Impact of Modular Architecture on Activity of Glycoside Hydrolase Family 5 Subfamily 8 Mannanases
Abstract
:1. Introduction
2. Results
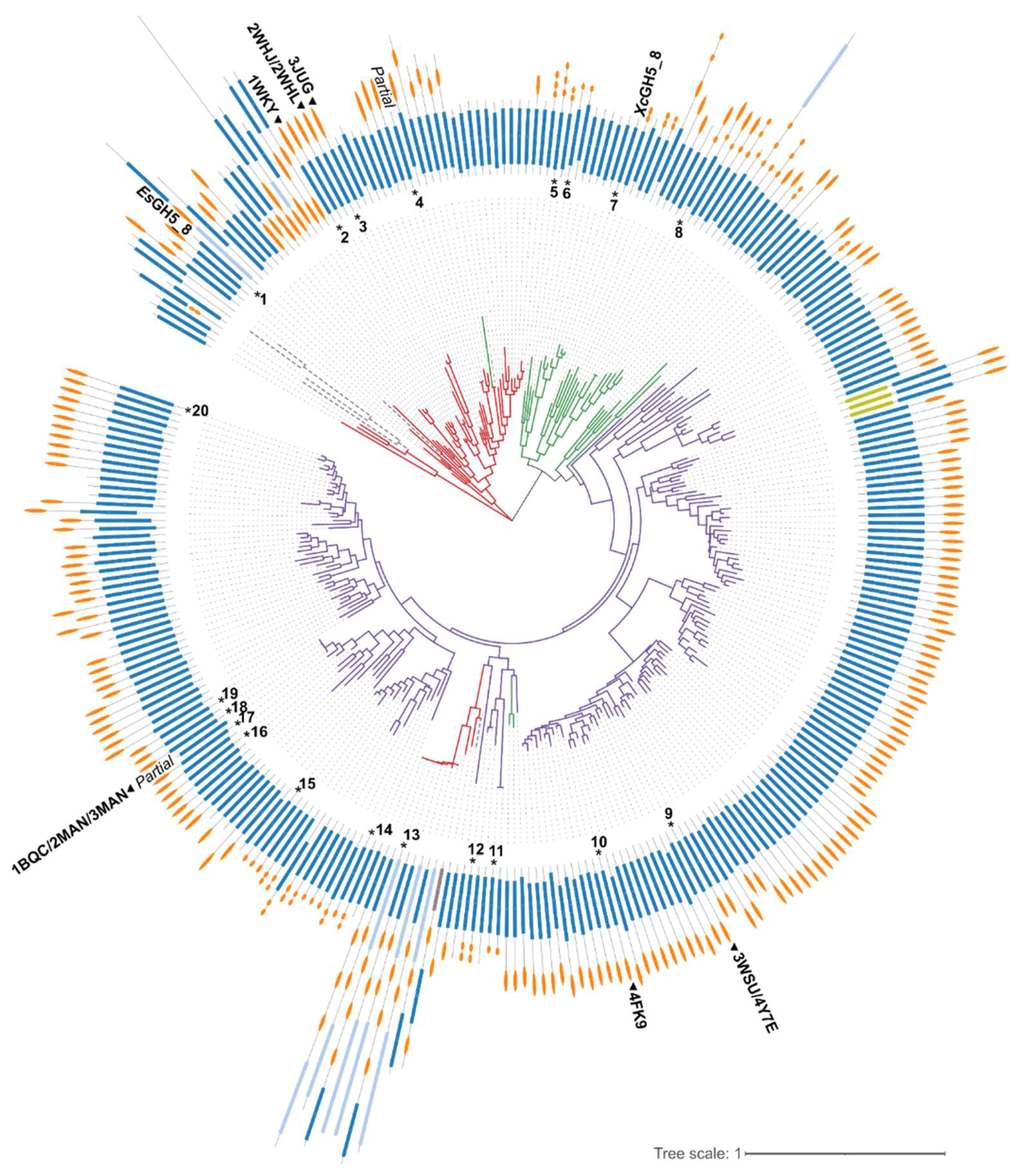
2.1. Bioinformatics Analysis
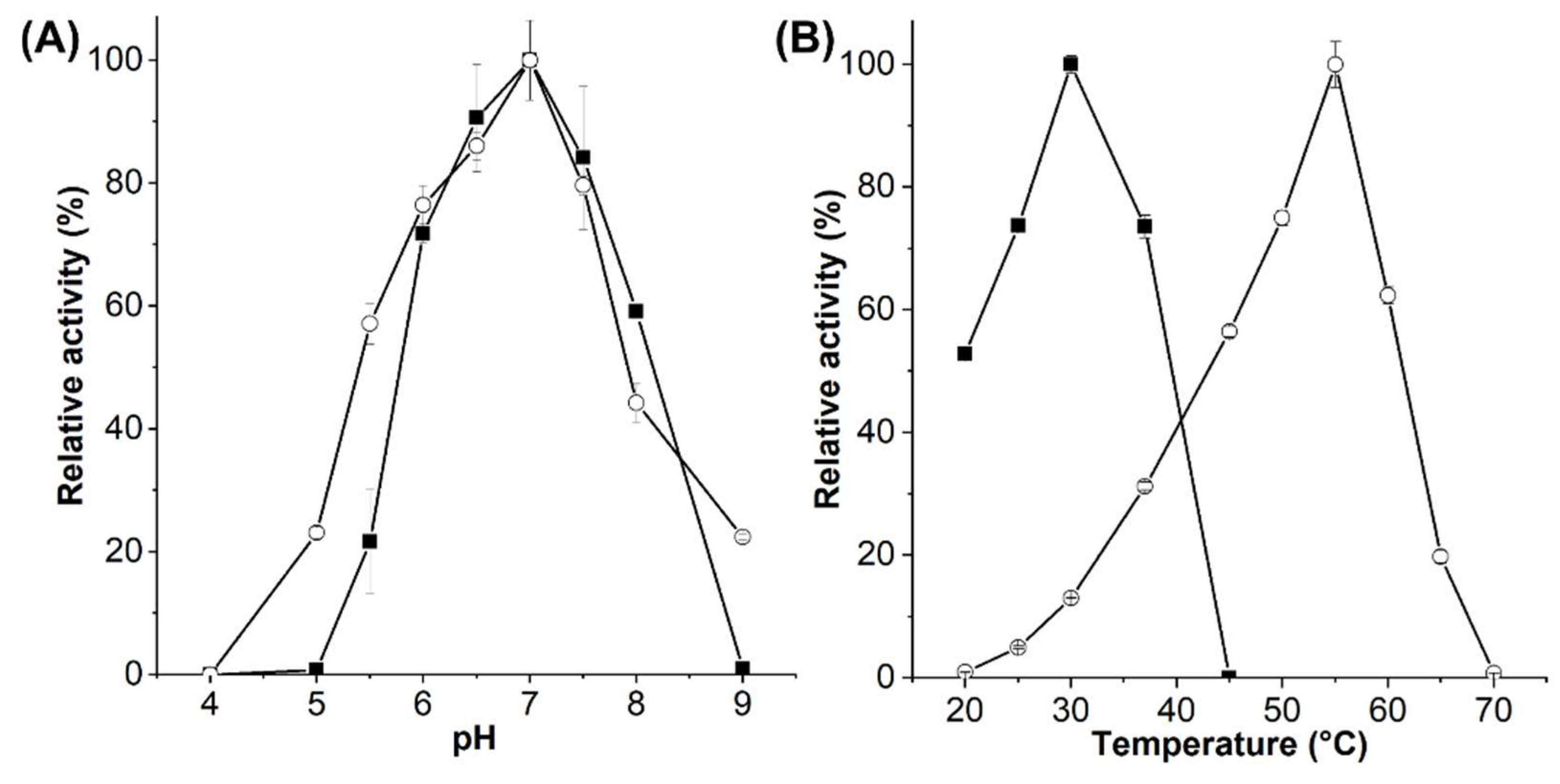
2.2. Enzymatic Activity of EsGH5_8 and XcGH5_8
| Origin (GenBank Accession; No. in Phylogenetic Tree) | Modular Structure of Charac. Protein | CGM-lv U/mg; 1/s (Relative 1, %) | CGM-hv U/mg; 1/s (Relative 1, %) | KGM U/mg 1/s (Relative 1, %) | GG U/mg; 1/s (Relative 1, %) | INM U/mg; 1/s (Relative 1, %) | Ref. |
|---|---|---|---|---|---|---|---|
| Eubacterium siraeum (CBK96294; #1) | GH5_8 | 625 ± 28.8; 397 ± 18.3 (97) | 578 ± 13.0; 367 ± 8.3 (90) | 644 ± 11.4; 409 ± 7.2 (100) | 3.6 ± 0.2; 2.3 ± 0.1 (0.6) | 7.5 ± 0.1; 4.8 ± 0.1 (1.2) | Present study |
| Saccharophagus degradans (ABD79918; #5) | GH5_8-CBM10x3 | 1972 ± 80; 1729 ± 70 (56) | 2212 ± 78; 1939 ± 68 (62) | 3544 ± 110; 3107 ± 96 (100) | 40 ± 8; 35 ± 7 (1.1) | 9 ± 1; 8 ± 1 (0.3) | [18] |
| GH5_8-ΔCBM10x3 | 2906 ± 53; 1695 ± 31 (64) | 3151 ± 304; 1838 ± 177 (69) | 4556 ± 108; 2658 ± 63 (100) | 108 ± 25; 63 ± 15 (2.4) | 81 ± 7; 47 ± 4 (1.8) | [18] | |
| Xanthomonas citri (AMU98328; #7) | GH5_8 | 713 ± 12.0; 414 ± 7.0 (100) | 709 ± 23.0; 411 ± 13,3 (99) | 247 ± 19.3; 143 ± 11.2 (35) | 1.1 ± 0.1; 0.6 ± 0.1 (0.2) | N.D. 2 | Present study |
| Bifidobacterium animalis (ACS46797; #11) | GH5_8-CBM10 | 1380; 872 (55) | 1920; 1213 (76) | 2520; 1592 (100) | N.D. 2 | 120; 76 (5) | [19] |
| Cellulosimicrobium sp. strain HY-13 (AEE43708; #12) | GH5_8-CBM10x2 | 8498 ± 105; 6232 ± 77 (58) | - | - | 967 ± 18; 709 ± 13 (6.6) | 14,711 ± 183; 10,788 ± 134 (100) | [21] |
| Streptomyces sp. S27 (ADK91085; #14) | GH5_8-CBM10 | 2107 ± 182; 1510 ± 130 (100) | - | 1312 ± 110; 940 ± 79 (62) | 74 ± 12; 53 ± 8.6 (3.5) | - | [22] |
| Streptomyces lividans (AAA26710; #15) | GH5_8-CBM10 | 141 ± 1.7 3,4 (100) | - | 55 ± 0.7 4 (39) | 21 ± 1.7 4 (15) | 18.8 ± 1.2 4 (13) | [23] |
| GH5_8-ΔCBM10 | 97 ± 1.4 3,4 (100) | - | 61 ± 0.45 4 (63) | 23 ± 3.1 4 (23) | 19 ± 0.7 4 (20) | [23] | |
| Streptomyces thermoluteus (BAM62868; #20) | GH5_8-CBM2 | 51 ± 1.6 3,4 (78) | - | 66 ± 1.5 4 (100) | 27 ± 1.4 4 (41) | 20.5 ± 0.4 4 (31) | [23] |
| StGH5_8-ΔCBM2 | 39 ± 0.6 3,4 (86) | - | 45 ± 0.5 4 (100) | 20 ± 3.7 4 (44) | 14 ± 1.3 4 (32) | [23] |
| Origin (Genbank Accession; No. in Phylogenetic Tree) | Modular Structure of Charac. Protein | KM (mg/mL) | kcat (1/s) | kcat/KM (mg/(mL s) | Binding CGM? | Ref. |
|---|---|---|---|---|---|---|
| Eubacterium siraeum (CBK96294; #1) | GH5_8 | 4.6 ± 0.5 | 850 ± 47 | 185 ± 23 | Yes, weakly | Present study |
| Bacillus sp. JAMB-602 (BAD99527; #2) | GH5_8-CBM59 | 3.1 | 135 1 | ND 2 | [24] | |
| Bacillus agaradhaerens (AAN27517; #3) | GH5_8-CBM59 | 1.8 | 633 | 250 | ND 2 | [13] |
| Bacillus nealsonii PN-11 (AGU71466; #4) | GH5_8-CBM59 | 7.2 ± 0.3 | 750 ± 55 3 | 104 ± 2 3 | ND 2 | [25] |
| Saccharophagus degradans (ABD79918; #5) | GH5_8-CBM10x3 | 2.1 ± 0.1 | 2333 ± 55 | 1096 ± 71 | Yes, Kd < 0.125 mg/mL | [18] |
| SdGH5_8-ΔCBM10x3 | 2.4 ± 0.1 | 3440 ± 75 | 1413 ± 82 | No | [18] | |
| Cellvibrio japonicus (ACE84673/AAO31759; #6) | GH5_8-ΔCBM10x2 | 8.5 ± 1.5 | 2381 ± 66 | 246.0 | No | [26] |
| Xanthomonas citri (AMU98328; #7) | GH5_8 | 2.6 ± 0.3 | 732 ± 36 | 282 ± 35 | ND 2 | Present study |
| Cellvibrio japonicus (AAO31760; #8) | GH5_8-ΔCBM10-CBM2 | 2.2 ± 0.3 | 1075 ± 27 | 446 | No | [26] |
| Streptomyces thermolilacinus (BAK26781; #9) | GH5_8-ΔCBM2 | 4.9 ± 1.0 | 21 ± 2 | 4 ± 1 | ND 2 | [15,27] |
| Streptomyces sp. SirexAA-E (AEN10237; #10) | GH5_8-Fn3-CBM2 | 2 ± 0.2 | 41 ± 2 | 21 ± 10 | Yes, weakly | [14] |
| GH5_8-ΔFn3-CBM2 | 2 ± 0.4 | 41 ± 3 | 21 ± 8 | No | [14] | |
| Bifidobacterium animalis (ACS46797; #11) | GH5_8-CBM10 | 1.6 ± 0.2 | 1828 ± 87 | 1157 ± 177 | Yes, Kd = 0.31 mg/ml | [19] |
| GH5_8-ΔCBM10 | 1.8 ± 0.5 | 2005 ± 179 | 1146 ± 324 | No | [19] | |
| Caldicellulosiruptor bescii (ACM60953; #13) | GH9-CBM3x3-GH5_8 | 0.6 ± 0.3 | 1420 ± 158 | 2290 | ND 2 | [28] |
| CBM3x3-GH5_8 | 1.8 ± 0.5 | 3446 ± 367 | 1893 | ND 2 | [28] | |
| Streptomyces sp. S27 (ADK91085; #14) | GH5_8-CBM10 | 0.16 | 3739 3 | 23369 3 | ND 2 | [22] |
| Streptomyces lividans (AAA26710; #15) | GH5_8-CBM10 | 3.5 ± 0.5 | 197 ± 11 | 60 ± 7 | ND 2 | [23] |
| GH5_8-ΔCBM10 | 4.3 ± 0.7 | 139 ± 9 | 33 ± 4 | ND 2 | [23] | |
| Thermobifida halotolerans (AHB89704; #16) | GH5_8-CBM2 | 1.3 ± 0.3 | 78 ± 9 | 60 | ND 2 | [29] |
| Thermobifida cellulosilytica (AHB89703; #17) | GH5_8-CBM2 | 0.8 ± 0.2 | 89 ± 5 | 106 | ND 2 | [29] |
| Thermobifida fusca (AAZ54938; #18) | GH5_8-ΔCBM2 | 10.4 ± 2.6 | 96 ± 14 | 9 ± 3 | ND 2 | [15] |
| Thermobifida fusca TM51 (AHB89702; #19) | GH5_8-CBM2 | 1.7 ± 0.4 | 122 ± 11 | 74 | ND 2 | [29] |
| Streptomyces thermoluteus (BAM62868; #20) | GH5_8-CBM2 | 5.5 ± 1.6 | 101 ± 17 | 18 ± 3 | ND 2 | [23] |
| GH5_8-ΔCBM2 | 5.5 ± 1.5 | 75 ± 12 | 14 ± 2 | ND 2 | [23] |
2.3. Polysaccharide Interaction
3. Discussion
4. Materials and Methods
4.1. Carbohydrates
4.2. Bioinformatics Analysis of GH5_8
4.3. Recombinant Protein Production
4.4. Protein Purification
4.5. Determination of Melting Temperature
4.6. Enzymatic Assays
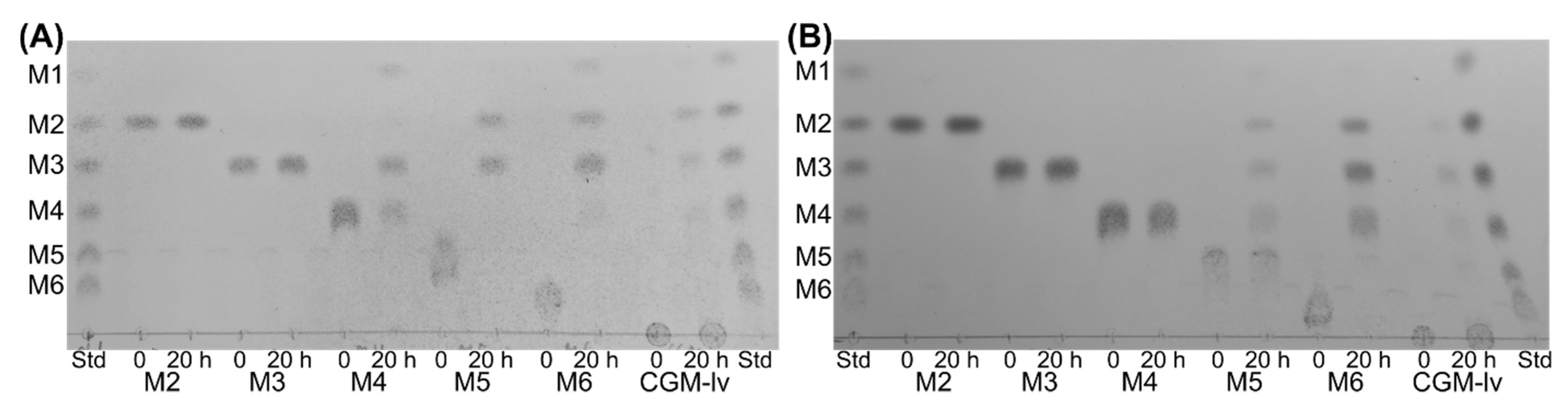
4.7. Thin Layer Chromatography (TLC)
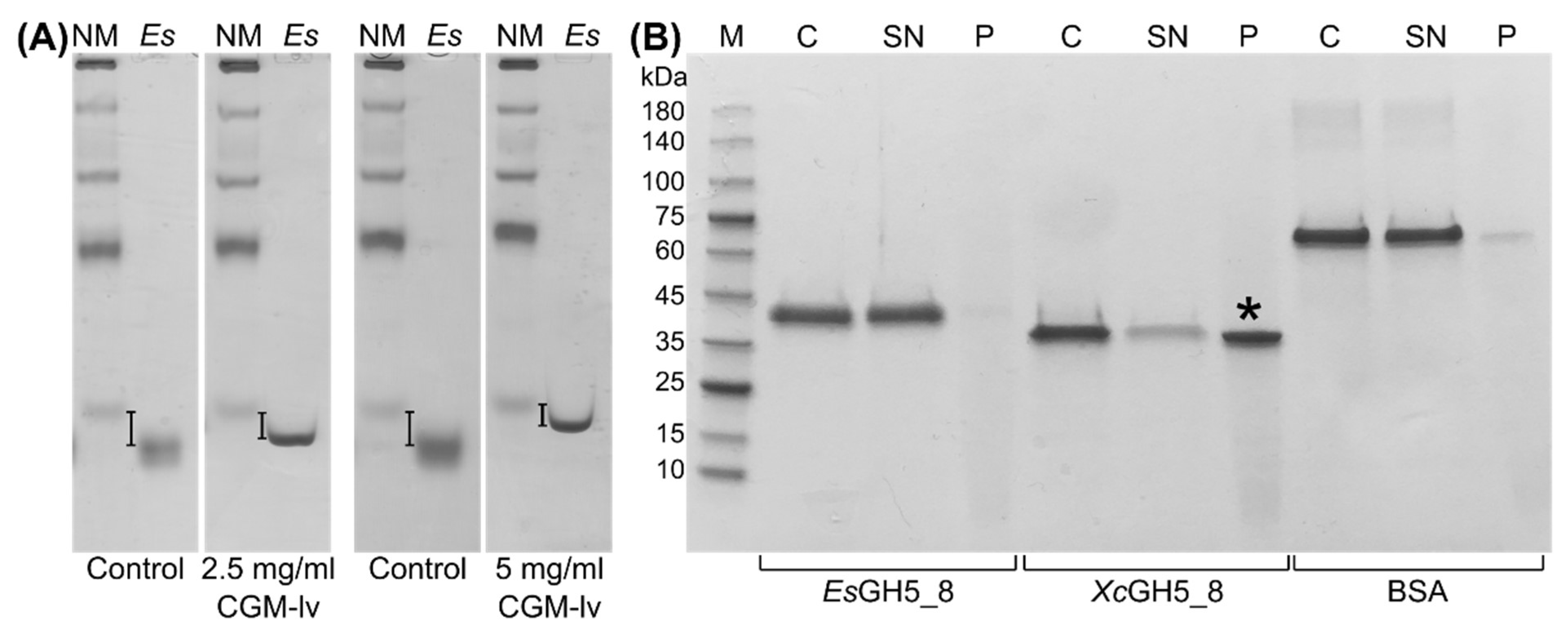
4.8. Affinity Gel-Electrophoresis (AGE)
4.9. Pull-Down Assay
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gilbert, H.J.; Knox, J.P.; Boraston, A.B. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr. Opin. Struct. Biol. 2013, 23, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Sidar, A.; Albuquerque, E.D.; Voshol, G.P.; Ram, A.F.J.; Vijgenboom, E.; Punt, P.J. Carbohydrate binding modules: Diversity of domain architecture in amylases and cellulases from filamentous microorganisms. Front. Bioeng. Biotechnol. 2020, 8, 871. [Google Scholar] [CrossRef] [PubMed]
- Guillén, D.; Sánchez, S.; Rodríguez-Sanoja, R. Carbohydrate-binding domains: Multiplicity of biological roles. Appl. Microbiol. Biotechnol. 2010, 85, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Cockburn, D.; Svensson, B. Surface binding sites in carbohydrate active enzymes: An emerging picture of structural and functional diversity. In Carbohydrate Chemistry; Rauter, A.P., Lindhorst, T., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 39, pp. 204–221. ISBN 9781849735872. [Google Scholar]
- Cuyvers, S.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Occurrence and functional significance of secondary carbohydrate binding sites in glycoside hydrolases. Crit. Rev. Biotechnol. 2012, 32, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.; Ma, K. Applications of microbial β-mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef]
- Aspeborg, H.; Coutinho, P.M.; Wang, Y.; Brumer, H.; Henrissat, B. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol. Biol. 2012, 12, 186. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.M.; Liu, Y.F.; Fang, M.C.; Tseng, Y.H. Transcriptional regulation and molecular characterization of the manA gene encoding the biofilm dispersing enzyme mannan endo-1,4-β-mannosidase in Xanthomonas campestris. J. Agric. Food Chem. 2010, 58, 1653–1663. [Google Scholar] [CrossRef]
- Akita, M.; Takeda, N.; Hirasawa, K.; Sakai, H.; Kawamoto, M.; Yamamoto, M.; Grant, W.D.; Hatada, Y.; Ito, S.; Horikoshi, K. Crystallization and preliminary X-ray study of alkaline mannanase from an alkaliphilic Bacillus isolate. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, D60, 1490–1492. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, Y.; Cao, Y.; Qi, J.; Mao, L.; Xue, Y.; Gao, F.; Peng, H.; Wang, X.; Gao, G.F.; et al. Structural analysis of alkaline β-Mannanase from alkaliphilic Bacillus sp. N16-5: Implications for adaptation to alkaline conditions. PLoS ONE 2011, 6, e14608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tailford, L.E.; Ducros, V.M.A.; Flint, J.E.; Roberts, S.M.; Morland, C.; Zechel, D.L.; Smith, N.; Bjørnvad, M.E.; Borchert, T.V.; Wilson, K.S.; et al. Understanding how diverse β-mannanases recognize heterogeneous substrates. Biochemistry 2009, 48, 7009–7018. [Google Scholar] [CrossRef] [PubMed]
- Takasuka, T.E.; Acheson, J.F.; Bianchetti, C.M.; Prom, B.M.; Bergeman, L.F.; Book, A.J.; Currie, C.R.; Fox, B.G. Biochemical properties and atomic resolution structure of a proteolytically processed β-mannanase from cellulolytic Streptomyces sp. SirexAA-E. PLoS ONE 2014, 9, e94166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, Y.; Yamashita, K.; Tagami, T.; Uraji, M.; Wan, K.; Okuyama, M.; Yao, M.; Kimura, A.; Hatanaka, T. The loop structure of Actinomycete glycoside hydrolase family 5 mannanases governs substrate recognition. FEBS J. 2015, 282, 4001–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilge, M.; Gloor, S.M.; Rypniewski, W.; Sauer, O.; Heightman, T.D.; Zimmermann, W.; Winterhalter, K.; Piontek, K. High-resolution native and complex structures of thermostable β-mannanase from Thermomonospora fusca-substrate specificity in glycosyl hydrolase family 5. Structure 1998, 6, 1433–1444. [Google Scholar] [CrossRef]
- You, X.; Qin, Z.; Yan, Q.; Yang, S.; Li, Y.; Jiang, Z. Structural insights into the catalytic mechanism of a novel glycoside hydrolase family 113 β-1,4-mannanase from Amphibacillus xylanus. J. Biol. Chem. 2018, 293, 11746–11757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, M.S.; El Bouaballati, S.; Henrissat, B.; Svensson, B. Functional diversity of three tandem C-terminal carbohydrate-binding modules of a β-mannanase. J. Biol. Chem. 2021, 296, 100638. [Google Scholar] [CrossRef] [PubMed]
- Morrill, J.; Kulcinskaja, E.; Sulewska, A.M.; Lahtinen, S.; Stålbrand, H.; Svensson, B.; Abou Hachem, M. The GH5 1,4-β-mannanase from Bifidobacterium animalis subsp. lactis Bl-04 possesses a low-affinity mannan-binding module and highlights the diversity of mannanolytic enzymes. BMC Biochem. 2015, 16, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, K.; Kimoto, S.; Shinzawa, Y.; Minezawa, M.; Suzuki, K.; Jindou, S.; Kato, M.; Shimizu, M. Characterization of pH-tolerant and thermostable GH 134 β-1,4-mannanase SsGH134 possessing carbohydrate binding module 10 from Streptomyces sp. NRRL B-24484. J. Biosci. Bioeng. 2018, 125, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Ham, S.J.; Lee, H.J.; Cho, H.Y.; Kim, J.H.; Kim, Y.J.; Shin, D.H.; Rhee, Y.H.; Son, K.H.; Park, H.Y. Cloning and characterization of a modular GH5 β-1,4-mannanase with high specific activity from the fibrolytic bacterium Cellulosimicrobium sp. strain HY-13. Bioresour. Technol. 2011, 102, 9185–9192. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Yuan, T.; Zhao, J.; Huang, H.; Luo, H.; Meng, K.; Wang, Y.; Yao, B. Genetic and biochemical characterization of a protease-resistant mesophilic β-mannanase from Streptomyces sp. S27. J. Ind. Microbiol. Biotechnol. 2011, 38, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kawakami, K.; Uraji, M.; Hatanaka, T. Binding of bivalent ions to actinomycete mannanase is accompanied by conformational change and is a key factor in its thermal stability. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Hirasawa, K.; Uchimura, K.; Nogi, Y.; Hatada, Y.; Akita, M.; Usami, R.; Yoshida, Y.; Grant, W.D.; Ito, S.; et al. Alkaline mannanase from a novel species of alkaliphilic Bacillus. J. Appl. Glycosci. 2004, 51, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, P.S.; Sharma, P.; Puri, N.; Gupta, N. Purification and characterization of an alkali-thermostable β-mannanase from Bacillus nealsonii PN-11 and its application in mannooligosaccharides preparation having prebiotic potential. Eur. Food Res. Technol. 2014, 238, 927–936. [Google Scholar] [CrossRef]
- Hogg, D.; Pell, G.; Dupree, P.; Goubet, F.; Martín-Orúe, S.M.; Armand, S.; Gilbert, H.J. The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation. Biochem. J. 2003, 371, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Uraji, M.; Wan, K.; Okuyama, M.; Kimura, A.; Hatanaka, T. Molecular insights into the mechanism of thermal stability of actinomycete mannanase. FEBS Lett. 2016, 590, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Mackie, R.I.; Cann, I.K.O. Biochemical and mutational analyses of a multidomain cellulase/mannanase from Caldicellulosiruptor bescii. Appl. Environ. Microbiol. 2012, 78, 2230–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, Á.; Barna, T.; Szabó, E.; Elek, R.; Hubert, Á.; Nagy, I.; Nagy, I.; Kriszt, B.; Táncsics, A.; Kukolya, J. Cloning, expression and biochemical characterization of endomannanases from Thermobifida species isolated from different niches. PLoS ONE 2016, 11, e155769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, D.; Wilkens, C.; Svensson, B. Chapter 9: Affinity electrophoresis for analysis of catalytic module-carbohydrate interactions. In Protein-Carbohydrate Interactions: Methods and Protocols; Abbott, D.W., Lammerts van Bueren, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1588, pp. 119–127. ISBN 978-1-4939-6898-5. [Google Scholar]
- Dow, J.M.; Crossman, L.; Findlay, K.; He, Y.Q.; Feng, J.X.; Tang, J.L. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10995–11000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef]
- Pei, J.; Tang, M.; Grishin, N. V PROMALS3D web server for accurate multiple protein sequence and structure alignments. Nucleic Acids Res. 2008, 36, W30–W34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhang, H.; Wu, P.; Entwistle, S.; Li, X.; Yohe, T.; Yi, H.; Yang, Z.; Yin, Y. dbCAN-seq: A database of carbohydrate-active enzyme (CAZyme) sequence and annotation. Nucleic Acids Res. 2018, 46, D516–D521. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Dilokpimol, A.; Nakai, H.; Gotfredsen, C.H.; Baumann, M.J.; Nakai, N.; Abou Hachem, M.; Svensson, B. Recombinant production and characterisation of two related GH5 endo-β-1,4-mannanases from Aspergillus nidulans FGSC A4 showing distinctly different transglycosylation capacity. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Brooke, D.; Movahed, N.; Bothner, B. Universal buffers for use in biochemistry and biophysical experiments. AIMS Biophys. 2015, 2, 336–342. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Møller, M.S. Impact of Modular Architecture on Activity of Glycoside Hydrolase Family 5 Subfamily 8 Mannanases. Molecules 2022, 27, 1915. https://doi.org/10.3390/molecules27061915
Møller MS. Impact of Modular Architecture on Activity of Glycoside Hydrolase Family 5 Subfamily 8 Mannanases. Molecules. 2022; 27(6):1915. https://doi.org/10.3390/molecules27061915
Chicago/Turabian StyleMøller, Marie Sofie. 2022. "Impact of Modular Architecture on Activity of Glycoside Hydrolase Family 5 Subfamily 8 Mannanases" Molecules 27, no. 6: 1915. https://doi.org/10.3390/molecules27061915
APA StyleMøller, M. S. (2022). Impact of Modular Architecture on Activity of Glycoside Hydrolase Family 5 Subfamily 8 Mannanases. Molecules, 27(6), 1915. https://doi.org/10.3390/molecules27061915






