Crab-Eating Monkey Acidic Chitinase (CHIA) Efficiently Degrades Chitin and Chitosan under Acidic and High-Temperature Conditions
Abstract
:1. Introduction
2. Results
2.1. Preparation of Recombinant Monkey CHIA
2.2. Monkey CHIA Efficiently Degrades Chitin Substrates at High Temperatures
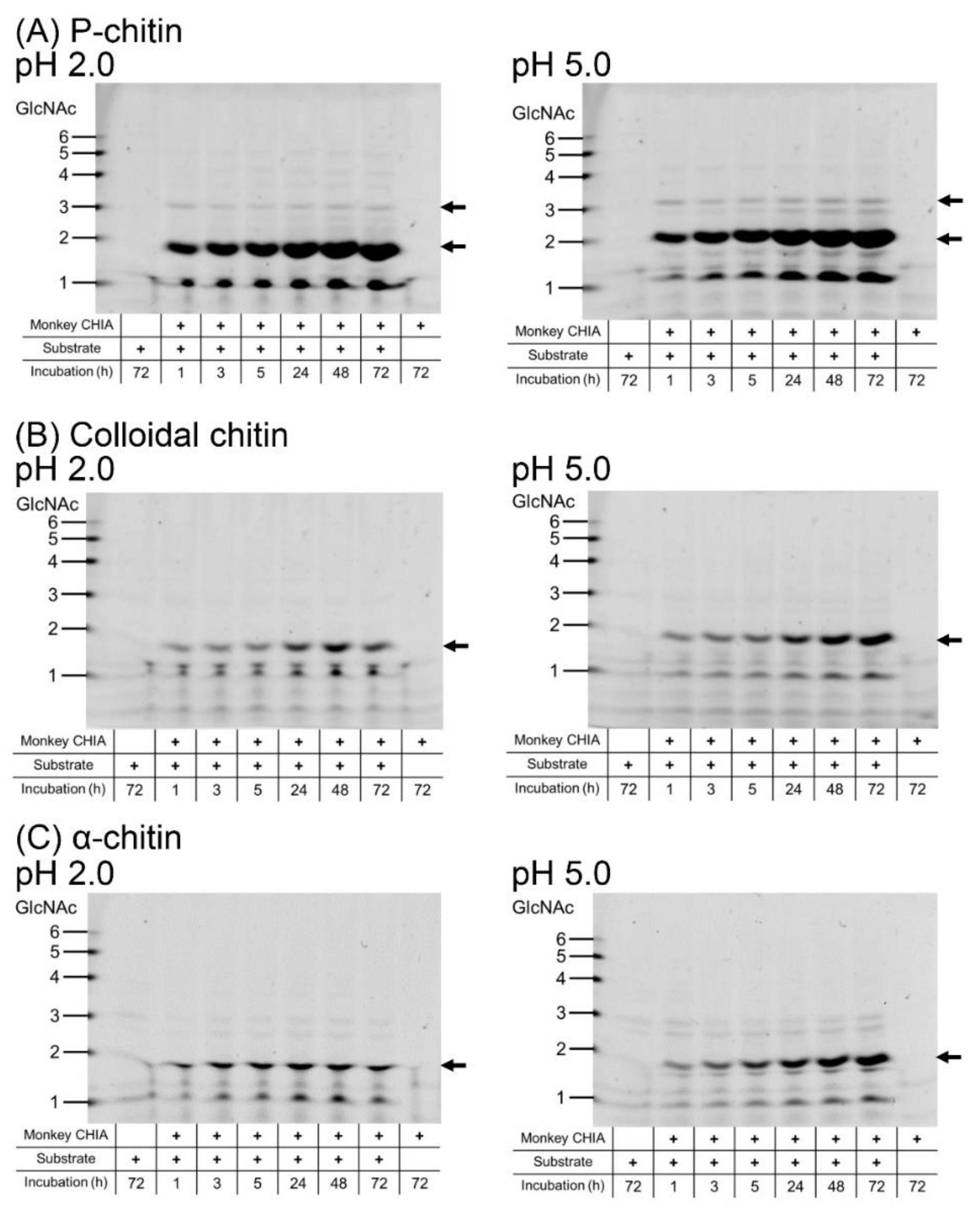
2.3. P-Chitin Is a Superior Substrate for GlcNAc Dimer Production
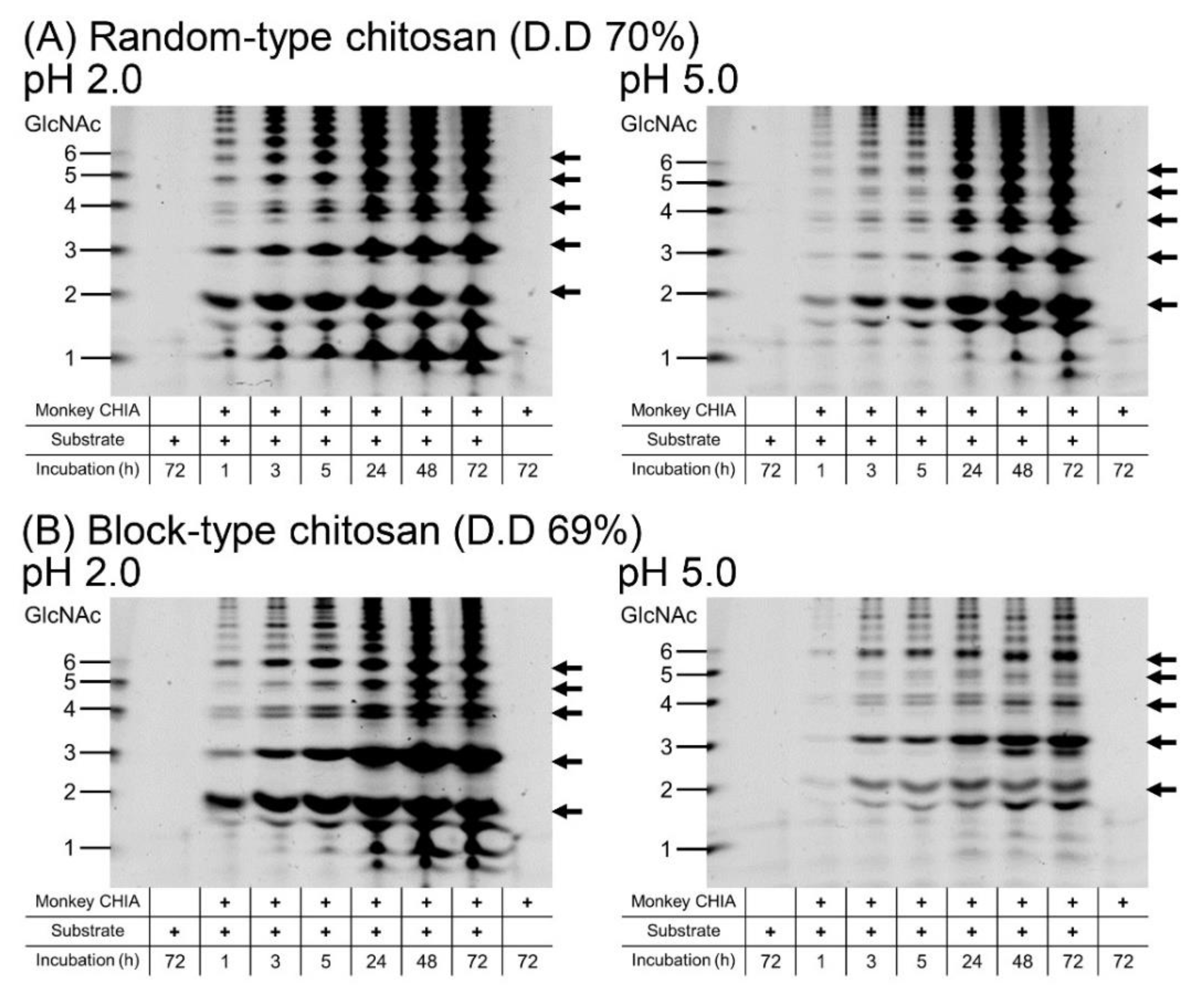
2.4. GlcNAc Dimer Production from Chitosan
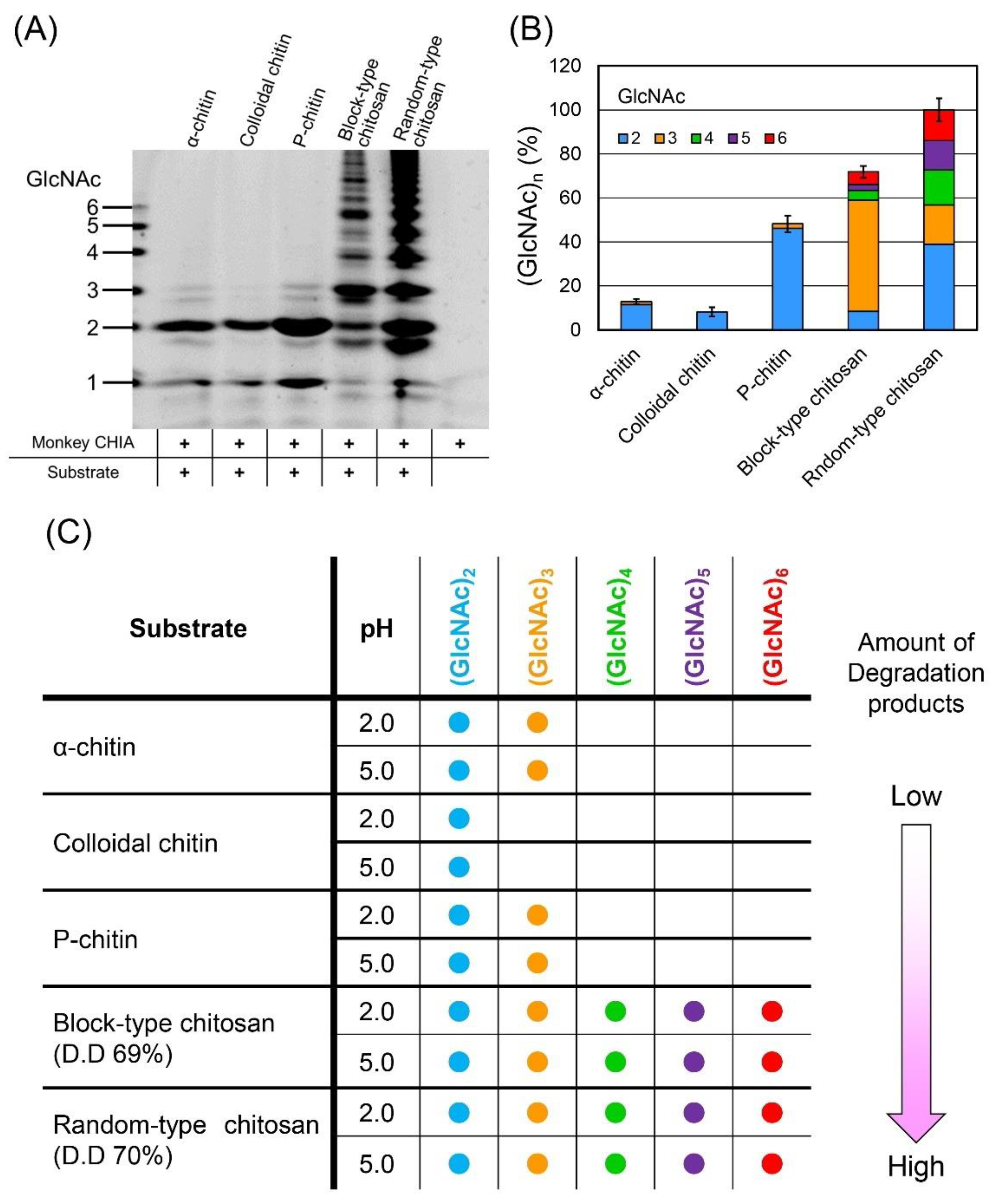
2.5. Pattern of the Chitooligosaccharides Produced by Monkey CHIA from Different Chitin and Chitosan Substrates
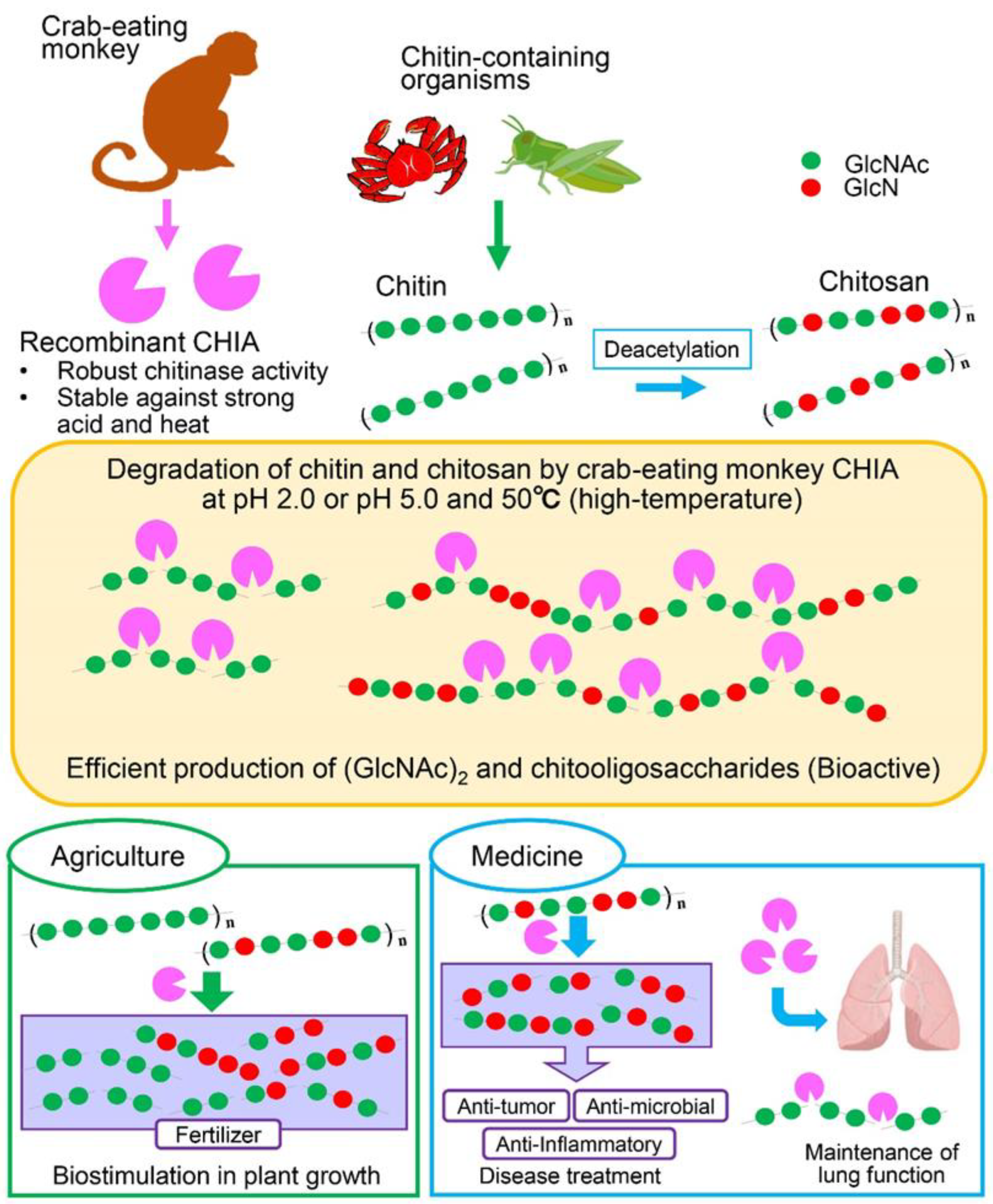
3. Discussion
4. Materials and Methods
4.1. Preparation of the Recombinant Monkey CHIA Expressed in E. coli
4.2. Chitin and Chitosan Substrates
4.3. Degradation of Chitin and Chitosan by Recombinant Monkey CHIA
4.4. Analysis of Chitooligosaccharides by FACE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef] [Green Version]
- Koch, B.E.V.; Stougaard, J.; Spaink, H.P. Keeping track of the growing number of biological functions of chitin and its interaction partners in biomedical research. Glycobiology 2015, 25, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Bueter, C.L.; Specht, C.A.; Levitz, S.M. Innate sensing of chitin and chitosan. PLoS Pathog. 2013, 9, e1003080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juang, R.S.; Shao, H.J. A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res 2002, 36, 2999–3008. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromol. Chem. Macromol. Chem. Phys. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Kurita, K.; Sannan, T.; Iwakura, Y. Studies on chitin, 4. Evidence for formation of block and random copolymers of N-acetyl-d-glucosamine and d-glucosamine by hetero- and homogeneous hydrolyses. Macromol Chem Phys. 1977, 178, 3197–3202. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Locksley, R.M. Chitins and chitinase activity in airway diseases. J. Allergy Clin. Immunol. 2018, 142, 364–369. [Google Scholar] [CrossRef]
- Boot, R.G.; Blommaart, E.F.C.; Swart, E.; der Vlugt, K.G.-V.; Bijl, N.; Moe, C.; Place, A.; Aerts, J. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 2001, 276, 6770–6778. [Google Scholar] [CrossRef] [Green Version]
- Boot, R.G.; Bussink, A.P.; Verhoek, M.; de Boer, P.A.; Moorman, A.F.; Aerts, J.M. Marked differences in tissue-specific expression of chitinases in mouse and man. J. Histochem. Cytochem. 2005, 53, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280 Pt 2, 309–316. [Google Scholar] [CrossRef]
- Bussink, A.P.; Speijer, D.; Aerts, J.M.; Boot, R.G. Evolution of mammalian chitinase (-like) members of family 18 glycosyl hydrolases. Genetics 2007, 177, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The carbohydrate-active enzymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.G.; Renkema, G.H.; Strijland, A.; van Zonneveld, A.J.; Aerts, J.M. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J. Biol. Chem. 1995, 270, 26252–26256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eide, K.B.; Norberg, A.L.; Heggset, E.B.; Lindbom, A.R.; Varum, K.M.; Eijsink, V.G.; Sorlie, M. Human chitotriosidase-catalyzed hydrolysis of chitosan. Biochemistry 2012, 51, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Reese, T.A.; Liang, H.E.; Tager, A.M.; Luster, A.D.; Van Rooijen, N.; Voehringer, D.; Locksley, R.M. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007, 447, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Bierbaum, S.; Nickel, R.; Koch, A.; Lau, S.; Deichmann, K.A.; Wahn, U.; Superti-Furga, A.; Heinzmann, A. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 1505–1509. [Google Scholar] [CrossRef] [Green Version]
- Seibold, M.A.; Reese, T.A.; Choudhry, S.; Salam, M.T.; Beckman, K.; Eng, C.; Atakilit, A.; Meade, K.; Lenoir, M.; Watson, H.G.; et al. Differential enzymatic activity of common haplotypic versions of the human acidic mammalian chitinase protein. J. Biol. Chem. 2009, 284, 19650–19658. [Google Scholar] [CrossRef] [Green Version]
- Okawa, K.; Ohno, M.; Kashimura, A.; Kimura, M.; Kobayashi, Y.; Sakaguchi, M.; Sugahara, Y.; Kamaya, M.; Kino, Y.; Bauer, P.O.; et al. Loss and gain of human acidic mammalian chitinase activity by nonsynonymous SNPs. Mol. Biol. Evol. 2016, 33, 3183–3193. [Google Scholar] [CrossRef] [Green Version]
- Van Dyken, S.J.; Liang, H.-E.; Naikawadi, R.P.; Woodruff, P.G.; Wolters, P.J.; Erle, D.J.; Locksley, R.M. Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 2017, 169, 497–509.e13. [Google Scholar] [CrossRef] [Green Version]
- Ohno, M.; Kimura, M.; Miyazaki, H.; Okawa, K.; Onuki, R.; Nemoto, C.; Tabata, E.; Wakita, S.; Kashimura, A.; Sakaguchi, M.; et al. Acidic mammalian chitinase is a proteases-resistant glycosidase in mouse digestive system. Sci. Rep. 2016, 6, 37756. [Google Scholar] [CrossRef] [PubMed]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Kino, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Gastric and intestinal proteases resistance of chicken acidic chitinase nominates chitin-containing organisms for alternative whole edible diets for poultry. Sci. Rep. 2017, 7, 6662. [Google Scholar] [CrossRef] [Green Version]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Imamura, Y.; Seki, S.; Ueda, H.; Matoska, V.; et al. Protease resistance of porcine acidic mammalian chitinase under gastrointestinal conditions implies that chitin-containing organisms can be sustainable dietary resources. Sci. Rep. 2017, 7, 12963. [Google Scholar] [CrossRef] [Green Version]
- Tabata, E.; Kashimura, A.; Uehara, M.; Wakita, S.; Sakaguchi, M.; Sugahara, Y.; Yurimoto, T.; Sasaki, E.; Matoska, V.; Bauer, P.; et al. High expression of acidic chitinase and chitin digestibility in the stomach of common marmoset (Callithrix jacchus), an insectivorous nonhuman primate. Sci. Rep. 2019, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Shen, K.-T.; Chen, M.-H.; Chan, H.-Y.; Jeng, J.-H.; Wang, Y.-J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, X.-F.; Du, Y.-G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Winkler, A.J.; Dominguez-Nuñez, J.A.; Aranaz, I.; Poza-Carrión, C.; Ramonell, K.; Somerville, S.; Berrocal-Lobo, M. Short-chain chitin oligomers: Promoters of plant growth. Mar. Drugs 2017, 15, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, K.; Liu, S.; Zou, P.; Xing, R.; Yu, H.; Chen, X.; Qin, Y.; Li, P. Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of wheat seedlings under salt stress. J. Agric. Food Chem. 2017, 65, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Jiang, S.; Jiang, S.; Zhou, Y.; Zhang, G. A Bacillus pumilus originated beta-N-acetylglucosaminidase for chitin combinatory hydrolysis and exploration of its thermostable mechanism. Int. J. Biol. Macromol. 2019, 132, 1282–1289. [Google Scholar] [CrossRef]
- Oyeleye, A.; Normi, Y.M. Chitinase: Diversity, limitations, and trends in engineering for suitable applications. Biosci. Rep. 2018, 38, BSR2018032300. [Google Scholar] [CrossRef]
- Barad, B.A.; Liu, L.; Diaz, R.E.; Basilio, R.; Van Dyken, S.J.; Locksley, R.M.; Fraser, J.S. Differences in the chitinolytic activity of mammalian chitinases on soluble and insoluble substrates. Protein Sci. 2020, 29, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Kazami, N.; Sakaguchi, M.; Mizutani, D.; Masuda, T.; Wakita, S.; Oyama, F.; Kawakita, M.; Sugahara, Y. A simple procedure for preparing chitin oligomers through acetone precipitation after hydrolysis in concentrated hydrochloric acid. Carbohydr. Polym. 2015, 132, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef]
- Roncal, T.; Oviedo, A.; de Armentia, I.L.; Fernández, L.; Villarán, M.C. High yield production of monomer-free chitosan oligosaccharides by pepsin catalyzed hydrolysis of a high deacetylation degree chitosan. Carbohydr. Res. 2007, 342, 2750–2756. [Google Scholar] [CrossRef] [PubMed]
- Tabata, E.; Wakita, S.; Kashimura, A.; Sugahara, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Residues of acidic chitinase cause chitinolytic activity degrading chitosan in porcine pepsin preparations. Sci. Rep. 2019, 9, 15609. [Google Scholar] [CrossRef] [PubMed]
- Wakita, S.; Sugahara, Y.; Nakamura, M.; Kobayashi, S.; Matsuda, K.; Takasaki, C.; Kimura, M.; Kida, Y.; Uehara, M.; Tabata, E.; et al. Mouse acidic chitinase effectively degrades random-type chitosan to chitooligosaccharides of variable lengths under stomach and lung tissue pH conditions. Molecules 2021, 26, 6706. [Google Scholar] [CrossRef]
- Huh, J.-W.; Kim, Y.-H.; Park, S.-J.; Kim, D.-S.; Lee, S.-R.; Kim, K.-M.; Jeong, K.-J.; Kim, J.-S.; Song, B.-S.; Sim, B.-W.; et al. Large-scale transcriptome sequencing and gene analyses in the crab-eating macaque (Macaca fascicularis) for biomedical research. BMC Genom. 2012, 13, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilham, K.; Rizaldi; Nurdin, J.; Tsuji, Y. Status of urban populations of the long-tailed macaque (Macaca fascicularis) in West Sumatra, Indonesia. Primates 2017, 58, 295–305. [Google Scholar] [CrossRef]
- Janiak, M.C.; Chaney, M.E.; Tosi, A.J. Evolution of acidic mammalian chitinase genes (CHIA) is related to body mass and insectivory in primates. Mol. Biol. Evol. 2018, 35, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Uehara, M.; Tabata, E.; Ishii, K.; Sawa, A.; Ohno, M.; Sakaguchi, M.; Matoska, V.; Bauer, P.O.; Oyama, F. Chitinase mRNA levels determined by qPCR in crab-eating monkey (Macaca fascicularis) tissues: Species-specific expression of acidic mammalian chitinase and chitotriosidase. Genes 2018, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Krykbaev, R.; Fitz, L.J.; Reddy, P.S.; Winkler, A.; Xuan, D.; Yang, X.; Fleming, M.; Wolf, S.F. Evolutionary and biochemical differences between human and monkey acidic mammalian chitinases. Gene 2010, 452, 63–71. [Google Scholar] [CrossRef]
- Uehara, M.; Tabata, E.; Okuda, M.; Maruyama, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Robust chitinolytic activity of crab-eating monkey (Macaca fascicularis) acidic chitinase under a broad pH and temperature range. Sci. Rep. 2021, 11, 15470. [Google Scholar] [CrossRef] [PubMed]
- Kashimura, A.; Okawa, K.; Ishikawa, K.; Kida, Y.; Iwabuchi, K.; Matsushima, Y.; Sakaguchi, M.; Sugahara, Y.; Oyama, F. Protein A-mouse acidic mammalian chitinase-V5-His expressed in periplasmic space of Escherichia coli possesses chitinase functions comparable to CHO-expressed protein. PLoS ONE 2013, 8, e78669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorbotten, A.; Horn, S.J.; Eijsink, V.G.; Varum, K.M. Degradation of chitosans with chitinase B from Serratia marcescens. Production of chito-oligosaccharides and insight into enzyme processivity. FEBS J. 2005, 272, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Sørbotten, A.; Synstad, B.; Sikorski, P.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G. Endo/exo mechanism and processivity of family 18 chitinases produced by Serratia marcescens. FEBS J. 2006, 273, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P. The use of polyacrylamide-gel electrophoresis for the high-resolution separation of reducing saccharides labelled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid. Detection of picomolar quantities by an imaging system based on a cooled charge-coupled device. Biochem. J. 1990, 270, 705–713. [Google Scholar]
- Wakita, S.; Kimura, M.; Kato, N.; Kashimura, A.; Kobayashi, S.; Kanayama, N.; Ohno, M.; Honda, S.; Sakaguchi, M.; Sugahara, Y.; et al. Improved fluorescent labeling of chitin oligomers: Chitinolytic properties of acidic mammalian chitinase under somatic tissue pH conditions. Carbohydr. Polym. 2017, 164, 145–153. [Google Scholar] [CrossRef]
- Villa-Lerma, G.; González-Márquez, H.; Gimeno, M.; López-Luna, A.; Bárzana, E.; Shirai, K. Ultrasonication and steam-explosion as chitin pretreatments for chitin oligosaccharide production by chitinases of Lecanicillium lecanii. Bioresour. Technol. 2013, 146, 794–798. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. Biomed. Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.-E.; Li, L.-M.; Jiang, H.-Y.; Zhang, X.-J.; Li, J.; Li, G.-Y.; Chen, J. Acidic mammalian chitinase gene is highly expressed in the special oxyntic glands of Manis javanica. FEBS Open Bio 2018, 8, 1247–1255. [Google Scholar] [CrossRef]
- Du, C.; Zhao, X.; Song, W.; He, N.; Jiang, S.; Zhou, Y.; Zhang, G. Combined strategies to improve the expression of acidic mammalian chitinase in Pichia pastoris for the production of N, N’-diacetylchitobiose. Biochem. Eng. J. 2021, 167, 107907. [Google Scholar] [CrossRef]
- Ohno, M.; Togashi, Y.; Tsuda, K.; Okawa, K.; Kamaya, M.; Sakaguchi, M.; Sugahara, Y.; Oyama, F. Quantification of chitinase mRNA levels in human and mouse tissues by real-time PCR: Species-specific expression of acidic mammalian chitinase in stomach tissues. PLoS ONE 2013, 8, e67399. [Google Scholar] [CrossRef]
- Poria, V.; Rana, A.; Kumari, A.; Grewal, J.; Pranaw, K.; Singh, S. Current perspectives on chitinolytic enzymes and their agro-industrial applications. Biology 2021, 10, 1319. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehara, M.; Takasaki, C.; Wakita, S.; Sugahara, Y.; Tabata, E.; Matoska, V.; Bauer, P.O.; Oyama, F. Crab-Eating Monkey Acidic Chitinase (CHIA) Efficiently Degrades Chitin and Chitosan under Acidic and High-Temperature Conditions. Molecules 2022, 27, 409. https://doi.org/10.3390/molecules27020409
Uehara M, Takasaki C, Wakita S, Sugahara Y, Tabata E, Matoska V, Bauer PO, Oyama F. Crab-Eating Monkey Acidic Chitinase (CHIA) Efficiently Degrades Chitin and Chitosan under Acidic and High-Temperature Conditions. Molecules. 2022; 27(2):409. https://doi.org/10.3390/molecules27020409
Chicago/Turabian StyleUehara, Maiko, Chinatsu Takasaki, Satoshi Wakita, Yasusato Sugahara, Eri Tabata, Vaclav Matoska, Peter O. Bauer, and Fumitaka Oyama. 2022. "Crab-Eating Monkey Acidic Chitinase (CHIA) Efficiently Degrades Chitin and Chitosan under Acidic and High-Temperature Conditions" Molecules 27, no. 2: 409. https://doi.org/10.3390/molecules27020409
APA StyleUehara, M., Takasaki, C., Wakita, S., Sugahara, Y., Tabata, E., Matoska, V., Bauer, P. O., & Oyama, F. (2022). Crab-Eating Monkey Acidic Chitinase (CHIA) Efficiently Degrades Chitin and Chitosan under Acidic and High-Temperature Conditions. Molecules, 27(2), 409. https://doi.org/10.3390/molecules27020409





