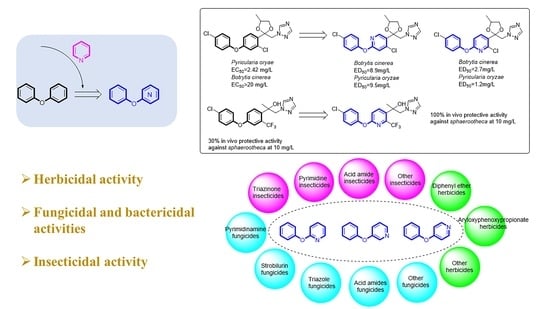
Advancement of Phenoxypyridine as an Active Scaffold for Pesticides
Abstract
1. Introduction
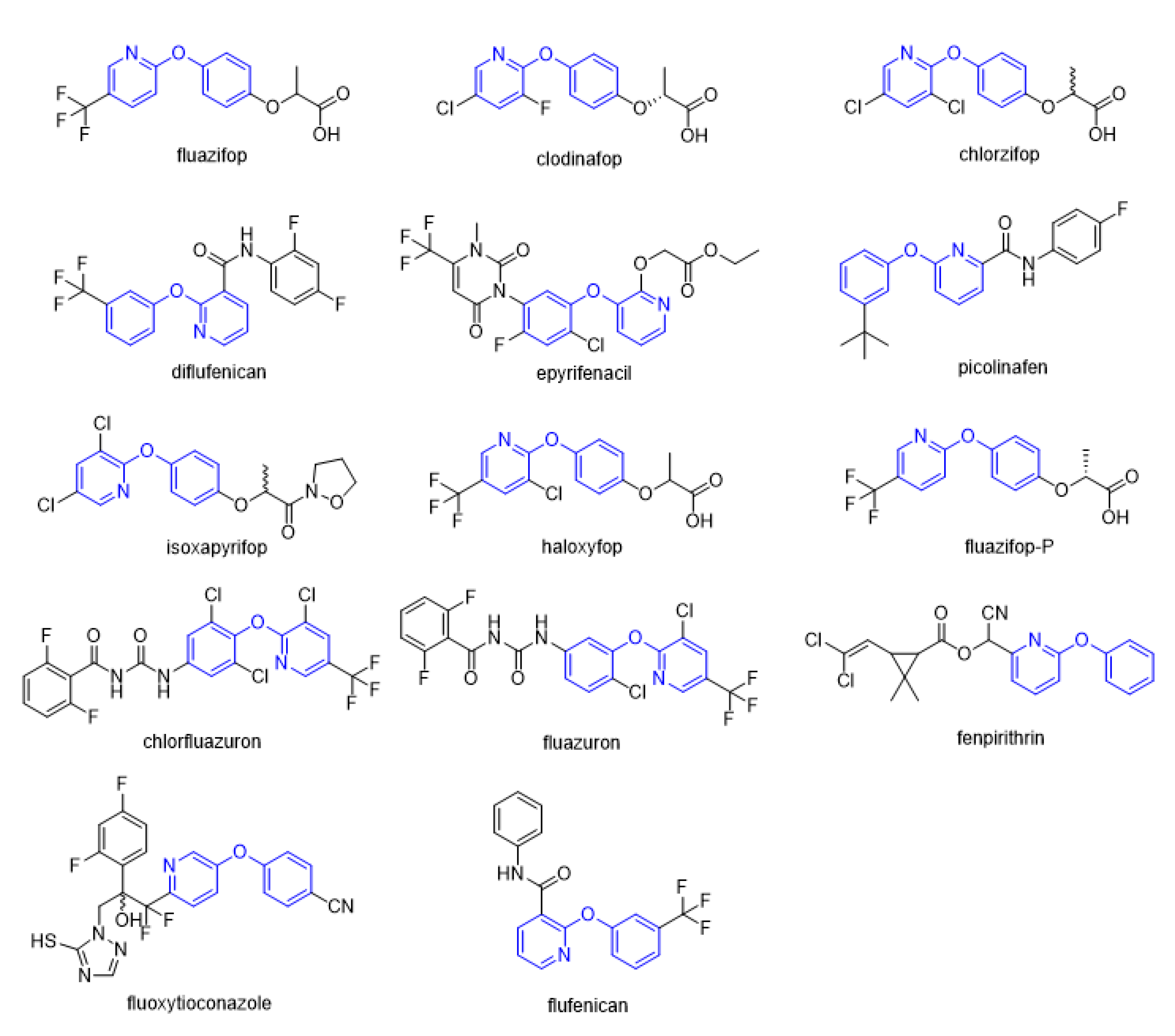
2. Herbicides Containing Phenoxypyridine Scaffold
2.1. Acetyl CoA Carboxylase Inhibitors
2.2. Protoporphyrinogen IX Oxidase Inhibitors
2.3. Other Herbicides
3. Fungicides and Bactericides Containing Phenoxypyridine Scaffold
3.1. Complex I Inhibitors
3.2. Complex III Inhibitors
3.3. Sterol Biosynthesis Inhibitors
3.4. Succinate Dehydrogenase Inhibitors
3.5. Other Fungicides and Bactericides
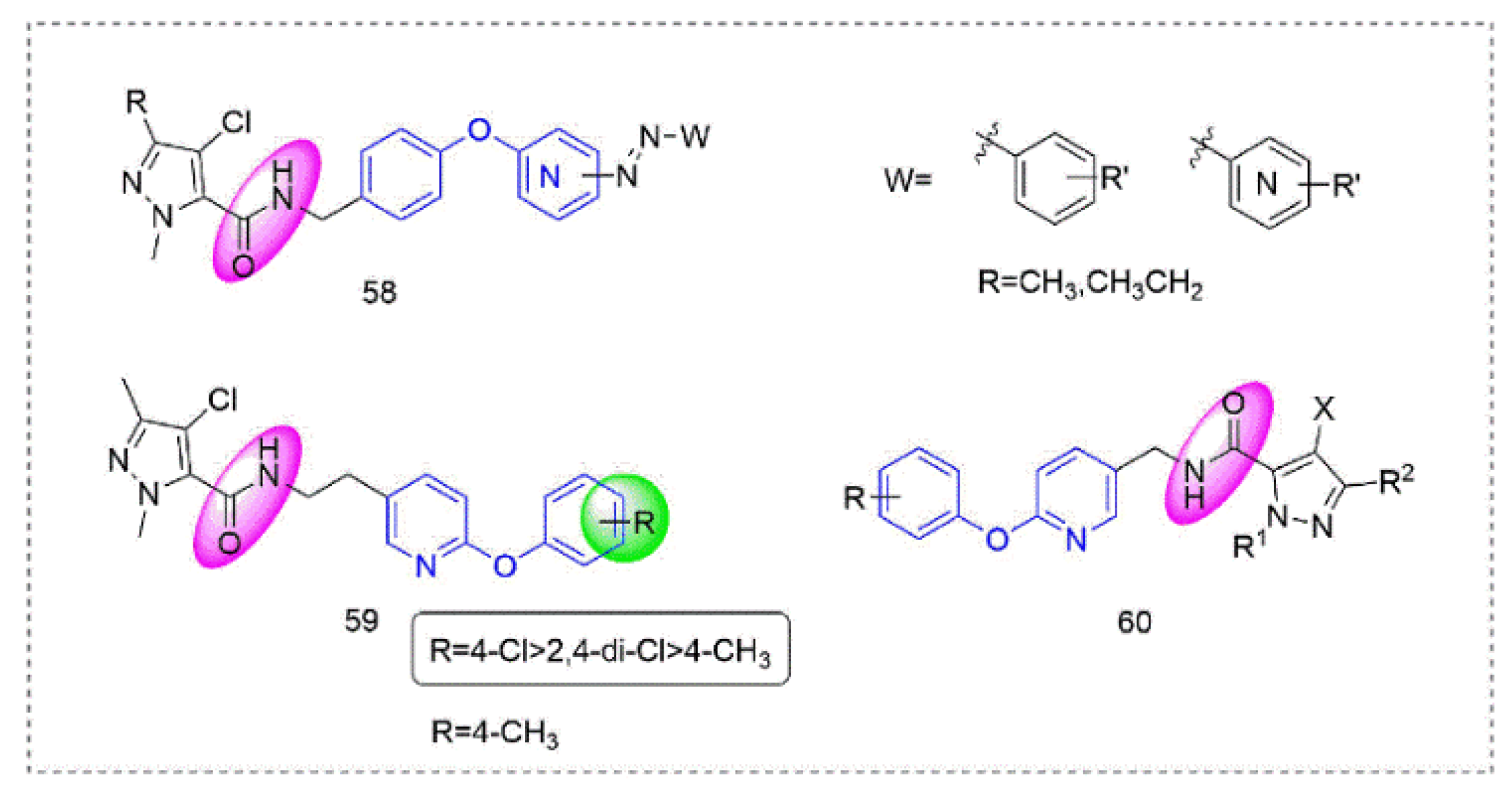
4. Insecticides Containing Phenoxypyridine Scaffold
4.1. Transient Receptor Potential Vanilloid Channel Blockers
4.2. Complex I Inhibitors
4.3. Other Insecticides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedos-Belval, F.; Rouch, A.; Vanucci-Bacque, C.; Baltas, M. Diaryl ether derivatives as anticancer agents—A review. Medchemcomm 2012, 3, 1356–1372. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, H.; Yang, J.F.; Zhu, X.L.; Qu, R.Y.; Yang, G.F. Diaryl Ether: A Privileged Scaffold for Drug and Agrochemical Discovery. J. Agric. Food Chem. 2020, 68, 9839–9877. [Google Scholar] [CrossRef] [PubMed]
- Ujihara, K. The history of extensive structural modifications of pyrethroids. J. Pestic. Sci. 2019, 44, 215–224. [Google Scholar] [CrossRef]
- Shimizu, S.; Watanabe, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. Pyridine and Pyridine Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 30, p. a22-399. [Google Scholar]
- Guan, A.Y.; Liu, C.L.; Sun, X.F.; Xie, Y.; Wang, M.A. Discovery of pyridine-based agrochemicals by using Intermediate Derivatization Methods. Bioorganic Med. Chem. 2016, 24, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Antiviral Activities of Pyridine Fused and Pyridine Containing Heterocy-cles, A Review (from 2000 to 2020). Mini-Rev. Med. Chem. 2021, 21, 2584–2611. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Li, Q.; Wen, K.; Ismail, I.; Liu, D.D.; Niu, C.W.; Wen, X.; Yang, G.F.; Xi, Z. Synthesis and Herbicidal Activity of Pyrido[2,3-d]pyrimidine-2,4-dione-Benzoxazinone Hybrids as Protoporphyrinogen Oxidase Inhibitors. J. Agric. Food Chem. 2017, 65, 5278–5286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Peng, J.F.; Liu, F.Y.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Design, Synthesis, and Herbicidal Activity of Diphenyl Ether Derivatives Containing a Five-Membered Heterocycle. J. Agric. Food Chem. 2022, 70, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.K.; Ovejero, R.F.L.; Belchior, G.G.; Maymone, G.P.L.; Dayan, F.E. ACCase-inhibiting herbicides: Mechanism of action, resistance evolution and stewardship. Sci. Agric. 2021, 78, e20190102. [Google Scholar] [CrossRef]
- Kukorelli, G.; Reisinger, P.; Pinke, G. ACCase inhibitor herbicides—Selectivity, weed resistance and fitness cost: A review. Int. J. Pest Manag. 2013, 59, 165–173. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, G.; Zhang, A.; Zheng, L.; Cao, S. Study on the enantioselectivity inhibition mechanism of acetyl-coenzyme A carboxylase toward haloxyfop by homology modeling and MM-PBSA analysis. J. Mol. Model. 2012, 18, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Pernich, D.J. Origin of enantiomeric selectivity in the aryloxyphenoxypropionic acid class of herbicidal acetyl coenzyme A carboxylase (ACCase) inhibitors. J. Agric. Food Chem. 2002, 50, 4554–4566. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Langelueddeke, P.; Leditschke, H.; Nahm, H.; Schwerdtle, F. Herbicidal α-(4-phenoxyphenoxy)alkanecarboxylic Acid Derivatives. DE Patent US3954442A, 4 May 1976. [Google Scholar]
- Huang, M.; Liu, A.; Nie, S.; Liu, Q.; Lei, M.; Gao, D.; Ren, Y.; Zhang, P.; Han, K.; He, L. N-(arylalkoxy)aryloxyphenoxy Carboxylic Acid Amide Compounds, and Preparation Method and Application Thereof. Chinese Patent CN104277034A, 14 January 2015. [Google Scholar]
- Wang, X.; Liu, A.; Liu, Q.; Lei, M.; Ren, Y.; Ou, X.; Huang, L.; Han, K.; Gao, G.; Wu, M. [N-(Aralkyl)aryloxy]phenoxycarboxylic Acid Amide Compounds as Agrochemicals and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Plant Diseases. Chinese Patent WO2015000392A1, 8 January 2015. [Google Scholar]
- Ying, Z.H.; Chen, A.Y.; Hu, A.X.; Ye, J. Synthesis, Crystal Structure and Herbicidal Activity of N-(Thiazol-2-yl)-2-(4-aryloxyphenoxy)propionamides. Chin. J. Org. Chem. 2017, 37, 149–156. [Google Scholar] [CrossRef]
- Yang, S.; Ding, C.R.; Liu, X.H.; Weng, J.Q.; Yuan, J.; Tan, C.X. Synthesis and Herbicidal Activity of Chiral Aryloxyphenoxypropionic Amides Compounds. Chin. J. Org. Chem. 2019, 39, 3588–3593. [Google Scholar] [CrossRef]
- Xiao, T.; Li, S.; Feng, Y.; Wang, X.; Tian, X.; Chen, Z. Preparation of Aryloxyphenoxypropionamide Compounds as Weedicides. Chinese Patent CN104876922A, 2 September 2015. [Google Scholar]
- Liu, A.; Ren, Y.; Lei, M.; Pang, H.; Liu, Q.; Huang, L.; He, L.; Han, K.; Gao, G.; He, L. N-pyridine Aryloxy Phenoxy Carboxylic Acid Derivative, Preparation Method and Application. Chinese Patent CN105315199A, 10 February 2016. [Google Scholar]
- Liu, Q.; Zhou, H.; Zhu, R.; Chen, L. Pyridine-3-yl Aryloxy Phenoxy Alkyl Acid Ester and Its Application as Agricultural Herbicide. Chinese Patent CN106632007A, 10 May 2017. [Google Scholar]
- Liu, Q.; Zhou, H.; Lu, G.; Peng, Y. Preparation of Aryloxyphenoxy Alkyl Acid Derivatives as Herbicides. Chinese Patent CN106632293A, 10 May 2017. [Google Scholar]
- Lin, D.; Xiao, M.W.; Yang, Z.H.; Li, B.B.; Hu, A.X.; Ye, J. Synthesis and Herbicidal Activity of N-(2,2-Dimethyl-7-alkoxy-2,3-dihydrobenzofuran-5-yl)-2-(4-arylxoyphenoxy)propionamides. Chem. Res. Chin. Univ. 2017, 33, 74–79. [Google Scholar] [CrossRef]
- Hu, A.; Lin, D.; Li, B.; Ye, J.; Ou, X. Process for Preparation and Application of 2-[4-(pyridyl-2-yloxy)phenoxy]amide Derivative. Chinese Patent CN106478613A, 8 March 2017. [Google Scholar]
- Hu, A.; Yang, Z.; Ye, J.; Li, Y. Preparation of N-[(dihydrobenzofuran-7-oxo)alkyl]-2-aryloxy Amide Derivatives as Herbicides. Chinese Patent CN107382980A, 24 November 2017. [Google Scholar]
- Yan, Z.Z.; Yang, Z.H.; Deng, X.L.; Lin, D.; Wu, M.F.; Li, J.M.; Chen, A.Y.; Ye, J.; Hu, A.X.; Liao, H.D. Novel aryloxyphenoxypropionate derivates containing benzofuran moiety: Design, synthesis, herbicidal activity, docking study and theoretical calculation. Pestic. Biochem. Physiol. 2019, 154, 78–87. [Google Scholar] [CrossRef]
- Krahmer, H.; Walter, H.; Jeschke, P.; Haaf, K.; Baur, P.; Evans, R. What makes a molecule a pre- or a post-herbicide—How valuable are physicochemical parameters for their design? Pest Manag. Sci. 2021, 77, 4863–4873. [Google Scholar] [CrossRef]
- Hu, A.X.; Chen, A.Y.; Li, B.B.; Yang, Z.H.; Li, Y.H. Preparation of N-(2-hydroxyiminoethyl)amide Derivatives Useful as Herbicides. Chinese Patent CN105859669A, 17 August 2016. [Google Scholar]
- Yang, Z.; Gu, L.; Wang, M.; Li, G.; Chang, X.; Wang, X.; Ma, M.; Yu, J.; Wang, X.; Wang, C. Preparation of Pyridine Derivative Compound as Herbicide. Chinese Patent CN108003142A, 8 May 2018. [Google Scholar]
- Xu, Z.H.; Zhang, T.; Wang, S.K.; Li, J.K. Synthesis and Herbicidal Activities of Novel Ethyl 2-(4-(Pyridin-2-yl-oxy)phenyl-amino)propanoates/acetates. Chin. J. Org. Chem. 2017, 37, 526–532. [Google Scholar] [CrossRef]
- Kalhor, M.; Dadras, A. Synthesis, Characterization, and Herbicidal Activities of New 1,3,4-Oxadiazoles, 1,3,4-Thiadiazoles, and 1,2,4-Triazoles Derivatives Bearing (R)-5-Chloro-3-fluoro-2-phenoxypyridine. J. Heterocycl. Chem. 2013, 50, 220–224. [Google Scholar] [CrossRef]
- Dayan, F.E.; Barker, A.; Tranel, P.J. Origins and structure of chloroplastic and mitochondrial plant protoporphyrinogen oxidases: Implications for the evolution of herbicide resistance. Pest Manag Sci. 2018, 74, 2226–2234. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Rouhollahi, A.; Ghasemi, J.B.; Babaee, E. Quantitative Structure Activity Relationship Modeling of Environmentally Important Diphenyl Ether Herbicides Using MLR and PLS. Curr. Anal. Chem. 2010, 6, 3–10. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.X.; Hu, J.J.; Wang, Z.X.; Yin, M.L.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Novel phenoxy-(trifluoromethyl)pyridine-2-pyrrolidinone-based inhibitors of protoporphyrinogen oxidase: Design, synthesis, and herbicidal activity. Pestic Biochem. Physiol. 2020, 170, 104684. [Google Scholar] [CrossRef]
- Zhao, L.X.; Peng, J.F.; Hu, J.J.; Zou, Y.L.; Yin, M.L.; Wang, Z.X.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, herbicidal activity, and the molecular docking study of novel diphenyl ether derivatives as protoporphyrinogen IX oxidase inhibitors. J. Mol. Struct. 2022, 1258, 132670. [Google Scholar] [CrossRef]
- Zhao, L.X.; Wang, Z.X.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Phenoxypyridine derivatives containing natural product coumarins with allelopathy as novel and promising proporphyrin IX oxidase-inhibiting herbicides: Design, synthesis and biological activity study. Pestic Biochem. Physiol. 2021, 177, 104897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Wang, Z.X.; Peng, J.F.; Zou, Y.L.; Hui, Y.Z.; Chen, Y.Z.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and herbicidal activity of novel phenoxypyridine derivatives containing natural product coumarin. Pest Manag. Sci. 2021, 77, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Peng, J.F.; Liu, F.Y.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Discovery of novel phenoxypyridine as promising protoporphyrinogen IX oxidase inhibitors. Pestic Biochem. Physiol. 2022, 184, 105102. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, Y.A.; Kuang, A.; Jin, D. Advances on development of tetrahydrophthalimide herbicides. Nongyao 2001, 40, 7–9, 11. [Google Scholar]
- Uclés, S.; Hakme, E.; Ferrer, C.; Fernández-Alba, A.R. Analysis of thermally labile pesticides by on-column injection gas chromatography in fruit and vegetables. Anal. Bioanal Chem. 2018, 410, 6861–6871. [Google Scholar] [CrossRef]
- Zhao, L.X.; Jiang, M.J.; Hu, J.J.; Zou, Y.L.; Cheng, Y.; Ren, T.; Gao, S.; Fu, Y.; Ye, F. Design, Synthesis, and Herbicidal Activity of Novel Diphenyl Ether Derivatives Containing Fast Degrading Tetrahydrophthalimide. J. Agric. Food Chem. 2020, 68, 3729–3741. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y.; Pang, L.L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Q.M. Recent Advances in the Pesticide Activities of 2-Cyanoacrylate Derivatives. J. Agric. Food Chem. 2021, 69, 12933–12946. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ni, Y.D.; Miao, H.Y.; Chen, J.; Zhang, Y.; Shi, L.; Zhang, H.J.; Zhou, H.Y.; Li, J.F.; Qian, H.W. Preparation of Trifluoromethylpyridyloxy Aryl Group-Containing Cyanoacrylate Derivative as Herbicide. Chinese Patent CN111410630A, 14 July 2020. [Google Scholar]
- Zhang, D.; Zhou, N.; Yang, L.J.; Yu, Z.L.; Ma, D.J.; Wang, D.W.; Li, Y.H.; Liu, B.; Wang, B.F.; Xu, H.; et al. Discovery of (5-(Benzylthio)-4-(3-(trifluoromethyl)phenyl)-4H-1,2,4-triazol-3-yl) Methanols as Potent Phytoene Desaturase Inhibitors through Virtual Screening and Structure Optimization. J. Agric. Food Chem. 2022, 70, 10144–10157. [Google Scholar] [CrossRef]
- Koschmieder, J.; Fehling-Kaschek, M.; Schaub, P.; Ghisla, S.; Brausemann, A.; Timmer, J.; Beyer, P. Plant-type phytoene desaturase: Functional evaluation of structural implications. PLoS ONE 2017, 12, e0187628. [Google Scholar] [CrossRef]
- Zhai, Y.E.; Shi, D.Q. Synthesis and Herbicidal Activity of 2-Alkyl(aryl)-4-amino-3-[alkyl(alkoxy)carbonyl]-5-cyano-6-[(3-trifluoromethyl)phenoxy]-pyridines. J. Heterocycl. Chem. 2013, 50, 1039–1042. [Google Scholar] [CrossRef]
- Bhonoah, Y.; Elliott, A.C.; Gaulier, S.; Ling, K.; Mitchell, G.; Morris, J.A.; Rzepa, P.R.; Viner, R.C. Preparation of Herbicidal Pyridazinone Derivatives. GB Patent WO2013050421A1, 11 April 2013. [Google Scholar]
- Reddy, R.P.; Balagopal, L. Bis(aryl)catechol Derivatives as Herbicides and Their Preparation. US Patent WO2016010731A1, 21 January 2016. [Google Scholar]
- Wailes, J.S.; Black, J.; Morris, J.A.; Briggs, E.; Tate, J.A.; Aspinall, M.B.; Ng, S. Aryloxypyridines as Herbicides and Their Preparation. GB Patent WO2020094524A1, 14 May 2020. [Google Scholar]
- McLeod, M.C.; Barber, D.M.; Braun, R.; Jakobi, H.; Asmus, E.; Schmutzler, D.; Machettira, A.B. Preparation of Substituted Pyridinyloxyanilines, Their Salts and Use of Said Compounds as Herbicidal Agents. DE Patent EP3747867A1, 9 December 2020. [Google Scholar]
- McLeod, M.C.; Barber, D.M.; Braun, R.; Jakobi, H.; Asmus, E.; Schmutzler, D.; Machettira, A.B. Substituted Phenoxypyridines, Their Salts and Use of Said Compounds as Herbicidal Agents. DE Patent EP3747868A1, 9 December 2020. [Google Scholar]
- Wang, W.; Liu, Y.; Xue, Z.; Li, J.; Wang, Z.; Liu, X. Activity of the Novel Fungicide SYP-34773 against Plant Pathogens and Its Mode of Action on Phytophthora infestans. J. Agric. Food Chem. 2021, 69, 11794–11803. [Google Scholar] [CrossRef]
- Walter, H. NADH Inhibitors (Complex I). Mod. Crop Prot. Compd. 2012, 2, 670–691. [Google Scholar]
- Sparks, T.C.; DeAmicis, C.V.; Dekeyser, M.A.; Furuya, T.; Nakano, M.; Fujioka, S. Inhibitors of Mitochondrial Electron Transport: Acaricides and Insecticides. Mod. Crop Prot. Compd. 2012, 3, 1156–1201. [Google Scholar]
- Guan, A.Y.; Liu, C.L.; Chen, W.; Yang, F.; Xie, Y.; Zhang, J.B.; Li, Z.N.; Wang, M.A. Design, Synthesis, and Structure-Activity Relationship of New Pyrimidinamine Derivatives Containing an Aryloxy Pyridine Moiety. J. Agric. Food Chem. 2017, 65, 1272–1280. [Google Scholar] [CrossRef]
- Guan, A.Y.; Wang, M.A.; Yang, J.L.; Wang, L.Z.; Xie, Y.; Lan, J.; Liu, C.L. Discovery of a New Fungicide Candidate through Lead Optimization of Pyrimidinamine Derivatives and Its Activity against Cucumber Downy Mildew. J. Agric. Food Chem. 2017, 65, 10829–10835. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Wang, M.A.; Chen, W.; Yang, F.; Yang, J.L.; Zhao, Y.; Li, Z.N.; Liu, C.L. Design, synthesis and antifungal activity of new substituted difluoromethylpyrimidinamine derivatives. J. Fluor. Chem. 2017, 201, 49–54. [Google Scholar] [CrossRef]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113716A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113720A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113773A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.M.W.; Lohmann, J.K.; Montag, J.; Vrettou-Schultes, M. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113778A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113787A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. Patent WO2013135671A1, 19 September 2013. [Google Scholar]
- Grammenos, W.; Craig, I.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. Patent WO2013113863A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.M.W.; Lohmann, J.K.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. Patent WO2013113715A1, 8 August 2013. [Google Scholar]
- Zhang, X.; Liu, H.; Gao, Y.; Wang, H.; Guo, B.; Li, J. Synthesis and Antifungal Activities of New Type β-Methoxyacrylate-Based Strobilurin Analogues. Chin. J. Chem. 2012, 30, 1517–1524. [Google Scholar] [CrossRef]
- Chai, B.S.; Liu, C.L.; Li, H.C.; He, X.M.; Luo, Y.M.; Huang, G.; Zhang, H.; Chang, J.B. Design, synthesis and acaricidal activity of novel strobilurin derivatives containing pyrimidine moieties. Pest Manag. Sci. 2010, 66, 1208–1214. [Google Scholar] [CrossRef]
- Chen, J.; Shi, J.; Yu, L.; Liu, D.; Gan, X.; Song, B.; Hu, D. Design, Synthesis, Antiviral Bioactivity, and Defense Mechanisms of Novel Dithioacetal Derivatives Bearing a Strobilurin Moiety. J. Agric. Food Chem. 2018, 66, 5335–5345. [Google Scholar] [CrossRef]
- Hao, Z.S.; Wang, W.B.; Yu, B.; Qi, X.; Lv, Y.; Liu, X.Y.; Chen, H.Y.; Kalinina, T.A.; Glukhareva, T.V.; Fan, Z.J. Design, Synthesis, and Evaluation of Fungicidal Activity of Novel Pyrazole-Containing Strobilurin Derivatives(dagger). Chin. J. Chem. 2021, 39, 1531–1537. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, D.Y.; Yin, F.H.; Li, J.Q.; Xiao, Y.M.; Fu, B.; Qin, Z.H. The application of “plug-in molecules” method in novel strobilurin fungicides screening. RSC Adv. 2020, 10, 42804–42809. [Google Scholar] [CrossRef]
- Mueller, B.; Sauter, H.; Roehl, F.; Doetzer, R.; Lorenz, G.; Ammermann, E. Preparation of Carbamates and Plant-Protecting Agents Containing Them. DE Patent WO9315046A1, 5 August 1993. [Google Scholar]
- Qin, Z.; Yang, D. High-Activity Imino Phenylacetate Compounds and Preparation Method and Application Thereof. Chinese Patent CN106946770A, 14 July 2017. [Google Scholar]
- Wang, L.L.; Zhao, S.S.; Kong, X.T.; Cao, L.L.; Tian, S.; Ye, Y.H.; Qiao, C.H. Design, synthesis and fungicidal evaluation of novel pyraclostrobin analogues. Bioorganic Med. Chem. 2018, 26, 875–883. [Google Scholar] [CrossRef]
- Hayase, Y.; Kataoka, T.; Takenaka, H.; Ichinari, M.; Masuko, M.; Takahashi, T.; Tanimoto, N. Preparation of Substituted phenyl(alkoxyimino)acetamides and Their Use as Fungicides. JP Patent EP398692A2, 1990. [Google Scholar]
- Qin, Z. Preparation of Pyra-Famoxadone as Agricultural Fungicide. Chinese Patent CN103396410A, 20 November 2013. [Google Scholar]
- Cools, H.J.; Fraaije, B.A. Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag. Sci. 2013, 69, 150–155. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole Fungicides Can Induce Cross-Resistance to Medical Triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef]
- Coqueron, P.; Miller, R.; Bernier, D.; Naud, S.; Genix, P.; Wittrock, S.; Kennel, P.; Brunet, S.; Hoffmann, S.; Meissner, R. Preparation of Triazole Derivatives for Use as Fungicides. DE Patent WO2018054832A1, 29 March 2018. [Google Scholar]
- Coqueron, P.; Miller, R.; Bernier, D.; Naud, S.; Genix, P.; Kennel, P.; Brunet, S.; Wittrock, S.; Hoffmann, S. Preparation of Triazole Derivatives for Use as Fungicides. DE Patent WO2018054829A1, 29 March 2018. [Google Scholar]
- Hoffmann, S.; Sudau, A.; Dahmen, P.; Wachendorff-Neumann, U.; Meissner, R.; Geist, J.; Bernier, D.; Vors, J.; Coqueron, P.; Wittrock, S.; et al. Preparation of Triazole Derivatives, Intermediates Thereof and Their Use as Fungicides. Patent WO2017029179A1, 23 February 2017. [Google Scholar]
- Goertz, A.; Meissner, R.; Miller, R.; Naud, S.; Bernier, D.; Genix, P.; Brunet, S.; Kennel, P.; Coqueron, P. Preparation of Triazolethione Derivatives as Fungicides for Crop Protection and Plant Growth Regulators. DE Patent WO2018145933A1, 16 August 2018. [Google Scholar]
- Jeanmart, S.; Gagnepain, J.; Maity, P.; Lamberth, C.; Cederbaum, F.; Rajan, R.; Jacob, O.; Blum, M.; Bieri, S. Synthesis and fungicidal activity of novel imidazole-based ketene dithioacetals. Bioorg. Med. Chem. 2018, 26, 2009–2016. [Google Scholar] [CrossRef]
- Aydinli, S.G.; Bulut, E.; Deniz, N.G.; Sayil, C.; Komarovska-Porokhnyavets, O.; Lubenets, V.; Zvarych, V.; Stasevych, M.; Nesterkina, M.; Kravchenko, I. New ketene dithioacetals generated from 2-nitroperchlorobutadiene and investigation of their antibacterial, antifungal, anticonvulsant and antidepressant activities. Chem. Biodivers. 2022, 19, e202100931. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Bi, X.H.; Liu, Q. Recent developments of ketene dithioacetal chemistry. Chem. Soc. Rev. 2013, 42, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Yang, F.; Wang, J.F.; Chen, W.; Li, K.K.; Sun, X.F.; Chen, X.M.; Xie, Y.; Song, Y.Q.; Liu, C.L. Pyrazole Amide Compound as Agrochemical Insecticides and Bactericide and Its Preparation. Chinese Patent CN104974136A, 14 October 2015. [Google Scholar]
- Liu, C.; Wang, L.; Lan, J.; Sun, X.; Sun, Q.; Zhang, J. Use of Compound of Pyrazole Amides as Agricultural Fungicide. CN Patent WO2013064079A1, 10 May 2013. [Google Scholar]
- Sun, S.S.; Chen, L.; Huo, J.Q.; Wang, Y.; Kou, S.; Yuan, S.T.; Fu, Y.N.; Zhang, J.L. Discovery of Novel Pyrazole Amides as Potent Fungicide Candidates and Evaluation of Their Mode of Action. J. Agric. Food Chem. 2022, 70, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, J.; Zhou, J.; Guan, A.; Wang, J.; Xu, Y.; Li, M.; Liu, C. Isothiazolinone Compound as Agrochemical Fungicide and Its Preparation. Chinese Patent CN103570642A, 12 February 2014. [Google Scholar]
- Xu, Y.; Zhang, J.; Li, M.; Zhou, J.-Z.; Wang, J.-F.; Liu, C.-L. Synthesis and biological activity of isothiazolone derivatives. Nongyao 2014, 53, 712–714. [Google Scholar]
- Inagaki, J.; Yamanaka, H. Preparation of Pyridines and Agricultural/Horticultural Microbicides Containing Them. JP Patent WO2014013951A1, 23 January 2014. [Google Scholar]
- Liu, D.; Zhang, J.; Zhao, L.; He, W.; Liu, Z.; Gan, X.; Song, B. First Discovery of Novel Pyrido[1,2-a]pyrimidinone Mesoionic Compounds as Antibacterial Agents. J. Agric. Food Chem. 2019, 67, 11860–11866. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Xu, X.; Guo, J.; Wang, Z.; Li, P. Aromatic (hetero)cyclic Ether Compound Having Insecticidal Activity, Preparation Method and Application. CN Patent WO2020156512A1, 6 August 2020. [Google Scholar]
- Wang, L.X.; Niu, C.D.; Salgado, V.L.; Lelito, K.; Stam, L.; Jia, Y.L.; Zhang, Y.; Gao, C.F.; Wu, S.F. Pymetrozine activates TRPV channels of brown planthopper Nilaparvata lugens. Pestic Biochem. Physiol. 2019, 153, 77–86. [Google Scholar] [CrossRef]
- Ghareeb, E.A.; Mahmoud, N.F.H.; El-Bordany, E.A.; El-Helw, E.A.E. Synthesis, DFT, and eco-friendly insecticidal activity of some N-heterocycles derived from 4-((2-oxo-1,2-dihydroquinolin-3-yl)methylene)-2-phenyloxazol-5(4H)-one. Bioorganic Chem. 2021, 112, 104945. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, H.; Yuan, T.; Li, S.; Gan, X.; Song, B. Novel vanillin derivatives containing a 1,3,4-thiadiazole moiety as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2020, 30, 127113. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.X.; Song, H.J.; Li, Y.Q.; Wang, Q.M. Additive effects on the improvement of insecticidal activity: Design, synthesis, and insecticidal activity of novel pymetrozine derivatives. Bioorg. Med. Chem. 2016, 24, 391–402. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Yang, Y.; Liu, Y.; Wang, Z. Triazinone Derivative Containing Acylhydrazone Structure and Preparation Method Therefor, and Insecticidal and Bactericidal Uses Thereof. CN Patent WO2019056247A1, 28 March 2019. [Google Scholar]
- Wang, Q.; Yang, Y.; Wang, Z.; Liu, Y. Triazone Derivatives Containing Acylhydrazone Structure, Preparation Method and Application Thereof in Insecticide and Antimicrobial Agent. Chinese Patent CN107266381A, 20 October 2017. [Google Scholar]
- Song, H.J.; Liu, Y.X.; Xiong, L.X.; Li, Y.Q.; Yang, N.; Wang, Q.M. Design, synthesis, and insecticidal activity of novel pyrazole derivatives containing alpha-hydroxymethyl-N-benzyl carboxamide, alpha-chloromethyl-N-benzyl carboxamide, and 4,5-dihydrooxazole moieties. J. Agric. Food Chem. 2012, 60, 1470–1479. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Xiong, L.; Li, Y.; Yang, N.; Wang, Q. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, oxime ester, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J. Agric. Food Chem. 2013, 61, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Gadhachanda, V.R.; Wu, B.; Wang, Z.; Kuhen, K.L.; Caldwell, J.; Zondler, H.; Walter, H.; Havenhand, M.; He, Y. 4-Aminopyrimidines as novel HIV-1 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Z.; Pan, S.; Zhu, M.; Guan, A.; Sun, X.; Zhang, J.; Song, Y.; Liu, C.; Yang, X. A new potential aphicide against Myzus persicae: Design, synthesis and 3D-QSAR of novel phenoxypyridine derivatives containing 4-aminopyrimidine. J. Mol. Struct. 2022, 1262, 132949. [Google Scholar] [CrossRef]
- Chai, B.S.; Zhang, J.B.; Song, Y.Q.; Yang, J.C.; Li, K.K.; Wang, L.Z.; Sun, X.F.; Liu, C.L. Preparation of Substituted Thienopyrimidinamine Compounds and Their Application as Insecticidal and Acaricidal Agents. Chinese Patent CN105218557A, 6 January 2016. [Google Scholar]
- Shao, X.; Li, Z.; Zhang, Y.; Zhou, C.; Cheng, J.; Xu, X.; Xu, Z. Preparation and Application of Pyrazolamide Compounds Containing Azo Structure. Chinese Patent CN113512002A, 19 October 2021. [Google Scholar]
- Chen, W.; Guan, A.Y.; Yang, L.; Cheng, Y.L.; Xu, Y.Q.; Ding, T.; Fang, X.M. The Synthesis and Biological Activity of 4-Chloro-1,3-dimethyl-N-(2-(6-phenoxypyridin-3-yl)ethyl)-1H-pyrazole-5-carboxamide. Agrochemicals 2014, 53, 316–318. [Google Scholar]
- Okada, I.; Suzuki, S.; Okui, S.; Takahashi, Y.; Fukuchi, T.; Nakajima, T. Preparation of [(5-pyrazolylcarboxamido)alkyl]pyridine Derivatives and Insecticidal, Miticidal, and Fungicidal Compositions Containing Them as Active Ingredients. JP Patent EP329020A1, 1989. [Google Scholar]
- Powell, G.F.; Ward, D.A.; Prescott, M.C.; Spiller, D.G.; White, M.R.H.; Turner, P.C.; Earley, F.G.P.; Phillips, J.; Rees, H.H. The molecular action of the novel insecticide, Pyridalyl. Insect Biochem. Mol. Biol. 2011, 41, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.; Liu, M.H.; Yu, H.; Ou, X.M.; Liu, X.P.; Ren, Y.G.; Long, C.Y.; Huang, L.; Yu, W.Q.; Shi, G.R.; et al. Discovery of nitropyridyl-based dichloropropene ethers as insecticides. Bioorg. Med. Chem. Lett. 2019, 29, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Domon, K.; Toriyabe, K.; Ogawa, Y.; Bessho, J.; Kawamoto, K.; Watanabe, A.; Komatsu, M.; Matsuda, T.; Ito, S. Preparation of Alkylphenylsulphide Derivatives as Pest Control Agents. JP Patent WO2013157229A1, 24 October 2013. [Google Scholar]
- Liu, Y.; Wang, M.; Xu, Y.; Wu, Y.; Fu, B.; Li, J.; Xiao, Y.; Qin, Z. Design, synthesis, and biological activity of sulfoximine derivatives. J. Heterocycl. Chem. 2022, 59, 729–738. [Google Scholar] [CrossRef]
- Du, S.; Yang, D.; Wan, C.; Zhao, F.; Qin, Z. Design, synthesis and insecticidal activities of aryloxypyridinyl ethanone oxime ethers. Nongyaoxue Xuebao 2021, 23, 62–68. [Google Scholar] [CrossRef]
- Tang, J.; Wang, R.; Liu, J.; Wu, X.; Niu, J. 4-(2-Phenylethylamino)pyrimidine-nicotine Derivatives as Pesticides and Their Preparation, Pharmaceutical Compositions and Use in the Pest Control. Chinese Patent CN103232434A, 7 August 2013. [Google Scholar]
- Nesterov, A.; Spalthoff, C.; Kandasamy, R.; Katana, R.; Rankl, N.B.; Andres, M.; Jahde, P.; Dorsch, J.A.; Stam, L.F.; Braun, F.J.; et al. TRP Channels in Insect Stretch Receptors as Insecticide Targets. Neuron 2015, 86, 665–671. [Google Scholar] [CrossRef]
- Min, L.J.; Wang, H.; Bajsa-Hirschel, J.; Yu, C.S.; Wang, B.; Yao, M.M.; Han, L.; Cantrell, C.L.; Duke, S.O.; Sun, N.B.; et al. Novel Dioxolane Ring Compounds for the Management of Phytopathogen Diseases as Ergosterol Biosynthesis Inhibitors: Synthesis, Biological Activities, and Molecular Docking. J. Agric. Food Chem. 2022, 70, 4303–4315. [Google Scholar] [CrossRef] [PubMed]
- Ulmschneider, S.; Dietz, J.; Renner, J.; Grote, T.; Grammenos, W.; Mueller, B.; Lohmann, J.K.; Vrettou-Schultes, M. Triazole Compounds Carrying a Sulfur Substituent and Their Use as Fungicides. DE Patent WO2010146116A1, 23 December 2010. [Google Scholar]
- Dietz, J.; Riggs, R.; Boudet, N.; Lohmann, J.; Craig, I.R.; Haden, E.; Lauterwasser, E.; Mueller, B.; Grammenos, W.; Grote, T. Preparation of halogenalkylphenoxyphenyltriazolylethanol Derivatives for Use as Fungicides. Patent WO2013007767A1, 17 January 2013. [Google Scholar]












| No. | Pesticides Containing Diphenyl Ether | Compounds Containing Phenoxypyridine | ||
|---|---|---|---|---|
| Structure | Activity | Structure | Activity | |
| 1 |  Famoxadone [9] | Rhizoctonia solani IC50 > 100 mg/L Pythium aphanidermatum IC50 > 100 mg/L Pyricularia grisea IC50 > 100 mg/L Phomopsis asparagi IC50 > 100 mg/L | 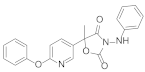 A [9] | Rhizoctonia solani IC50 = 6.53 mg/L Pythium aphanidermatum IC50 = 8.62 mg/L Pyricularia grisea IC50 = 11.46 mg/L Phomopsis asparagi IC50 = 16.2 mg/L |
| 2 |  Difenoconazole [10] | Rhizoctonia solani EC50 = 8.93 mg/L Pyricularia oryae EC50 = 2.42 mg/L Gibberella zeae EC50 = 4.40 mg/L Botrytis cinerea EC50 > 20 mg/L |  B [11] | Botrytis cinerea ED50 = 2.7 mg/L Septoria tritici ED50 = 0.008 mg/L Pyricularia oryzae ED50 = 1.2 mg/L |
 C [12] | Botrytis cinerea ED50 = 8.9 mg/L Septoria tritici ED50 = 0.013 mg/L Pyricularia oryzae ED50 = 9.5 mg/L | |||
| 3 | 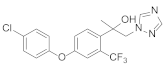 Mefentrifluconazole [13] | 30% in vivo protective activity against Sphaerootheca at 10 mg/L |  D [14] | 100% in vivo protective activity against Sphaerootheca at 10 mg/L |
| 4 |  E [15] | EC50 = 8.62 mg/L |  F [15] | EC50 = 0.19 mg/L |
| 5 |  G [15] | Cucumber Downy Mildew EC50 = 6.25–25 mg/L |  H [15] | Cucumber Downy Mildew EC50 = 2.65 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fu, B.; Xu, Y.; Ren, B.; Qin, Z. Advancement of Phenoxypyridine as an Active Scaffold for Pesticides. Molecules 2022, 27, 6803. https://doi.org/10.3390/molecules27206803
Liu Y, Fu B, Xu Y, Ren B, Qin Z. Advancement of Phenoxypyridine as an Active Scaffold for Pesticides. Molecules. 2022; 27(20):6803. https://doi.org/10.3390/molecules27206803
Chicago/Turabian StyleLiu, Yanfei, Bin Fu, Yanjun Xu, Bo Ren, and Zhaohai Qin. 2022. "Advancement of Phenoxypyridine as an Active Scaffold for Pesticides" Molecules 27, no. 20: 6803. https://doi.org/10.3390/molecules27206803
APA StyleLiu, Y., Fu, B., Xu, Y., Ren, B., & Qin, Z. (2022). Advancement of Phenoxypyridine as an Active Scaffold for Pesticides. Molecules, 27(20), 6803. https://doi.org/10.3390/molecules27206803