Structure-Based Bioisosterism Design, Synthesis, Biological Activity and Toxicity of 1,2,4-Oxadiazole Substituted Benzamides Analogues Containing Pyrazole Rings
Abstract
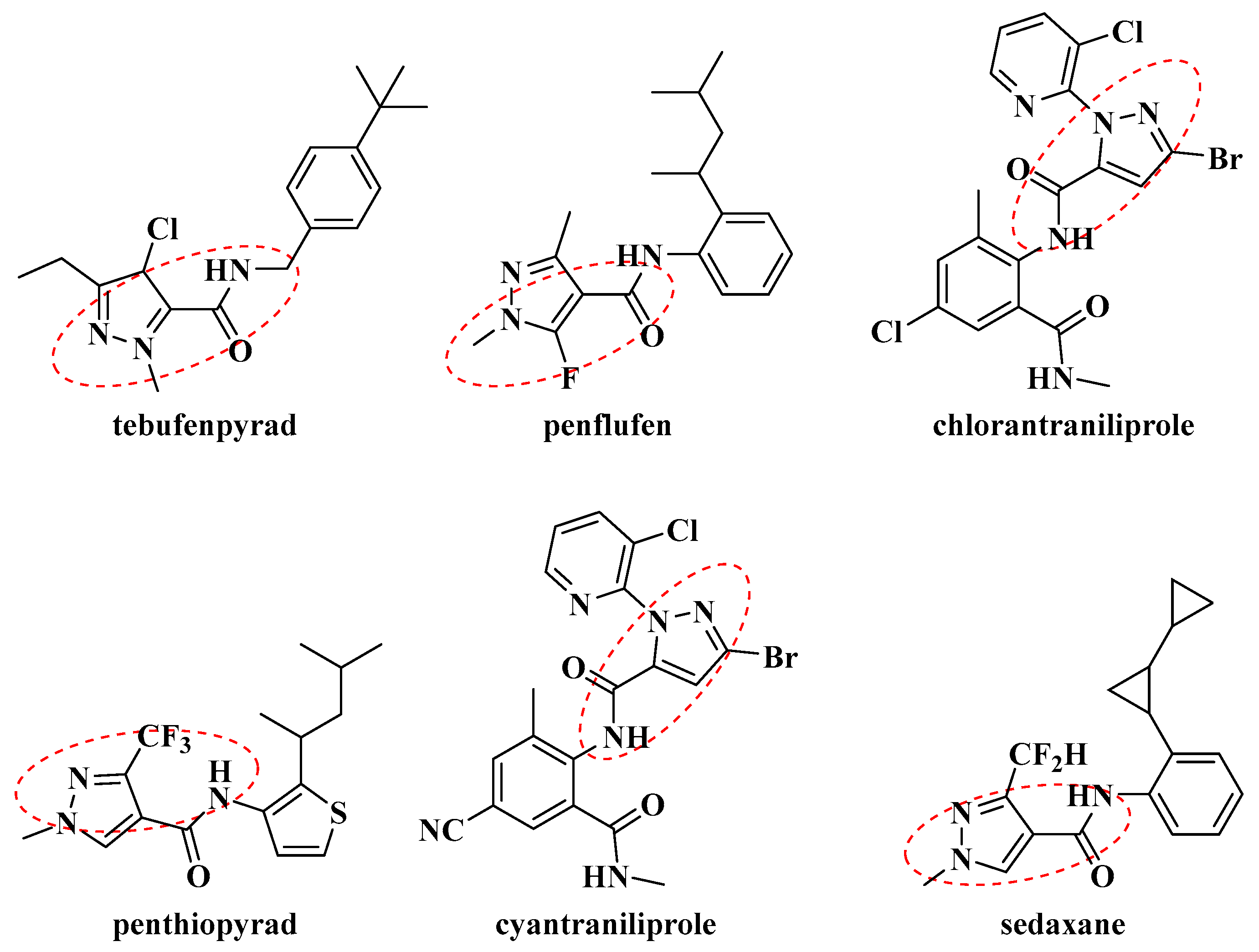
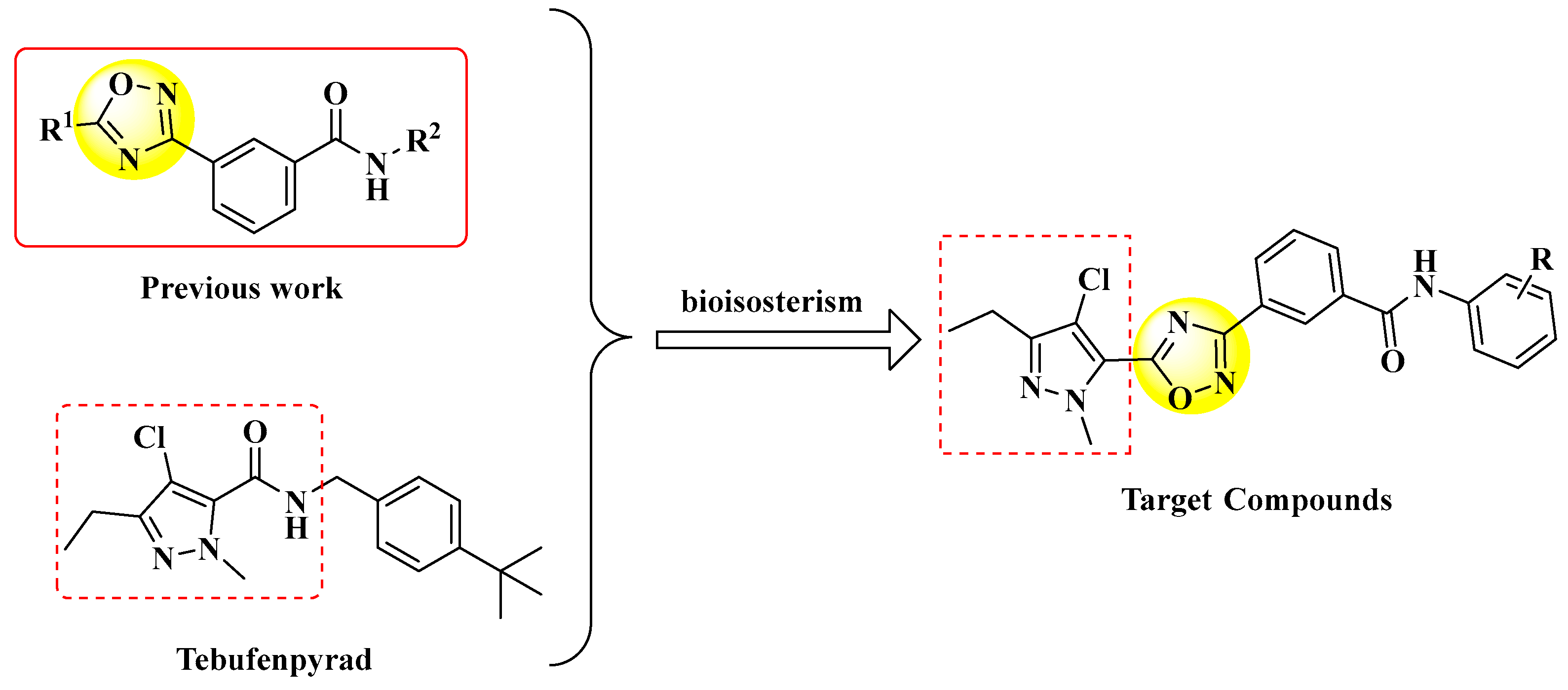
:1. Introduction
2. Results and Discussion
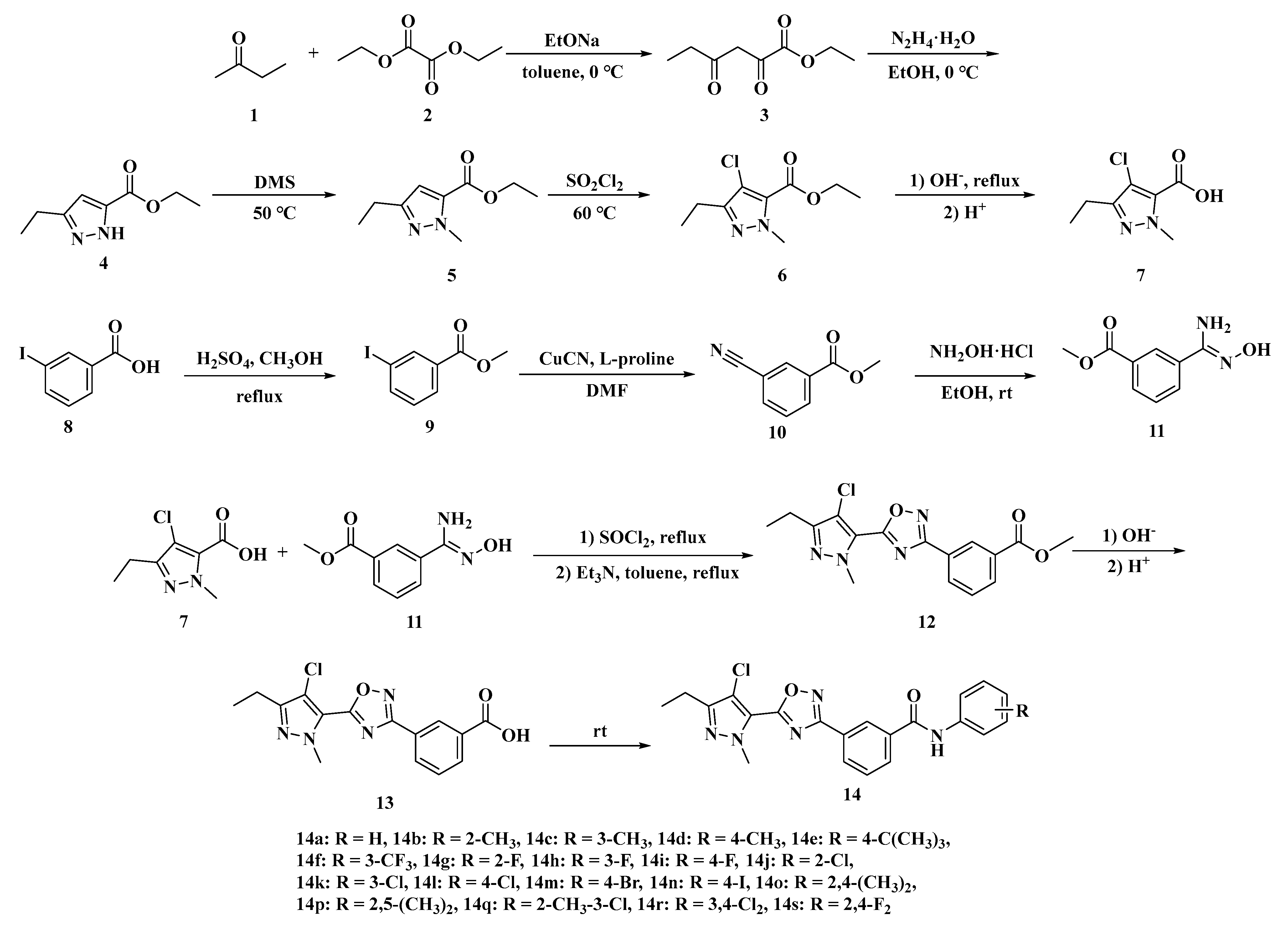
2.1. Synthesis of Target Compounds
2.2. Spectral Analysis of Target Compounds
2.3. Biological Activities of Target Compounds
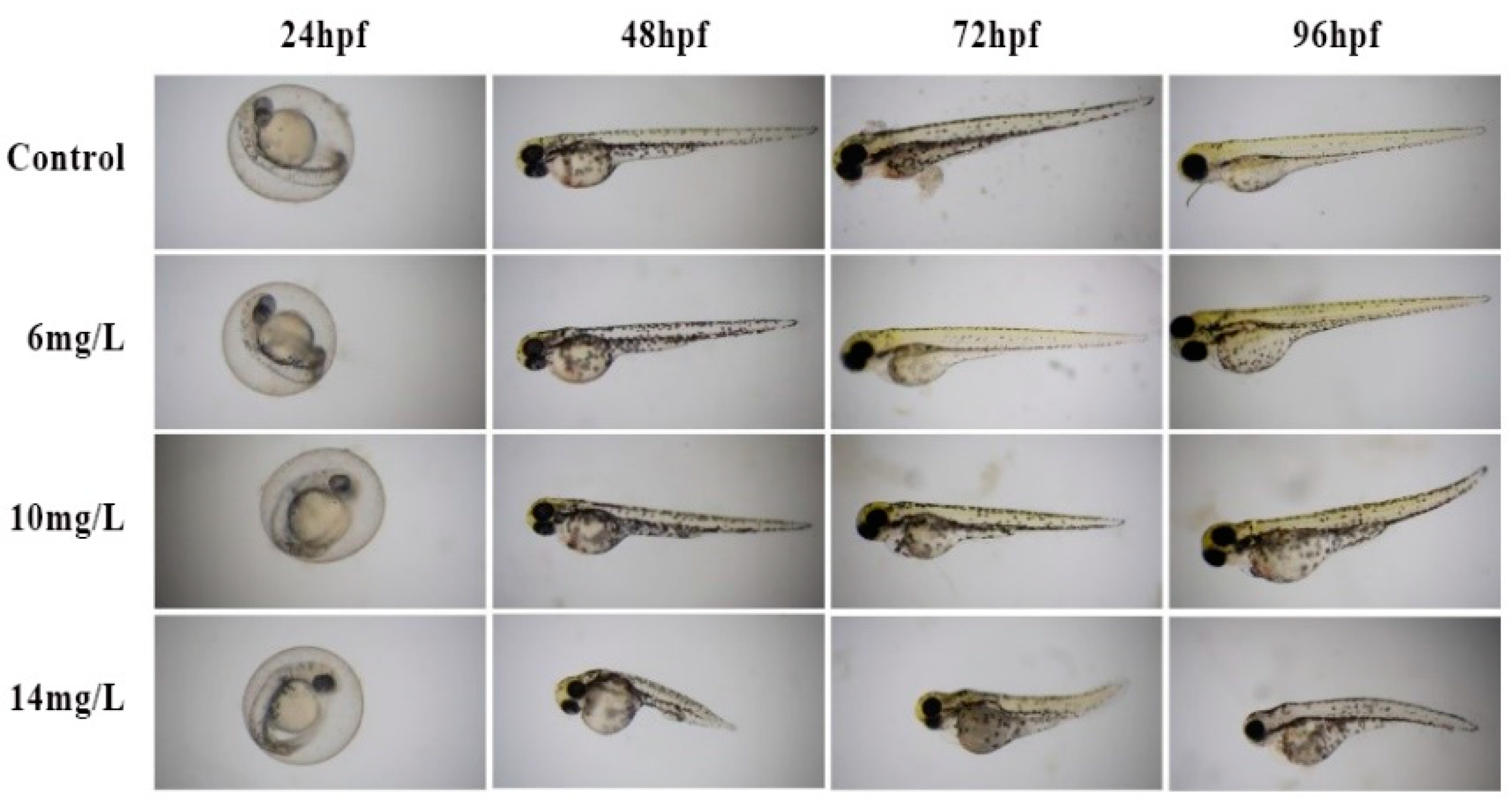
2.4. Toxicity to Zebrafish Embryo
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Ethyl 2,4-Dioxohexanoate 3
3.2.2. Ethyl 3-Ethyl-1H-pyrazole-5-carboxylate 4
3.2.3. Ethyl 3-Ethyl-1-methyl-1H-pyrazole-5-carboxylate 5
3.2.4. Ethyl 4-Chloro-3-ethyl-1-methyl-1H-pyrazole-5-carboxylate 6
3.2.5. Intermediate 7
3.2.6. Intermediate 11
3.2.7. Methyl 3-(5-(4-Chloro-3-ethyl-1-methyl-1H-pyrazol-5-yl)-1,2,4-oxadiazol-3-yl)benzoate 12
3.2.8. 3-(5-(4-Chloro-3-ethyl-1-methyl-1H-pyrazol-5-yl)-1,2,4-oxadiazol-3-yl)benzoic Acid 13
3.2.9. Target Compounds 14
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, X.H.; Qiao, L.; Zhai, Z.W.; Cai, P.P.; Cantrell, C.L.; Tan, C.X.; Weng, J.Q.; Han, L.; Wu, H.K. Novel 4-pyrazole carboxamide derivatives containing flexible chain motif: Design, synthesis and antifungal activity. Pest Manag. Sci. 2019, 75, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, E.; Kashige, N.; Nagabuchi, H.; Okabe-Nakahara, F.; Maruoka, H. Synthesis and Evaluation for Biological Activities of 2-Thio-Acylated Thiazoles Containing Pyrazole Moiety. Heterocycles 2019, 98, 1736–1746. [Google Scholar] [CrossRef]
- Shi, J.J.; Ren, G.H.; Wu, N.J.; Weng, J.Q.; Xu, T.M.; Liu, X.H.; Tan, C.X. Design, synthesis and insecticidal activities of novel anthranilic diamides containing polyfluoroalkyl pyrazole moiety. Chin. Chem. Lett. 2017, 28, 1727–1730. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhang, H.Q.; Liu, J.; Yang, X.P.; Liu, Z. Stereoselective Synthesis and Antifungal Activities of (E)-r-(Methoxyimino)benzeneacetate Derivatives Containing 1,3,5-Substituted Pyrazole Ring. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef]
- Fu, Q.; Cai, P.P.; Cheng, L.; Zhong, L.K.; Tan, C.X.; Shen, Z.H.; Han, L.; Xu, T.M.; Liu, X.H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020, 76, 868–879. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Yuan, J.; Yang, G.; Xu, T.M.; Peng, W.L. Synthesis, nematocidal activity and docking study of novel chiral 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3626–3628. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Ren, C.L.; Li, C.; Zhong, L.K.; Xu, T.M.; Tan, C.X. Synthesis and Insecticidal Activity of 3-Ethyl Sulfone Pyridine Substituted Aryl Triazole Compounds. Chin. J. Org. Chem. 2021, 41, 2055–2062. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, R.; Gao, S.; Ma, S.H.; Tang, H.J.; Yang, J.J.; Diao, Y.M.; Wang, H.L.; Zhu, H.J. Structure-based bioisosterism design, synthesis, insecticidal activity and structure-activity relationship (SAR) of anthranilic diamide analogues containing 1,2,4-oxadiazole rings. Pest Manag. Sci. 2017, 73, 917–924. [Google Scholar] [CrossRef]
- Haugwitz, R.D.; Martinez, A.J.; Venslavsky, J.; Angel, R.G.; Maurer, B.V.; Jacobs, G.A.; Narayanan, V.L.; Cruthers, L.R.; Szanto, J. Antiparasitic agents. 6. Synthesis and anthelmintic activities of novel isothiocyanatophenyl-1,2,4-oxadiazoles. J. Med. Chem. 1985, 28, 1234–1241. [Google Scholar] [CrossRef]
- King, W.F.; Wheeler, R.E. Substituted Oxadiazoles and Their Use as Corn Root Worm Insecticides. U.S. Patent US4237121A, 2 December 1981. [Google Scholar]
- Sangshetti, J.N.; Nagawade, R.R.; Shinde, D.B. Synthesis of novel 3-(1-(1-substituted piperidin-4-yl)-1H-1,2,3-triazol-4-yl)-1,2,4-oxadiazol-5(4H)-one as antifungal agents. Bioorg. Med. Chem. Lett. 2009, 19, 3564–3567. [Google Scholar] [CrossRef]
- Wiebe, C.; Terteryan-Seiser, V.; Grammenos, W.; Craig, I.R.; Quintero Palomar, M.A.; Mentzel, T.; Fehr, M.; Escribano Cuesta, A.; Grote, T.; Lohmann, J.K.; et al. New Substituted Oxadiazoles Useful for Combating Phytopathogenic Harmful Fungi. WO Patent WO2017178245A1, 19 October 2017. [Google Scholar]
- Liu, D.; Luo, L.; Wang, Z.X.; Ma, X.Y.; Gan, X.H. Design, Synthesis and Antifungal/Nematicidal Activity of Novel 1,2,4-Oxadiazole Derivatives Containing Amide Fragments. Int. J. Mol. Sci. 2022, 23, 2607. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.K.; Chung, K.H.; Lee, W.H.; Kim, J.N.; Hong, K.S. Herbicidal Quinolinyloxadiazoles. U.S. Patent US5489687, 6 February 1996. [Google Scholar]
- Vijaya Bhargavi, M.; Shashikala, P.; Sumakanth, M.; Krishna, C. Synthesis, Molecular Docking, Analgesic, and Anti-Inflammatory Activities of New 1,2,4-Oxadiazolo-Sulfonamides. Russ. J. Gen. Chem. 2018, 88, 804–811. [Google Scholar] [CrossRef]
- Maftei, C.V.; Fodor, E.; Jones, P.G.; Daniliuc, C.G.; Franz, M.H.; Kelter, G.; Fiebig, H.-H.; Tamm, M.; Neda, I. Novel 1,2,4-oxadiazoles and trifluoromethylpyridines related to natural products: Synthesis, structural analysis and investigation of their antitumor activity. Tetrahedron 2016, 72, 1185–1199. [Google Scholar] [CrossRef]
- Zhang, S.; Meng, S.Q.; Xie, Y.; Yang, Y.G.; Zhang, Y.M.; He, L.; Wang, K.; Qi, Z.Q.; Ji, M.S.; Qin, P.W.; et al. Synthesis, Fungicidal Activity and SAR of 2-Thiazolamide/Pyrazolamide-Cyclohexylsulfonamides against Botrytis cinerea. Molecules 2019, 24, 14. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.B.; Jin, X.Y.; Dong, Y.W.; Guo, B.B.; Cui, L.; Deng, X.L.; Zhang, L.; Yang, Q.; Li, Y.X.; Yang, X.L.; et al. Design, Synthesis, and Biological Activity of Novel Heptacyclic Pyrazolamide Derivatives: A New Candidate of Dual-Target Insect Growth Regulators. J. Agric. Food Chem. 2020, 68, 6347–6354. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J.; Liao, M.; Chu, M.J.; Ren, Z.L.; Zhang, X.; Lv, X.H.; Cao, H.Q. Design, synthesis and anti-tobacco mosaic virus (TMV) activity of 5-chloro-N-(4-cyano-1-aryl-1H-pyrazol-5-yl)-1-aryl-3-methyl-1H-pyrazole-4-carboxa mide derivatives. Molecules 2015, 20, 807–821. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Hu, D.; Kuang, J.; Cai, H.; Wu, S.; Xue, W. Synthesis and antifungal activity of N-(substituted pyridinyl)-1-methyl(phenyl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Molecules 2012, 17, 14205–14218. [Google Scholar] [CrossRef] [Green Version]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M. A retrospective look at anthranilic diamide insecticides: Discovery and lead optimization to chlorantraniliprole and cyantraniliprole. Pest Manag. Sci. 2017, 73, 658–665. [Google Scholar] [CrossRef]
- Culbreath, A.K.; Brenneman, T.B.; Kemerait, R.C.; Hammes, G.G. Effect of the new pyrazole carboxamide fungicide penthiopyrad on late leaf spot and stem rot of peanut. Pest Manag. Sci. 2009, 65, 66–73. [Google Scholar] [CrossRef]
- Oostendorp, M.; Zeun, R. Sedaxane, a new experimental active ingredient from Syngenta for seed treatment use. Phytopathology 2011, 101, S133. [Google Scholar]
- Yang, S.; Tian, X.Y.; Ma, T.Y.; Dai, L.; Ren, C.L.; Mei, J.C.; Liu, X.H.; Tan, C.X. Synthesis and Biological Activity of Benzamides Substituted with Pyridine-Linked 1,2,4-Oxadiazole. Molecules 2020, 25, 3500. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ren, C.L.; Ma, T.Y.; Zou, W.Q.; Dai, L.; Tian, X.Y.; Liu, X.H.; Tan, C.X. 1,2,4-Oxadiazole-Based Bio-Isosteres of Benzamides: Synthesis, Biological Activity and Toxicity to Zebrafish Embryo. Int. J. Mol. Sci. 2021, 22, 2367. [Google Scholar] [CrossRef] [PubMed]
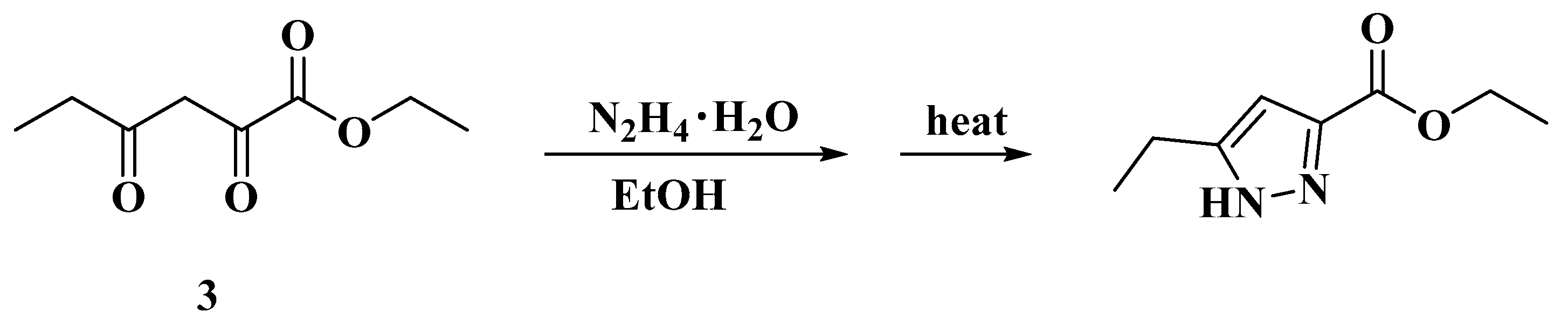

| Compounds | R | Insecticidal Activities (Death Rates %) | ||||
|---|---|---|---|---|---|---|
| Mythimna separate 500 mg/L | Helicoverpa armigera 500 mg/L | Ostrinia nubilalis 500 mg/L | Spodoptera frugiperda 500 mg/L | Culex pipiens pallens 10 mg/L | ||
| 14a | H | 50 | 20 | 10 | 0 | 10 |
| 14b | 2-CH3 | 20 | 10 | 5 | 10 | 0 |
| 14c | 3-CH3 | 30 | 10 | 10 | 5 | 25 |
| 14d | 4-CH3 | 10 | 15 | 30 | 0 | 0 |
| 14e | 4-t-Bu | 20 | 25 | 40 | 0 | 5 |
| 14f | 3-CF3 | 50 | 35 | 50 | 15 | 20 |
| 14g | 2-F | 25 | 30 | 45 | 0 | 5 |
| 14h | 3-F | 30 | 35 | 50 | 20 | 0 |
| 14i | 4-F | 35 | 20 | 30 | 70 | 30 |
| 14j | 2-Cl | 15 | 25 | 40 | 25 | 0 |
| 14k | 3-Cl | 40 | 10 | 0 | 10 | 15 |
| 14l | 4-Cl | 30 | 15 | 20 | 30 | 5 |
| 14m | 4-Br | 5 | 0 | 0 | 0 | 0 |
| 14n | 4-I | 20 | 10 | 5 | 0 | 10 |
| 14o | 2,4-di-CH3 | 10 | 15 | 15 | 20 | 25 |
| 14p | 2,6-di-CH3 | 20 | 15 | 10 | 30 | 0 |
| 14q | 3-Cl-2-CH3 | 70 | 45 | 15 | 40 | 25 |
| 14r | 3,4-di-Cl | 40 | 10 | 5 | 10 | 0 |
| 14s | 2,4-di-F | 50 | 20 | 10 | 0 | 15 |
| Tebufenpyrad | 60 | 45 | 40 | 30 | 45 | |
| Compounds | R | Fungicidal Activities (Inhibition Rate %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS | GZ | PO | PC | SS | BC | RS | FO | CA | PP | ||
| 14a | H | 21.4 | 17.6 | 33.3 | 18.8 | 16.1 | 11.4 | 24.4 | 17.4 | 13.3 | 19.4 |
| 14b | 2-CH3 | 21.4 | 26.5 | 22.2 | 9.4 | 29.0 | 13.6 | 22.0 | 8.7 | 6.7 | 8.3 |
| 14c | 3-CH3 | 21.4 | 32.4 | 55.6 | 18.8 | 32.3 | 18.2 | 4.9 | 8.7 | 20.0 | 25.0 |
| 14d | 4-CH3 | 28.6 | 44.1 | 44.4 | 18.8 | 9.7 | 4.5 | 12.2 | 17.4 | 13.3 | 8.3 |
| 14e | 4-t-Bu | 21.4 | 38.2 | 55.6 | 12.5 | 16.1 | 18.2 | 4.9 | 8.7 | 6.7 | 8.3 |
| 14f | 3-CF3 | 21.4 | 23.5 | 11.1 | 12.5 | 29.0 | 22.7 | 7.3 | 8.7 | 6.7 | 11.1 |
| 14g | 2-F | 21.4 | 23.5 | 33.3 | 9.4 | 45.2 | 13.6 | 22.0 | 8.7 | 40.0 | 11.1 |
| 14h | 3-F | 21.4 | 35.3 | 77.8 | 3.1 | 38.7 | 13.6 | 4.9 | 8.7 | 6.7 | 2.8 |
| 14i | 4-F | 7.1 | 44.1 | 33.3 | 3.1 | 32.3 | 18.2 | 12.2 | 8.7 | 26.7 | 30.6 |
| 14j | 2-Cl | 21.4 | 14.7 | 44.4 | 3.1 | 41.9 | 22.7 | 36.6 | 4.3 | 40.0 | 33.3 |
| 14k | 3-Cl | 14.3 | 35.3 | 66.7 | 12.5 | 38.7 | 13.6 | 4.9 | 4.3 | 6.7 | 13.9 |
| 14l | 4-Cl | 14.3 | 32.4 | 11.1 | 12.5 | 32.3 | 18.2 | 36.6 | 4.3 | 33.3 | 11.1 |
| 14m | 4-Br | 7.1 | 17.6 | 11.1 | 12.5 | 38.7 | 13.6 | 12.2 | 4.3 | 26.7 | 30.6 |
| 14n | 4-I | 50.0 | 55.9 | 66.7 | 12.5 | 58.1 | 31.8 | 65.9 | 13.0 | 53.3 | 30.6 |
| 14o | 2,4-di-CH3 | 21.4 | 35.3 | 11.1 | 12.5 | 58.1 | 31.8 | 48.8 | 8.7 | 60.6 | 25.0 |
| 14p | 2,6-di-CH3 | 28.6 | 38.2 | 33.3 | 9.4 | 51.6 | 27.3 | 4.9 | 8.7 | 46.7 | 22.2 |
| 14q | 3-Cl-2-CH3 | 21.4 | 32.4 | 44.4 | 18.8 | 48.4 | 40.9 | 31.7 | 4.3 | 40.0 | 22.2 |
| 14r | 3,4-di-Cl | 21.4 | 17.6 | 55.6 | 9.4 | 25.8 | 31.8 | 24.4 | 8.7 | 6.7 | 11.1 |
| 14s | 2,4-di-F | 21.4 | 23.5 | 44.4 | 3.1 | 32.3 | 22.7 | 46.3 | 8.7 | 40.0 | 11.1 |
| Bixafen | 92.9 | 70.6 | 100.0 | 40.6 | 100.0 | 72.7 | 92.7 | 73.9 | 86.7 | 77.8 | |
| Compounds | y = a + bx | r2 | EC50/(mg·L−1) |
|---|---|---|---|
| 14h | y = 1.6022x + 3.0305 | 0.9968 | 16.95 |
| bixafen | y = 1.7973x + 3.2716 | 0.9766 | 9.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, M.-T.; Shao, Y.-Y.; Yang, S.; Sun, B.-L.; Wang, Y.-Y.; Tan, C.-X.; Wang, X.-D. Structure-Based Bioisosterism Design, Synthesis, Biological Activity and Toxicity of 1,2,4-Oxadiazole Substituted Benzamides Analogues Containing Pyrazole Rings. Molecules 2022, 27, 4692. https://doi.org/10.3390/molecules27154692
Tu M-T, Shao Y-Y, Yang S, Sun B-L, Wang Y-Y, Tan C-X, Wang X-D. Structure-Based Bioisosterism Design, Synthesis, Biological Activity and Toxicity of 1,2,4-Oxadiazole Substituted Benzamides Analogues Containing Pyrazole Rings. Molecules. 2022; 27(15):4692. https://doi.org/10.3390/molecules27154692
Chicago/Turabian StyleTu, Min-Ting, Ying-Ying Shao, Sen Yang, Bin-Long Sun, Ying-Ying Wang, Cheng-Xia Tan, and Xue-Dong Wang. 2022. "Structure-Based Bioisosterism Design, Synthesis, Biological Activity and Toxicity of 1,2,4-Oxadiazole Substituted Benzamides Analogues Containing Pyrazole Rings" Molecules 27, no. 15: 4692. https://doi.org/10.3390/molecules27154692
APA StyleTu, M.-T., Shao, Y.-Y., Yang, S., Sun, B.-L., Wang, Y.-Y., Tan, C.-X., & Wang, X.-D. (2022). Structure-Based Bioisosterism Design, Synthesis, Biological Activity and Toxicity of 1,2,4-Oxadiazole Substituted Benzamides Analogues Containing Pyrazole Rings. Molecules, 27(15), 4692. https://doi.org/10.3390/molecules27154692






