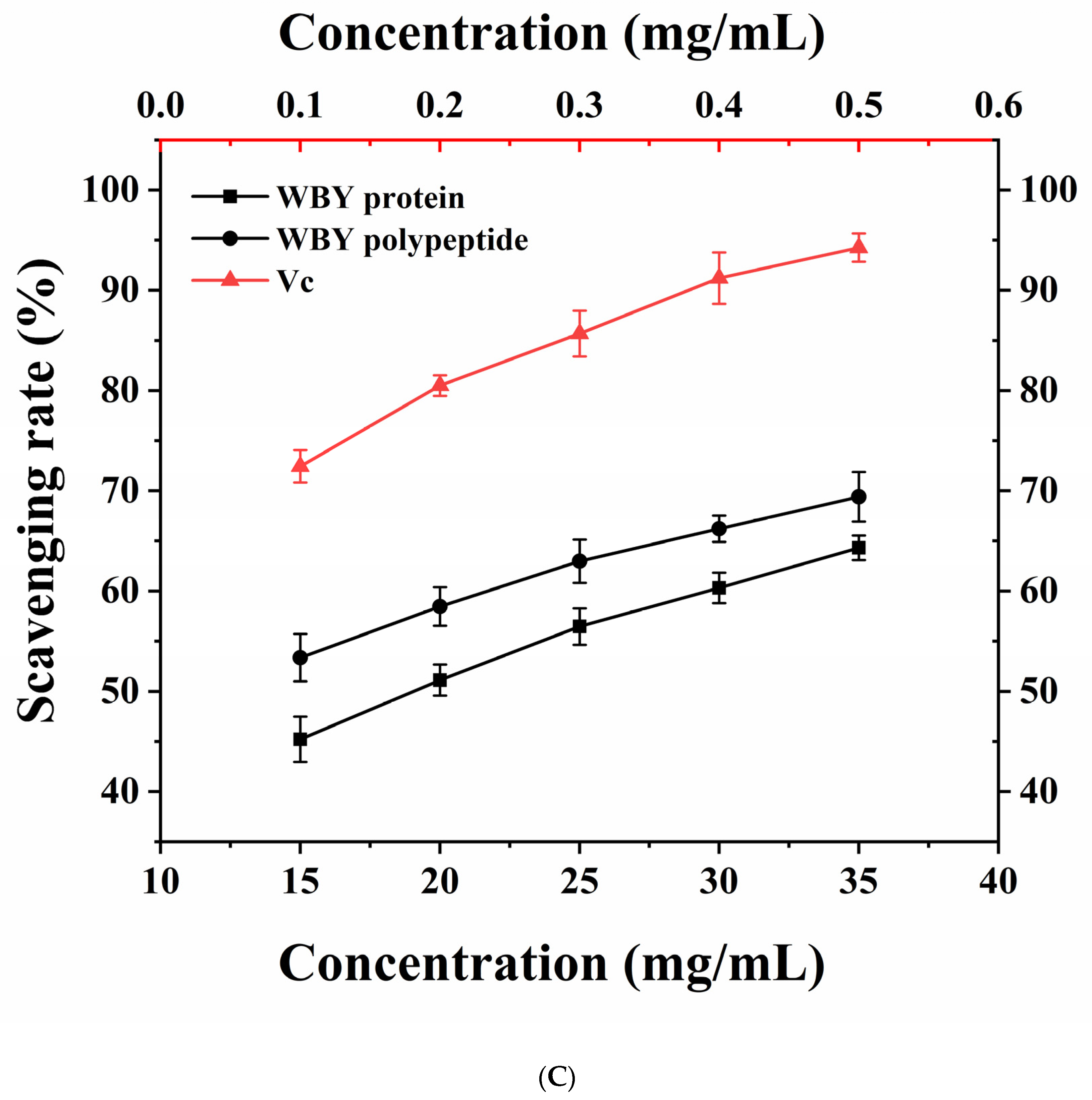
2.5.1. Hydroxyl Radical Scavenging Assay
2.5.2. DPPH Radical Scavenging Assay
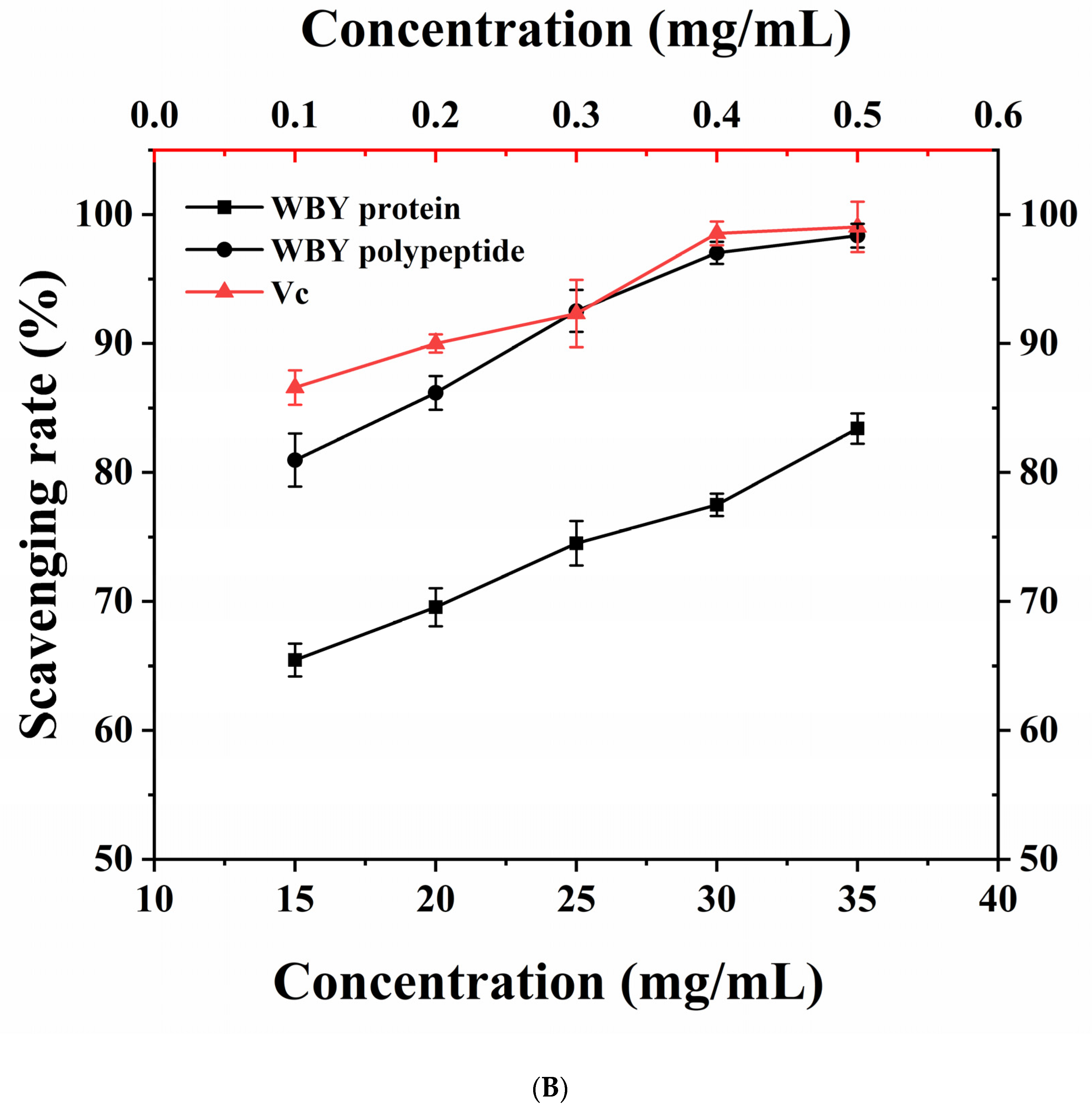
2.5.3. ABTS Radical Scavenging Assay
3. Materials and Methods
3.1. Materials
3.2. Process Optimization of Extracting Protein
3.2.1. Pretreatment
3.2.2. Ultrasonic-Assisted Extraction
3.2.3. Extraction Rate and Purity
3.2.4. Single-Factor Experimental Design
3.2.5. Experimental Design of Response Surface Method (RSM)
3.3. Process Optimization of Preparing Polypeptide
3.3.1. Enzymatic Hydrolysis and Degree of Hydrolysis (DH)
3.3.2. Extraction Rate and Purity
3.3.3. Single-Factor Experimental Design
3.3.4. RSM Experimental Design
3.4. Molecular Weight
3.5. Amino Acid Composition
3.6. Antioxidant Activity
3.6.1. Hydroxyl Radical Scavenging Assay
3.6.2. DPPH Radical Scavenging Assay
3.6.3. ABTS Scavenging Assay
3.6.4. IC50 Values
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast protein as an easily accessible food source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, C.G.; Yang, Z.Z.; Hu, X.C.; Liu, Z.C.; Song, W.L. Assessing the potential of Chlorella sp. for treatment and resource utilization of brewery wastewater coupled with bioproduct production. J. Clean. Prod. 2022, 367, 132939. [Google Scholar] [CrossRef]
- Adam, V. Eat microbial protein to spare the forests. New Sci. 2022, 254, 22. [Google Scholar] [CrossRef]
- Humpenöder, F.; Bodirsky, B.L.; Weindl, I.; Lotze-Campen, H.; Linder, T.; Popp, A. Projected environmental benefits of replacing beef with microbial protein. Nature 2022, 605, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Abou Saada, O.; Tsouris, A.; Large, C.; Friedrich, A.; Dunham, M.J.; Schacherer, J. Phased polyploid genomes provide deeper insight into the multiple origins of domesticated Saccharomyces cerevisiae beer yeasts. Curr. Biol. 2022, 32, 1350–1361. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, X.; Zhang, S.J.; Wang, X.Q.; Guo, C.H.; Guo, X.W.; Xiao, D.G. Development of Saccharomyces cerevisiae producing higher levels of sulfur dioxide and glutathione to improve beer flavor stability. Appl. Biochem. Biotechnol. 2012, 166, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Tarna, R.; Vrabie, E.; Paladii, I.; Sturza, R. Recovery of residual brewer’s yeast by electroactivation. Food Nutr. Sci. 2021, 12, 1177–1190. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s spent yeast (BSY), an underutilized brewing by-product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Estévez, A.; Padrell, L.; Iñarra, B.; Orive, M.; San Martin, D. Brewery by-products (yeast and spent grain) as protein sources in gilthead seabream (Sparus aurata) feeds. Aquaculture 2021, 543, 736921. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- da Silva Araújo, V.B.; de Melo AN, F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- Pancrazio, G.; Cunha, S.C.; Pinho, P.G.d.; Loureiro, M.; Meireles, S.; Ferreira, I.M.P.L.V.O.; Pinho, O. Spent brewer’s yeast extract as an ingredient in cooked hams. Meat Sci. 2016, 121, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Kumar, B.; Arora, N.; Singh, S.; Kumar, A.; Han, S.S.; Negi, Y.S. Novel bio-based solid acid catalyst derived from waste yeast residue for biodiesel production. Renew. Energy 2020, 159, 127–139. [Google Scholar] [CrossRef]
- Stafussa, A.P.; Maciel, G.M.; da Silva Anthero, A.G.; da Silva, M.V.; Zielinski, A.A.F.; Haminiuk, C.W.I. Biosorption of anthocyanins from grape pomace extracts by waste yeast: Kinetic and isotherm studies. J. Food Eng. 2016, 169, 53–60. [Google Scholar] [CrossRef]
- Szende, T.; Boldizsár, N.; Anamaria, T.; Cerasella, I.; Cornelia, M. Cd(II), Zn(II) and Cu(II) bioadsorption on chemically treated waste brewery yeast biomass: The role of functional groups. Acta Chim. Slov. 2015, 62, 736–746. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wang, D.F.; Xie, H.Z.; Wook, W.S.; Cui, L.Z.; Wu, G.P. Adsorption of Ag (I) from aqueous solution by waste yeast: Kinetic, equilibrium and mechanism studies. Bioprocess Biosyst. Eng. 2015, 38, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Orive, M.; Iñarra, B.; Castelo, J.; Estévez, A.; Nazzaro, J.; Iloro, I.; Elortza, F.; Zufía, J. Brewers’ spent yeast and grain protein hydrolysates as second-generation feedstuff for aquaculture feed. Waste Biomass Valorization 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Chollom, P.F.; Agbo, E.B.; Doma, U.D.; Okojokwu, O.J. The effect of graded levels of spent brewers’ yeast (Saccharomyces cerevisiae) on growth performance of broiler chickens. N. Y. Sci. J. 2017, 9, 9–13. [Google Scholar] [CrossRef]
- Lapsongphon, N.; Rodtong, S.; Yongsawatdigul, J. Spent brewery yeast sludge as a single nitrogen source for fibrinolytic enzyme production of Virgibacillus sp. SK37. Food Sci. Biotechnol. 2013, 22, 71–78. [Google Scholar] [CrossRef]
- Amorim, M.; Marques, C.; Pereira, J.O.; Guardão, L.; Martins, M.J.; Osório, H.; Moura, D.; Calhau, C.; Pinheiro, H.; Pintado, M. Antihypertensive effect of spent brewer yeast peptides. Process Biochem. 2018, 76, 213–218. [Google Scholar] [CrossRef]
- David, S.M.; Jone, I.; Bruno, I.; Nagore, L.; Jorge, F.; Carmen, A.; Carlos, B.; Monica, G.; Jaime, Z. Valorisation of brewer’s spent yeasts’ hydrolysates as high-value bioactive molecules. Sustainability 2021, 13, 6520. [Google Scholar] [CrossRef]
- Natchaya, D.; Weerachai, C.; Chitsiri, R.; Pitiporn, R.; Suvimol, C. Antimicrobial and functional properties of duckweed (Wolffia globosa) protein and peptide extracts prepared by ultrasound-assisted extraction. Foods 2022, 11, 2348. [Google Scholar] [CrossRef]
- Lamoolphak, W.; Goto, M.; Sasaki, M.; Suphantharika, M.; Muangnapoh, C.; Prommuag, C.; Shotipruk, A. Hydrothermal decomposition of yeast cells for production of proteins and amino acids. J. Hazard. Mater. 2006, 137, 1643–1648. [Google Scholar] [CrossRef]
- Amorim, M.; Pereira, J.O.; Gomes, D.; Pereira, C.D.; Pinheiro, H.; Pintado, M. Nutritional ingredients from spent brewer’s yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process. J. Food Eng. 2016, 185, 42–47. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Utilisation of spent brewer’s yeast for yeast extract production by autolysis: The effect of temperature. Food Bioprod. Process. 2007, 86, 317–321. [Google Scholar] [CrossRef]
- Zong, X.Y.; Li, L.; Feng, X.Q.; Luo, H.B.; Zhou, J.; Liu, C.J. Antioxidant Activity of Peptides Extracted from Brewers’ Spent Grain Peptides. Adv. Mater. Res. 2012, 1915, 891–895. [Google Scholar] [CrossRef]
- Lisboa, C.R.; Pereira, A.M.; Costa, J.A.V. Biopeptides with antioxidant activity extracted from the biomass of Spirulina sp. LEB 18. Afr. J. Microbiol. Res. 2016, 10, 79–86. [Google Scholar] [CrossRef][Green Version]
- Ballatore, M.B.; del Rosario Bettiol, M.; Braber, N.L.V.; Aminahuel, C.A.; Rossi, Y.E.; Petroselli, G.; Erra-Balsells, R.; Cavaglieri, L.R.; Montenegro, M.A. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin. Food Chem. 2020, 319, 126472. [Google Scholar] [CrossRef]
- Liang, L.L.; Wang, C.; Li, S.G.; Chu, X.M.; Sun, K.L. Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr. 2019, 7, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, H.; Wang, L.X.; Chai, L.Q.; Tian, C.R. Nutritional evaluation and functional properties of the antioxidant polypeptide from Zanthoxylum bungeanum Maxim seeds kernel protein hydrolysate. CyTA-J. Food 2017, 15, 425–432. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Guo, T.; Lu, Y.L.; Hadiatullah, H.; Li, P.; Ding, K.L.; Zhao, G.Z. Effects of amino acid composition of yeast extract on the microbiota and aroma quality of fermented soy sauce. Food Chem. 2022, 393, 133289. [Google Scholar] [CrossRef] [PubMed]
- Witten, S.; Böhm, H.; Aulrich, K. Effect of variety and environment on the contents of crude nutrients and amino acids in organically produced cereal and legume grains. Org. Agric. 2019, 10, 199–219. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Zhao, F.; Qian, J.; Liu, H.; Wang, C.; Wang, X.J.; Wu, W.X.; Wang, D.H.; Cai, C.P.; Lin, Y. Quantification, identification and comparison of oligopeptides on five tea categories with different fermentation degree by Kjeldahl method and ultra-high performance liquid chromatography coupled with quadrupole-orbitrap ultra-high resolution mass spectrometry. Food Chem. 2022, 378, 132130. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.K.; Fan, J.; Yang, Y.; Xu, Y.; Chen, F.L.; Bian, X.; Xing, T.L.; Liu, L.L.; Yu, D.H.; Zhang, N. Enzymatic Hydrolysis of Broken Rice Protein: Antioxidant Activities by Chemical and Cellular Antioxidant Methods. Front. Nutr. 2021, 8, 788078. [Google Scholar] [CrossRef]
- Oliveira, A.M.B.; Viganó, J.; Sanches, V.L.; Rostagno, M.A.; Martínez, J. Extraction of potential bioactive compounds from industrial Tahiti lime (Citrus latifólia Tan.) by-product using pressurized liquids and ultrasound-assisted extraction. Food Res. Int. 2022, 157, 111381. [Google Scholar] [CrossRef]


| Methods | Yield/Content | DH | Purity | Antioxidant Activity | ||||
|---|---|---|---|---|---|---|---|---|
| ⋅OH | DPPH⋅ | O2−⋅ | ABTS+⋅ | |||||
| Protein | Bienzymatic hydrolysis [21] | 29.67% (Y) | 28.88% | NR | NR | 1.59 gTEAC | ||
| Hydrothermal decomposition (250 °C, 20 min) [23] | 78% (Y) | NR | NR | NR | NR | |||
| Autolysis (70 °C, 4 h) [24] | 49% (C) | NR | NR | NR | NR | |||
| Antolysis (45 °C, 72 h) [25] | 76.4% (Y) | NR | NR | NR | NR | |||
| Peptide | Alcalase hydrolysis [26] | NR | NR | NR | IC50 < 0.8 mg/mL | NR | ||
| CCE hydrolysis [24] | NR | NR | 30–69% | NR | NR | |||
| Run | X1 | X2 | X3 | Extraction Rate% |
|---|---|---|---|---|
| 1 | 250 | 7.5 | 12 | 50.92 |
| 2 | 350 | 7.5 | 12 | 49.93 |
| 3 | 250 | 9.5 | 12 | 53.16 |
| 4 | 350 | 9.5 | 12 | 68.84 |
| 5 | 250 | 8.5 | 10 | 53.75 |
| 6 | 350 | 8.5 | 10 | 57.23 |
| 7 | 250 | 8.5 | 14 | 50.63 |
| 8 | 350 | 8.5 | 14 | 70.71 |
| 9 | 300 | 7.5 | 10 | 49.86 |
| 10 | 300 | 9.5 | 10 | 55.64 |
| 11 | 300 | 7.5 | 14 | 55.62 |
| 12 | 300 | 9.5 | 14 | 68.55 |
| 13 | 300 | 8.5 | 12 | 78.48 |
| 14 | 300 | 8.5 | 12 | 70.40 |
| 15 | 300 | 8.5 | 12 | 72.92 |
| 16 | 300 | 8.5 | 12 | 73.66 |
| 17 | 300 | 8.5 | 12 | 71.54 |
| Source of Variance | Sum of Squares | Free Degree | Mean Square Deviation | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1591.21 | 9 | 176.80 | 21.67 | 0.0003 | ** |
| X1 | 183.36 | 1 | 183.36 | 22.47 | 0.0021 | ** |
| X2 | 198.01 | 1 | 198.01 | 24.26 | 0.0017 | ** |
| X3 | 105.85 | 1 | 105.85 | 12.97 | 0.0087 | ** |
| X1X2 | 69.72 | 1 | 69.72 | 8.54 | 0.0222 | * |
| X1X3 | 68.89 | 1 | 68.89 | 8.44 | 0.0228 | * |
| X2X3 | 12.60 | 1 | 12.60 | 1.54 | 0.2540 | N |
| X12 | 304.57 | 1 | 304.57 | 37.32 | 0.0005 | ** |
| X22 | 354.83 | 1 | 354.83 | 43.48 | 0.0003 | ** |
| X32 | 194.98 | 1 | 194.98 | 23.89 | 0.0018 | ** |
| Residual | 57.12 | 7 | 8.16 | |||
| Lack of Fit | 19.23 | 3 | 6.41 | 0.6767 | 0.6103 | N |
| Pure Error | 37.89 | 4 | 9.47 | |||
| Cor Total | 1648.34 | 16 | ||||
| R2 | 0.9653 |
| Run | X1 | X2 | X3 | Extraction Rate% |
|---|---|---|---|---|
| 1 | 7 | 35 | 1 | 51.68 |
| 2 | 8 | 35 | 1 | 54.26 |
| 3 | 7 | 45 | 1 | 55.31 |
| 4 | 8 | 45 | 1 | 55.03 |
| 5 | 7 | 40 | 0.5 | 54.79 |
| 6 | 8 | 40 | 0.5 | 55.21 |
| 7 | 7 | 40 | 1.5 | 55.87 |
| 8 | 8 | 40 | 1.5 | 56.65 |
| 9 | 7.5 | 35 | 0.5 | 51.2 |
| 10 | 7.5 | 45 | 0.5 | 53.93 |
| 11 | 7.5 | 35 | 1.5 | 52.26 |
| 12 | 7.5 | 45 | 1.5 | 53.51 |
| 13 | 7.5 | 40 | 1 | 61.92 |
| 14 | 7.5 | 40 | 1 | 62.15 |
| 15 | 7.5 | 40 | 1 | 61.48 |
| 16 | 7.5 | 40 | 1 | 61.67 |
| 17 | 7.5 | 40 | 1 | 62.88 |
| Source of Variance | Sum of Squares | Free Degree | Mean Square Deviation | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 250.16 | 9 | 27.80 | 108.35 | < 0.0001 | ** |
| X1 | 1.53 | 1 | 1.53 | 5.97 | 0.0446 | * |
| X2 | 8.78 | 1 | 8.78 | 34.22 | 0.0006 | ** |
| X3 | 1.25 | 1 | 1.25 | 4.87 | 0.0632 | N |
| X1X2 | 2.04 | 1 | 2.04 | 7.97 | 0.0256 | * |
| X1X3 | 0.0324 | 1 | 0.0324 | 0.1263 | 0.7328 | N |
| X2X3 | 0.5476 | 1 | 0.5476 | 2.13 | 0.1874 | N |
| X12 | 26.79 | 1 | 26.79 | 104.44 | < 0.0001 | ** |
| X22 | 124.03 | 1 | 124.03 | 483.50 | < 0.0001 | ** |
| X32 | 62.98 | 1 | 62.98 | 245.51 | < 0.0001 | ** |
| Residual | 1.80 | 7 | 0.2565 | |||
| Lack of Fit | 0.6151 | 3 | 0.2050 | 0.6947 | 0.6018 | N |
| Pure Error | 1.18 | 4 | 0.2952 | |||
| Cor Total | 251.95 | 16 | ||||
| R2 | 0.9929 |
| Amino Acids | Protein (%) | Polypeptide (%) | |
|---|---|---|---|
| EAA | Thr | 0.78 ± 0.14 | 0.69 ± 0.03 |
| Val # | 2.06 ± 0.06 | 2.01 ± 0.12 | |
| Ile # | 1.45 ± 0.11 | 1.40 ± 0.03 | |
| Leu # | 3.11 ± 0.09 | 3.11 ± 0.14 | |
| Phe #,a | 1.79 ± 0.05 | 1.77 ± 0.05 | |
| Lys * | 3.92 ± 0.10 | 3.21 ± 0.02 | |
| Met # | 0.70 ± 0.03 | 0.44 ± 0.03 | |
| NEAA | Asp *,b | 2.37 ± 0.04 | 1.96 ± 0.01 |
| Ser | 1.82 ± 0.06 | 1.01 ± 0.09 | |
| Glu *,b | 6.46 ± 0.15 | 6.13 ± 0.13 | |
| Gly | 5.26 ± 0.02 | 6.02 ± 0.15 | |
| Ala # | 3.31 ± 0.12 | 3.30 ± 0.10 | |
| Tyr #,a | 2.24 ± 0.08 | 2.21 ± 0.06 | |
| His * | 2.03 ± 0.02 | 2.01 ± 0.13 | |
| Arg * | 1.99 ± 0.07 | 2.02 ± 0.07 | |
| Pro # | 1.61 ± 0.11 | 1.54 ± 0.11 | |
| Cys # | 0.05 ± 0.02 | 0.03 ± 0.01 | |
| EAA | 13.81 ± 0.47 | 12.63 ± 0.34 | |
| NEAA | 27.14 ± 0.56 | 26.23 ± 0.70 | |
| TAA | 40.95 ± 1.04 | 38.86 ± 1.05 | |
| HAA | 16.32 ± 0.55 | 15.81 ± 0.53 | |
| CAA | 16.77 ± 0.31 | 15.33 ± 0.29 | |
| AAA | 4.03 ± 0.11 | 3.98 ± 0.09 | |
| FAA | 8.83 ± 0.16 | 8.09 ± 0.11 | |
| Samples | Hydroxyl Radical | DPPH Radical | ABTS Radical |
|---|---|---|---|
| IC50 (mg/mL) | IC50 (mg/mL) | IC50 (mg/mL) | |
| protein | 2.83 ± 0.13 b | 8.67 ± 0.15 c | 18.75 ± 0.35 c |
| polypeptide | 2.68 ± 0.11 b | 5.63 ± 0.31 b | 12.86 ± 0.26 b |
| Vc | 0.018 ± 0.08 a | 0.015 ± 0.06 a | 0.038 ± 0.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Wang, J.; Feng, Y.; Yin, H.; Lai, H.; Xiao, R.; He, S.; Yang, Z.; He, Y. Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast. Molecules 2022, 27, 6825. https://doi.org/10.3390/molecules27206825
Zhu L, Wang J, Feng Y, Yin H, Lai H, Xiao R, He S, Yang Z, He Y. Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast. Molecules. 2022; 27(20):6825. https://doi.org/10.3390/molecules27206825
Chicago/Turabian StyleZhu, Lisha, Jianfeng Wang, Yincheng Feng, Hua Yin, Huafa Lai, Ruoshi Xiao, Sijia He, Zhaoxia Yang, and Yi He. 2022. "Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast" Molecules 27, no. 20: 6825. https://doi.org/10.3390/molecules27206825
APA StyleZhu, L., Wang, J., Feng, Y., Yin, H., Lai, H., Xiao, R., He, S., Yang, Z., & He, Y. (2022). Process Optimization, Amino Acid Composition, and Antioxidant Activities of Protein and Polypeptide Extracted from Waste Beer Yeast. Molecules, 27(20), 6825. https://doi.org/10.3390/molecules27206825




