Abstract
Fungus continues to attract great attention as a promising pool of biometabolites. Aspergillus ochraceus Wilh (Aspergillaceae) has established its capacity to biosynthesize a myriad of metabolites belonging to different chemical classes, such as isocoumarins, pyrazines, sterols, indole alkaloids, diketopiperazines, polyketides, peptides, quinones, polyketides, and sesquiterpenoids, revealing various bioactivities that are antimicrobial, cytotoxic, antiviral, anti-inflammatory, insecticidal, and neuroprotective. Additionally, A. ochraceus produces a variety of enzymes that could have variable industrial and biotechnological applications. From 1965 until June 2022, 165 metabolites were reported from A. ochraceus isolated from different sources. In this review, the formerly separated metabolites from A. ochraceus, including their bioactivities and biosynthesis, in addition, the industrial and biotechnological potential of A. ochraceus are highlighted.
Keywords:
Aspergillus ochraceus; Aspergillaceae; fungi; metabolites; bioactivities; biosynthesis; enzymes 1. Introduction
Recently, a dramatic shift has increased towards the utilization of eco-friendly and sustainable sources for discovering therapeutic agents against various health concerns to promote human health and wellbeing. Fungi have a prolonged and close relationship with human beings, especially at the chemical level. Particularly, they have drawn great interest for their capacity to biosynthesize a variety of structurally unique metabolites that possess promising bioactivities [1,2,3,4,5]. Additionally, the current advances in genetics, synthetic biology, natural product chemistry, and bioinformatics have considerably reinforced the capability to mine their genomes for discovering novel drugs [6].
The genus Aspergillus (Aspergillaceae) is one of the most widespread, diversified genera, comprising 400 filamentous species of substantial pharmaceutical, biotechnological, and commercial values [7,8,9,10]. Some of its species have promising enzyme production capacity, as well as causing various illnesses in humans and animals [7,11,12]. They cause various clinical infections that range from allergic and chronic infections to acute invasive aspergillosis [13]. On the other hand, its species are renowned, prolific producers of various metabolites, such as butyrolactones, polyketides, xanthones, sterols, anthraquinones, terpenoids, peptides, and alkaloids, which demonstrate various bioactivities [7,14,15].
Aspergillus ochraceus Wilh is a widely distributed fungus that was isolated from various sources, such as decaying vegetation, soils, a variety of agricultural commodities, and moldy grains [16]. The fungus was also known as an important food pathogen that is responsible for the production of carcinogenic mycotoxins, such as ochratoxins, penicillic acid, dihydropenicillic acid, and viomellein [17]. The toxicological importance of this fungus is highlighted by a disease known as “Balkan nephropathy”, which has been linked to the consumption of food products contaminated with penicillic acid and ochratoxin A (14) [17,18,19]. On the other hand, this fungus is capable of the biosynthesis of other metabolites, such as isocoumarins, pyrazines, sterols, indole alkaloids, diketopiperazines, polyketides, peptides, quinones, benzodiazepines, and nitro-benzoyl sesquiterpenoids [16,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Most of them demonstrate promising bioactivities, that are antimicrobial, cytotoxic, antiviral, anti-inflammatory, antioxidant, anti-Parkinson’s disease, insecticidal, and neuroprotective [16,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Due to their intriguing structural features and remarkable diverse bioactivities, they have become a fascinating target for biosynthesis, chemical synthesis, and bioactivity investigations. Therefore, this work aimed at comprehensively exploring the diverse metabolites separated and characterized from A. ochraceus associated with different sources regarding their biosynthesis and bioactivities, which were categorized here according to their chemical classes. Additionally, the enzymes produced by this fungus and their possible applications have been discussed [36,37,38,39,40,41,42]. The reported data including the metabolites, sources, and activities have been illustrated. This work could provide the researchers and readers with an overview of the uninvestigated perspectives of A. ochraceus as a producer of novel biometabolites. The collected data were obtained via searching through different databases, such as Science-Direct, Web of Knowledge, Scopus, Wiley Online Library, Taylor & Francis, JACS, PubMed, Google Scholar, and Springer.
2. Enzymes of A. ochraceus and Their Applications
Fungi are fascinating producers of various enzymes that have beneficial contributions in the industrial field. A. ochraceus produces a variety of enzymes, which are reviewed in this work, along with their possible biotechnological and industrial values.
2.1. Hydrolases
2.1.1. Glycoside Hydrolases
Lignocellulosic biomass is one of the alternative energy sources to fossil fuel that is composed of hemicelluloses, cellulose, and lignin [4,5,43,44]. Cellulases are the principal catalytic enzymes for lignocellulosic biomass hydrolysis, involving β-D-glucosidase, cellobiohydrolase, and endoglucanase, that synergistically hydrolyze cellulose into glucose [4,5,43,44]. They are applicable in the fermentation industry, which needs stability under extreme bioprocessing conditions and high yield. Coir pith or coconut pith is a byproduct of the coir industry with 25% cellulose that is possibly utilized as substrate for saccharification.
Asha et al. purified and characterized β-glucosidase (AS-HT-CeluzB) and processive-type endoglucanase (AS-HT-CeluzA) from A. ochraceus MTCC1810, which bio-converted delignified coir pith to glucose for subsequent bioethanol production [37]. These enzymes possessed optimal total cellulase (28.15 FPU/mL), endoglucanase (35.63 U/mL), and β-glucosidase (15.19 U/mL) capacities at pH 6/40 °C. Accordingly, these enzymes could be utilized as synergistic cellulases for complete cellulose saccharification in bio-refineries [37].
Xylan is a major component of the plant cell wall. It consists of 1,4-connected β-D-xylopyranose residues [4,5,43,44]. Xylanases facilitate xylan hydrolysis, primarily utilized in the kraft operation for removing the generated LCC (lignin–carbohydrate complex), which is a physical barrier towards bleaching chemicals entry [45]. Chemical bleaching relies on the utilization of a large quantity of chlorine and chlorine-related chemicals that results in bio-accumulating, mutagenic, toxic, and bio-harmful byproducts [46]. Alternatively, xylanases used in the paper and pulp industry is an eco-friendly method that minimizes pulp fibers’ damage and generates superior quality dissolving pulps [47]. The production of microbial xylanases using various lignocellulosic residues as growth substrates have received great attention because of its low cost and high yield [45,46,47].
Betini et al. reported the production of xylanases from A. ochraceus under SSF (solid-state fermentation) using agro-industrial residues (e.g., wheat bran, rice straw, oatmeal, corncob, and Eucalyptus grandis sawdust) [39]. It was found that xylanase production (20%) was favored when a mixture of corncob and wheat bran was utilized. The bio-bleaching assay of these enzymes revealed twice to thrice times increased brightness and maintained viscosity, indicating that their use could assist the reduction of chlorine compound concentration in cellulose pulp treatment [39]. In another study, A. ochraceus produced high levels of cellulase-free xylanase in oat spelt or birchwood xylan media using wheat bran residue. The enzyme had maximal activity at 65 °C and 5.0 pH [48]. It caused the bleaching of eucalyptus kraft pulp. The results could improve the economic characteristics of bio-bleaching technology and minimize the pollutant compounds used in the process [48]. In 2012, Michelin et al. studied the production of xylanase by A. ochraceus utilizing wheat straw autohydrolysis liquor as a carbon source [49]. It was found that the best yield of β-xylosidase and xylanase was obtained when A. ochraceus was cultivated with 1% wheat bran added to 10% wheat straw liquor in a stirred tank bioreactor, suggesting the possibility of scaling up this process for commercial production [49]. The enhancement of xylanase and p-xylosidase productivity by A. ochraceus using various chemical and physical mutagenesis was assessed [40]. It was found that the NG-13 (N-methyl-N′-nitro-N-nitrosoguanidine) mutant strain secreted high levels of β-xylosidase and xylanase during growth on agricultural waste and commercial xylan, which were stable with optimal activity at pH 5–10 and temperature 45–50 °C [40].
Invertases (β-D-fructo-furanosidases) hydrolyze polysaccharides and sucrose to produce glucose and fructose [50]. The resulting glucose and fructose mixture is called inverted sugar. Invertases are substantial in the food industry, particularly in confectionery for artificial sweetener preparation and increasing sweetening properties [50].
Ghosh et al. (2001) purified invertase enzymes from A. ochraceus that was thermotolerant with high sucrose and raffinose affinity [41]. A. ochraceus also produced high levels of an extracellular thermostable β-D-fructofuranosidase using sugar cane bagasse-supplemented Khanna medium at 40 °C [51]. This enzyme had a hydrolytic activity with no trans-fructosylating potential. Furthermore, it was positively affected by glucose, which distinguished it from the other β-d-fructofuranosidases, supporting its application for fructose syrup production and sucrose hydrolysis [51].
2.1.2. Proteolytic Enzymes
Medical interest has been drawn to the thrombolytic enzymes of microbial origin. These enzymes act directly by dissolving blood clots as plasmin or as blood plasminogen tissue activators [52]. Protein C prevents blood hyper-clotting in hemostasis [53]. Protein C activator’s addition to the blood inactivates clotting factors VIII and V, which are necessary for thrombin formation, resulting in the prolongation of the partial thromboplastin time [54]. Discovering protein C activators from microbes could be beneficial for clinical practice use because of the low cost.
From A. ochraceus 513, proteinase belonging to protein C activator types was separated and assessed for anti-coagulant and fibrinolytic potential [38]. This enzyme was efficient as Agkistrodon snake venom protein C activator in thrombin formation time prolongation [38]. Osmolovskiy et al. stated that extracellular proteinases produced by submerged cultures of a micromycete A. ochraceus L-1 exhibited specific fibrinogenolytic and fibrinolytic potential, whereas the highest effectiveness was observed at pH 7.0 and 28 °C [55].
2.1.3. Tannases
Tannases (tannin acyl hydrolase) catalyze the hydrolysis of depside and the ester bonds of hydrolyzable tannins [56]. They have been utilized as clarifying agents in the industrial processing of coffee-flavored soft drinks and fruit juices, instant teas manufacture, gallic acid production, and treatment of polyphenolics-contaminated wastewaters, as well as the removal of tannin from foodstuffs and animal feeds [57,58].
A. ochraceus was reported to yield extracellular thermostable tannase with distinctive monomeric structural characteristics. This enzyme was activated by manganese, revealing its biotechnological potential for gallic acid production [42]. Furthermore, it was found that A. ochraceus biofilm produced tannase in Khanna medium containing tannic acid (1.5% w/v, carbon source), which was higher than that obtained using conventional submerged fermentation. This enzyme exhibited potent effectiveness at pH 6.0 and 30 °C and was not affected by detergent and surfactant addition [36]. It had different biotechnological applications in propyl gallate production, tannin-rich leather effluent treatment, and sorghum feed formulation [36]. Thus, fungal biofilm is an interesting alternative to produce high levels of tannase with the biotechnological potential to be applied in different industrial sectors.
2.2. Oxidases
Alcohol oxidases catalyze the oxidation of alcohols to the corresponding carbonyl compounds with a concomitant release of hydrogen peroxide [59]. They have potential applications in biosensors and the biocatalytic production of different carbonyl compounds that are beneficial in pharmaceutical, flavor, and clinical industries [59]. A. ochraceus AIU031 secreted alcohol oxidase (AOD), belonging to the same group as methylotrophic yeast AOD. It oxidized short-chain primary alcohols and ethylene glycol [60]. It was found to possess an optimal ethanol oxidation capacity at 50–55 °C and pH 5–7 [60].
3. Applications of A. ochraceus
3.1. Bioethanol Production
Bioethanol is a liquid biofuel that could be produced from various biomass resources (corn, wheat, paddy straw, sugar beet, wood, municipal waste, forestry byproducts, etc.) through microbial fermentation [61]. This process provides an economically competitive source of energy for biofuel production from cellulosic and lignocellulosic materials.
A. ochraceus cellulase demonstrated high hydrolyzing potential of sawdust and its ethanol yield in shaking fermentation than in stationary fermentation. The results supported the use of sawdust as a potential substrate for bioethanol production [62].
3.2. Dye Decolorization
Kadam et al. developed a consortium of A. ochraceus NCIM-1146 and Pseudomonas sp. SUK1 to decolorize adsorbed dyes from textile effluent wastewater under solid-state fermentation [63]. This consortium had a remarkable potential to decolorize adsorbed textile dyes from CPTDE (chemical precipitate of textile dye effluent) and textile wastewater on rice bran. This influence was strongly referred to as the synergism of the excreted extracellular enzymes, such as laccase, azoreductase, NADH-DCIP reductase, and tyrosinase [63]. This approach could be effective in reducing effluent volume through adsorbing textile dye on available agricultural waste residues at a low cost, as well as its disposal or bioremediation to nontoxicity by solid-state fermentation [63]. Saratale et al. reported that A. ochraceus remarkably decolorized cotton blue and malachite green dyes, while it had less decolorization capacity on methyl violet and crystal violet [64]. This effect was found to be due to microbial metabolism, not biosorption. Therefore, A. ochraceus could be used for bioremediation of cotton blue and malachite green dye-containing wastewater [64]. In addition, the intracellular blue laccase produced by A. ochraceus NCIM1146 had maximum substrate specificity toward ABTS (2,2′-azinobis, 3-ethylbenzothiazoline-6-sulfonic acid) and decolorized azo dyes with an optimal effect at 60 °C and 4.0 pH [65].
3.3. Kerosene Biodegradation
Accidental spillage of petroleum and its byproducts represents an environmental problem as it causes acute toxicity in some forms of aquatic life [66]. Fungi and bacteria are known for their capacity for petroleum hydrocarbon consumption [67]. Due to their degrading potential, they can be a promising alternative for reducing the environmental impacts of oil spills.
A. ochraceus was found to possess kerosene-degrading potential when the previously grown mycelium was incubated in kerosene-containing broth due to its alkane-oxidizing capacity [68]. It also revealed high levels of kerosene biodegradation, aminopyrine N-demethylase, and NADPH-DCIP reductase. The production of acetaldehyde could be utilized as an indicator for kerosene biodegradation [68]. In another study, A. ochraceus also showed a noticeable ability to utilize and degrade gasoline and crude oil [69].
3.4. Nanoparticles (NPs) of A. ochraceus
Intracellular silver nanoparticles synthesized by exposure of Ag+ ions to A. ochraceus were heat-treated in an N2 environment to yield AgNPs embedded in carbonaceous supports. These carbonaceous AgNPs had an antibacterial capacity versus B. subtilis and E. coli, and an antiviral potential towards the M-13 phage virus [70].
4. Secondary Metabolites from A. ochraceus and Their Bioactivities
4.1. Isocoumarin Derivatives
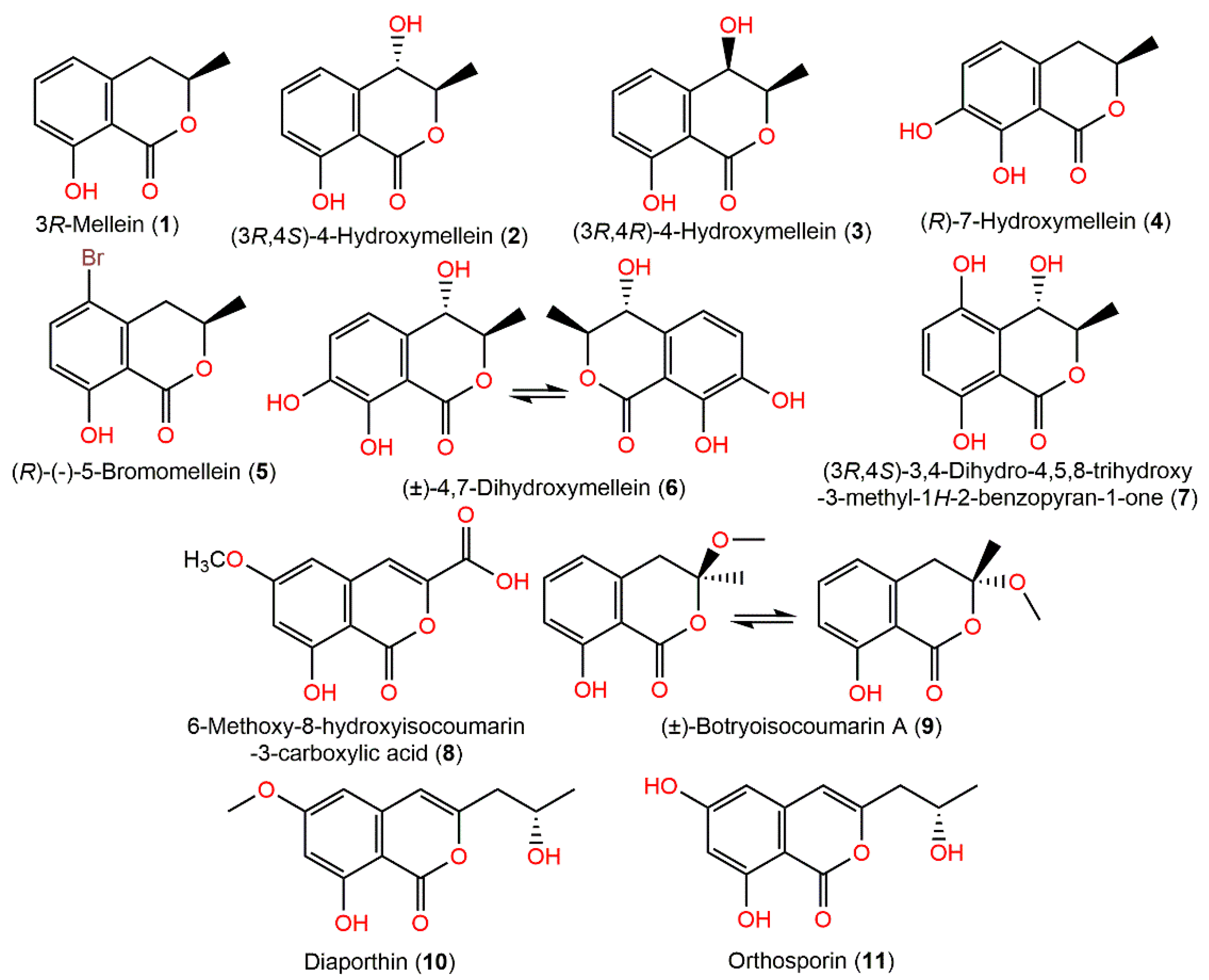
Dai et al. stated that 1 exhibited anti-HCV potential by prohibiting HCV protease (IC50 35 µM) [20]. Compounds 2–4 and 9 were separated from a marine mangrove Bruguiera gymnorrhiza rhizospheric soil-associated strain by Liu et al. [71]. The X-ray of 9 proved its racemic nature. They had no potential in the brine shrimp assay; however 4 displayed a selectivity towards V. harveyi with MIC 8 μg/mL as that of chloramphenicol [71]. Chromatographic investigation of the EtOAc fraction of A. ochraceus MCCC3A00521 resulted in a new pair of enantiomers (±)-4,7-dihydroxymellein (6), together with (3R,4S)-4-hydroxymellein (2) by SiO2, RP-18 CC, and HPLC. Their configurations: 3R/4S and 3S/4R for 6 and 3R/4S for 2 were established by NMR, ECD, and XRD (X-ray diffraction) analyses [21]. These metabolites were evaluated for their antioxidant capacity in ABTS, DPPH, and FRAP assays and cytotoxic activity versus SH-SY5Y cells in CCK-8, as well as neuroprotective effectiveness by measuring the GSH level using ELISA assay. The antioxidant results showed that 6 revealed more effectiveness (IC50 62.9 and 70.92 μM for DPPH and ABTS, respectively) than BHT (DPPH, IC50 91.35 μM) and Trolox (ABTS, IC50 101.23 μM). Moreover, 6 had promising cytoprotective potential on H2O2-induced oxidative damage in SHSY5Y cells without cytotoxic effect. Furthermore, it exerted its cytoprotection efficacy via the GSH level upregulation and ROS elimination, thus protecting SH-SY5Y cells from H2O2 damage and suggesting its protective role on neurodegenerative illnesses with oxidative stress [21]. Interestingly, NaBr addition to Chondria crassicualis-associated A. ochraceus fermentation culture induced the production of a new mellein-brominated analog, (R)-(–)-5-bromomellein (5), along with (3R)-mellein (1) (Figure 1). They possessed antioxidant potential (IC50 24 and 25 μM, respectively) compared to l-ascorbic acid (IC50, 20.0 μM) [72]. Yamazaki et al. purified and characterized 8 colorless needles by crystallization from MeOH and elucidated by spectral analysis [73].
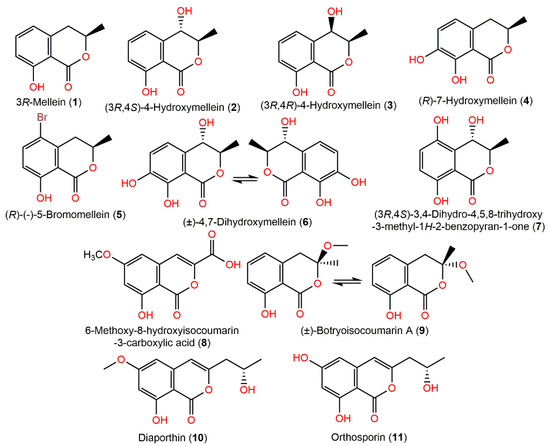
Figure 1.
Isocoumarin derivatives (1–11) reported from A. ochraceus.
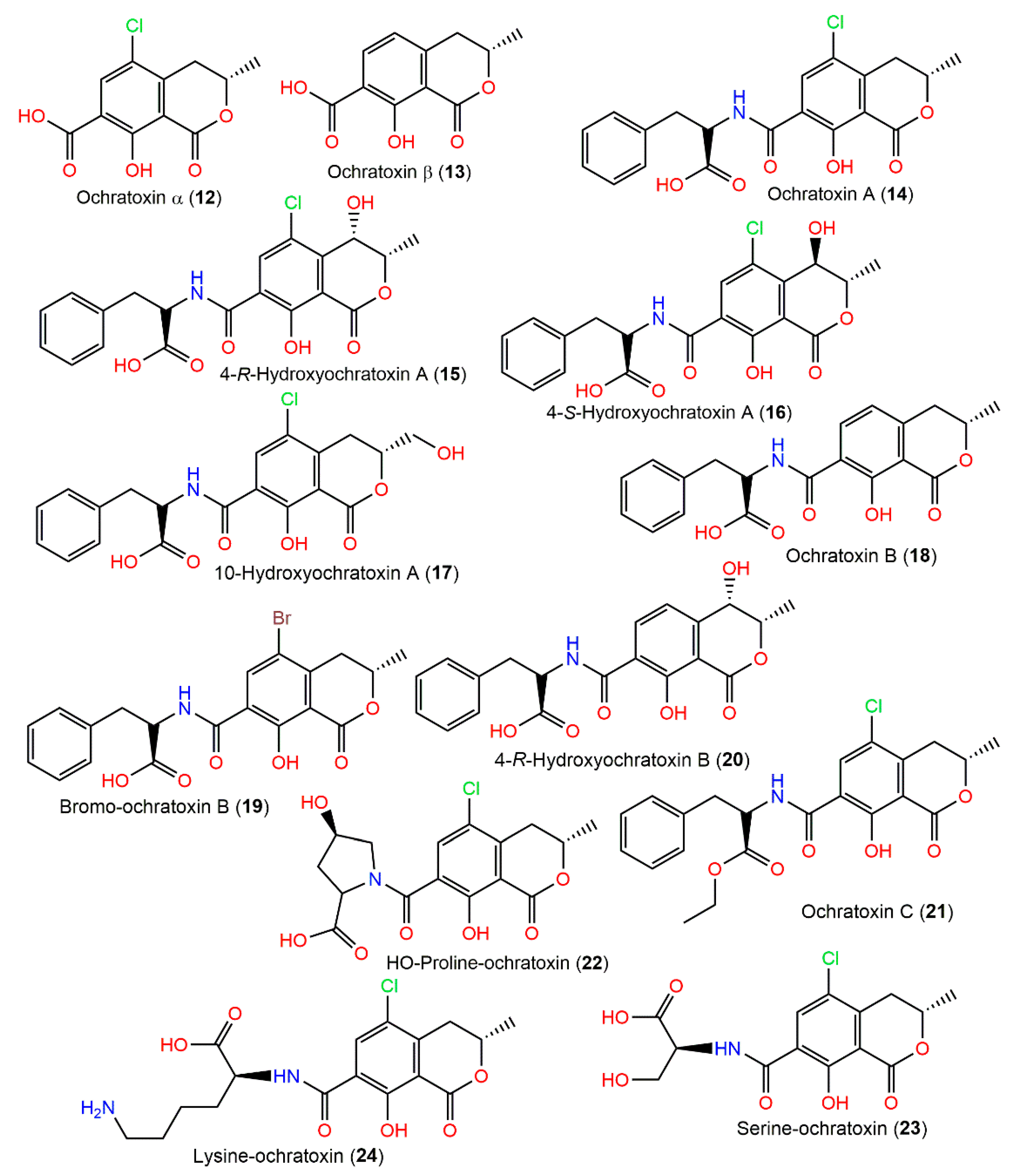
Ochratoxins are toxic metabolites, biosynthesized by this fungus, that contaminate a variety of foodstuffs, resulting in serious effects on both animals and humans. Ochratoxin A (14) is the highly toxic one of this group. It is implicated in nephrotoxic syndromes in various animals, as well as its hepatotoxic, carcinogenic, genotoxic, immune-toxic, and teratogenic effects on animal species and potent carcinogenic effects on humans. It is worth mentioning that ochratoxins A (14) and B (18) are well-known mycotoxins that are associated with diverse animal and human diseases, including porcine nephropathy, poultry ochratoxicosis, and human endemic nephropathies [17]. Ochratoxin A (14) features 7-carboxy-5-chloro-8-hydroxy-3,4-dihydro-3R-methyl isocoumarin, connected by an amide bond to L-β-phenylalanine at 7-carboxy group and, together with its dechloro and ethyl ester derivatives (ochratoxins B (18) and C (12), respectively) these were purified by SiO2 and ion exchange (Dowex I) CC from the fungal CHCl3-MeOH extract and characterized by NMR and chemical method [74] (Figure 2). Moreover, 14 was found as a major metabolite, in addition to 18 in the CHCl3 extract of A. ochraceus NRRL-3174 [75]. In 1971, Cole et al. reported the separation of a new metabolite, 4-hydroxymellein (2), along with 1 from the CHCl3 extract utilizing SiO2 CC, pTLC (acetone/CHCl3 7:93), and crystallization (CHCl3:hexane) [16]. Compound 18 was reported to show strong cytotoxicity versus A2780 (IC50 3.0 μM) compared to cisplatin (IC50 2.2 µM) [17]. The substitution of proton in 18 by chlorine in 14 decreased its cytotoxic effect versus A2780 cells [17].
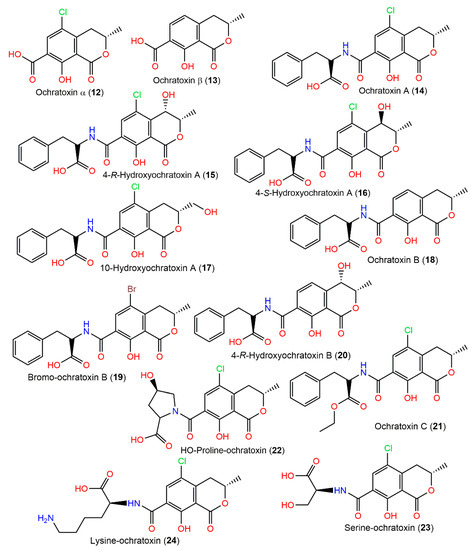
Figure 2.
Isocoumarin derivatives (12–24) reported from A. ochraceus.
In 1995, ochratoxins 12, 13, 15, 17, and 20 were purified from A. ochraceus NRRL3174 culture and structurally elucidated by HPLC, MS, and NMR (Table 1) [76].

Table 1.
Secondary metabolites reported from Aspergillus ochraceus (chemical class, molecular weight and formulae, fungal source, host, and place).
4.2. Pyrazine Derivatives
These metabolites possess pyrazin-2(1H)-one core that are biosynthesized from two amino acid molecules, such as isoleucine, leucine, norvaline, and valine. These compounds were reported from bacteria and fungi and revealed cytotoxic, antibacterial, and brine shrimp toxicity capacities.
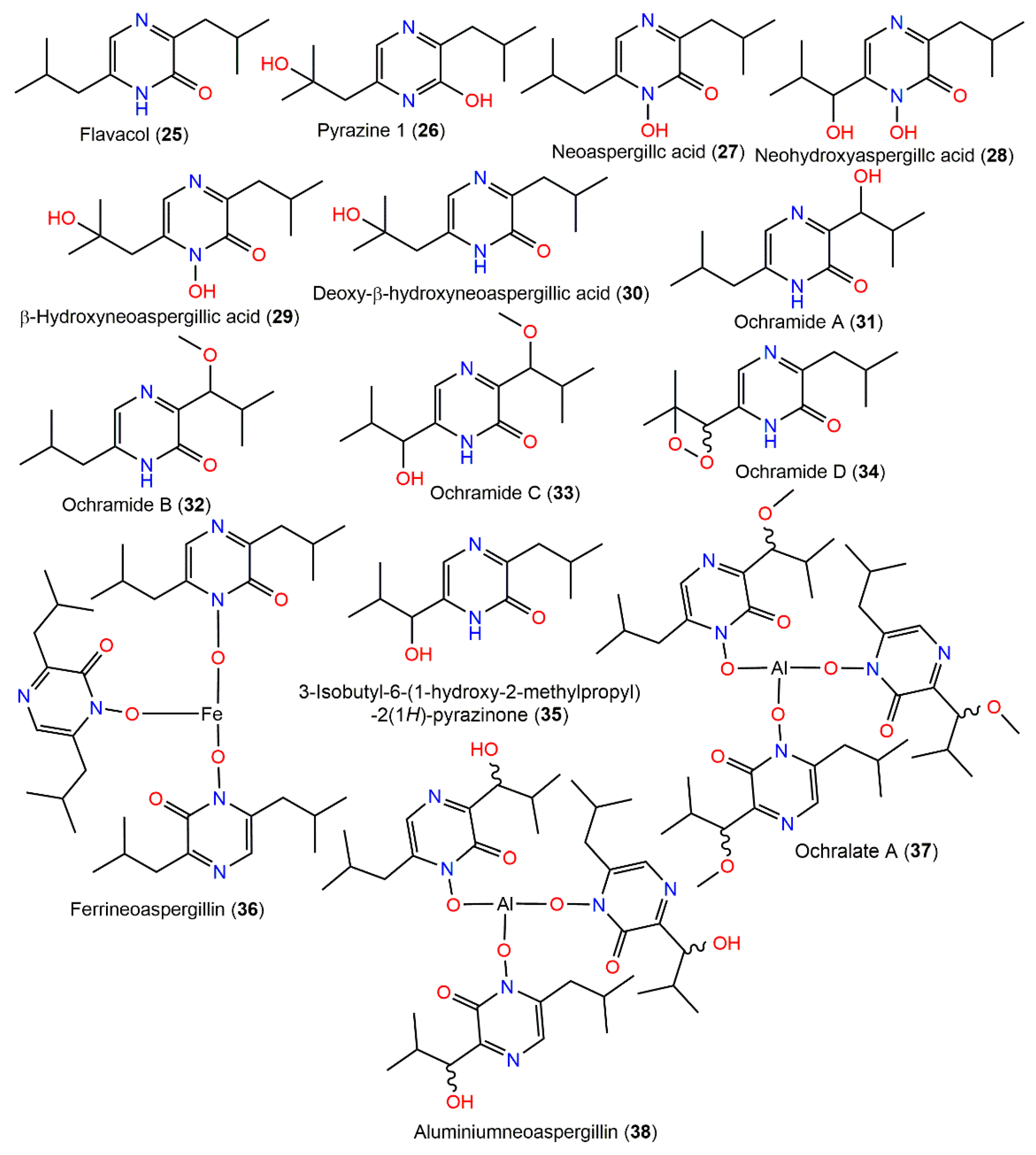
Compound 26, 25, and 27 were separated from fungus EtOAc extract by SiO2 CC. Compound 26 is structurally similar to 25 with the existence of a β-hydroxyl group in the isobutyl side chain [84] (Figure 3).
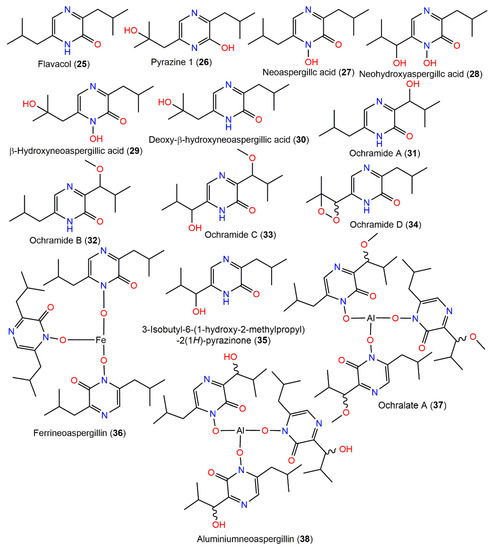
Figure 3.
Pyrazine derivatives (25–38) reported from A. ochraceus.
Additionally, from the moldy rice-associated strain, neoaspergillic acid (27) and five related metabolites (25, 28, 29, 30, and 36) derived from leucine were characterized. Ferrineoaspergillin (36) is a red pigment consisting of a complex of iron with neoaspergillic acid [85]. Compound 25 was reported to inhibit NADH oxidase activity with IC50 13 μM by López-Gresa et al. [86]. From the fermentation broth of A. ochraceus LCJ11/102 cultured on a 10% NaI-containing nutrient-limited medium, five new pyrazine derivatives, ochramides A–D (31–34) and ochralate A (37), in addition to the formerly reported 25, 35, and 38, were isolated utilizing Sephadex LH-20 and Rp-18 CC and HPLC, and their structures were determined using NMR and X-ray data. Compounds 31–33 are 7-hydroxy, 7-methoxy, and 7-methoxy and 11-OH derivative of 25, respectively, while 34 possesses peroxide linkage among C11 and C12 and 37 is similar to 38 with an additional 7-methoxy group. Compounds 32, 37, and 38 possess antibacterial potential towards E. aerogenes (MICs 40, 18.9, and 20.1 μM, respectively) compared to ciprofloxacin lactate (MIC 0.93 μM); however, they had no effectiveness versus K562, A549, and Hela cells in the SRB method [22].
4.3. Diketopiperazines
Diverse prenylated alkaloids, such as brevianamides, sclerotiamides, stephacidins, and notoamides, which possess bicyclo-[2,2,2]diazaoctane ring or diketopiperazine core, have been separated from A. ochraceus. These alkaloids are biosynthetically derived from a second cyclic amino acid residue, L-tryptophan, and two or one isoprene units.
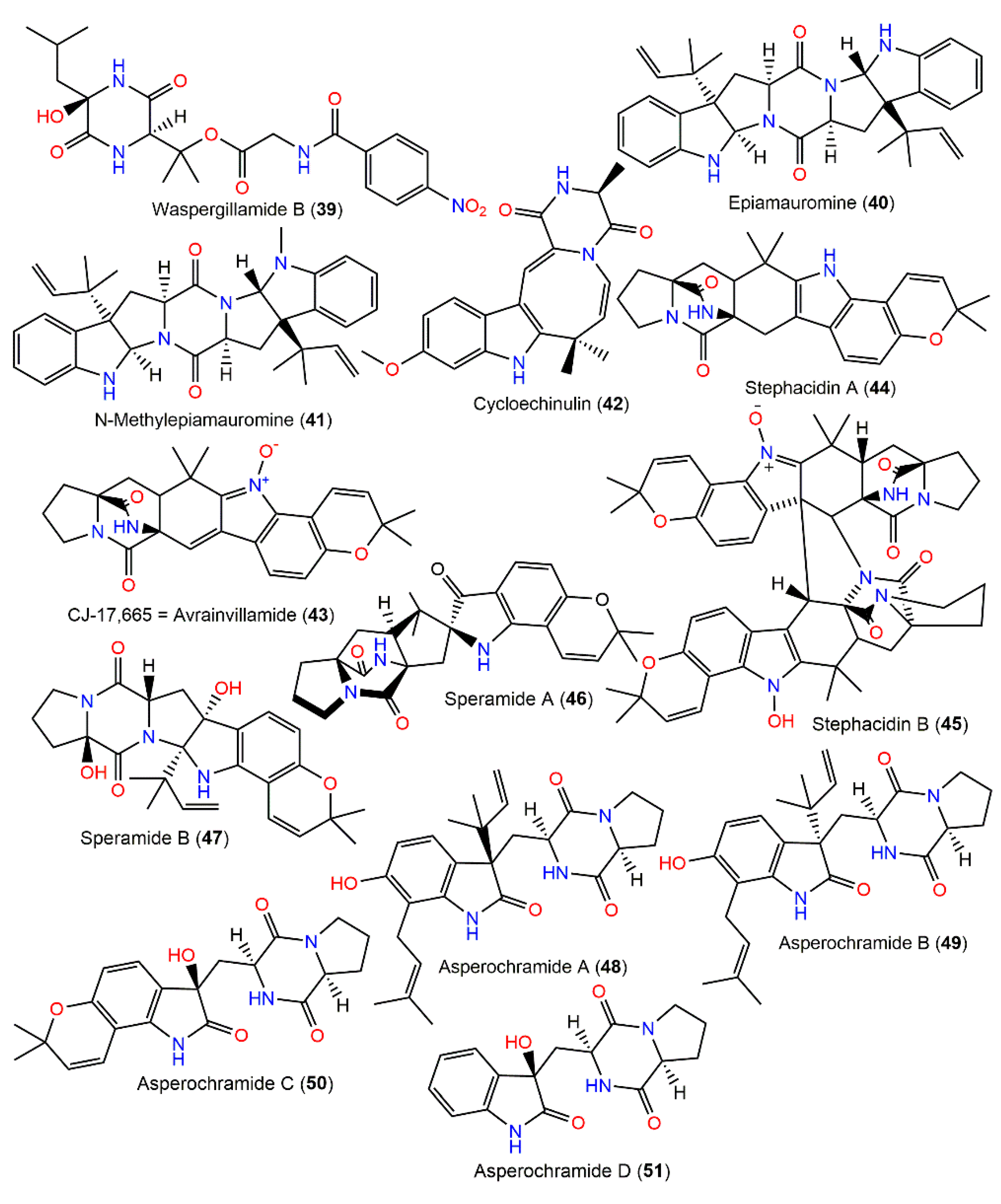
Using the OSMAC technique through cultivating Mediterranean sponge Agelas-oroides-derived A. ochraceus on white beans yielded a new diketopiperazine, waspergillamide B (39), featuring an uncommon p-nitrobenzoic acid unit and diketopiperazine moiety that was originated from 2-hydroxy leucine and 3-hydroxy valine residues (Figure 4). It was characterized by NMR and its 9R configuration was established by Marfey’s analysis [17].
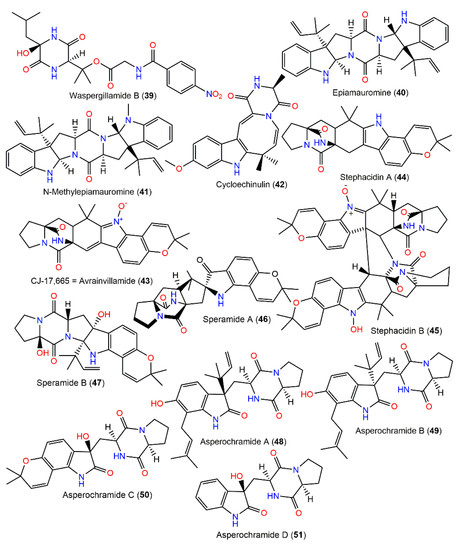
Figure 4.
Diketopiperazines derivatives (39–51) reported from A. ochraceus.
Three new diketopiperazine derivatives, epiamauromine (40), N-methylepiamauromine (41), and cycloechinulin (42), were obtained from A. ochraceus NRRL-3519 sclerotia using Sephadex LH-20 and reversed-phase HPLC and assigned using NMR, whereas their stereochemistry was assured by relying on Marfey’s experiment and alpha D (Figure 4). Compounds 40 and 41 are similar to amauromine obtained from Amuroascus sp. [110], possessing dihydroindole and N-methyl dihydroindole moieties, respectively, whereas 42 features an eight-membered ring and indole moiety. These metabolites revealed moderate weight gain reduction on Helicoverpa zea (corn earworm) with 30, 17, and 33% reductions, respectively, relative to controls after one week [28]. Compound 42, a non-basic Trp–Ala-derived alkaloid was separated from A. ochraceus D2306 mycelia CHCl3 extract by pTLC and characterized by X-ray analysis [87]. A new antibiotic, CJ-17,665 (avrainvillamide, 43), was isolated from the fermentation broth of soil-associated A. ochraceus CL41582 by Sephadex LH-20 CC/HPLC and characterized by NMR tools [88]. This compound is a diketopiperazine metabolite, possessing indole N-oxide moiety. It demonstrated antibacterial capacity versus multi-drug-resistant S. pyogenes, S. aureus, and E. faecalis (MICs 12.5, 12.5, and 25 μg/mL, respectively) in microdilution assay and cytotoxicity towards HeLa cell (IC90 1.1 μg/mL) in the MTT assay [88] (Table 2).

Table 2.
Biological activity of reported metabolites from Aspergillus ochraceus.
In 2002, new alkaloids, stephacidins A (44) and B (45), in addition to 43, were purified from A. ochraceus WC76466 EtOAc fraction by Sephadex LH-20 and RP-HPLC and established by NMR and X-ray. They demonstrated cytotoxic effectiveness versus LNCaP, PC-3, A2780/DDP, A2780, A2780/Tax, HCT116/mdr+, HCT-116, MCF-7, HCT116/topo, SKBR3, and LX-1 (IC50 ranged from 13.1 to 1.0 µM for 44 and 0.46 to 0.06 µM for 45), with observed selectivity towards LNCaP cells (IC50 0.06 and 1.0 µM, respectively) [89]. Compound 44 was found to have mild mitochondrial respiratory inhibition capacity (IC50 34 μM) by inhibiting NADH oxidase activity [86].
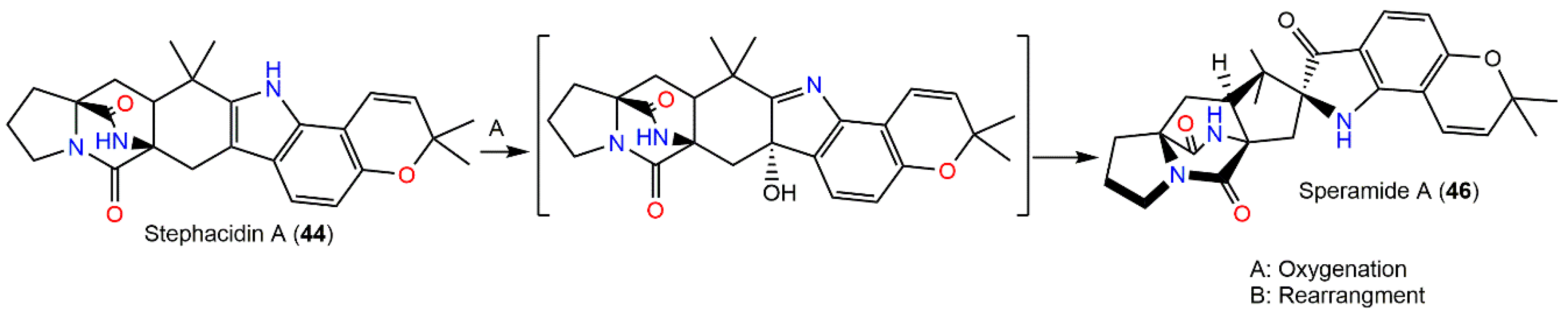
In 2016, Chang et al. reported two new prenylated indole alkaloids, speramides A (46) and B (47) from A. ochraceus KM-007 associated with fresh-water, found using various chromatographic and spectral tools. Compound 46 firstly reported prenylated brevianamide derivatives with a heptacyclic framework possessing azaspiro skeleton with a bicyclo[2.2.2]diazaoctane-linked 2,2-dimethyl-2Hfuran moiety connected to a 3-ox-indole unit. Its bicyclo[2.2.2]diazaoctane diketopiperazine core absolute configuration was 2S/11S/17S/21S based on the CD exciton chirality method. Meanwhile, 47 is related to notoamide D with an additional C-17-hydroxyl group, possessing a 2S/3R/11S/17R configuration. They had no marked effectiveness versus DU145, PC-3, and Lncap cell lines in the MTT assay (IC50 > 40 µM). On the other hand, only 46 revealed moderate potential versus P. aeruginosa (MIC 0.8 µM) in the 2-fold dilution method [23]. Compound 46 was proposed to be biosynthesized from 44 via oxidation and subsequent rearrangement (Scheme 1) [23].
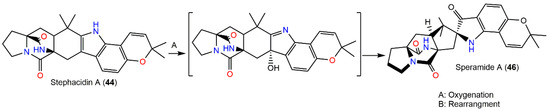
Scheme 1.
Biosynthesis pathway of speramide A (46) from (+)-stephacidin A (44) via oxidation and rearrangement [23].
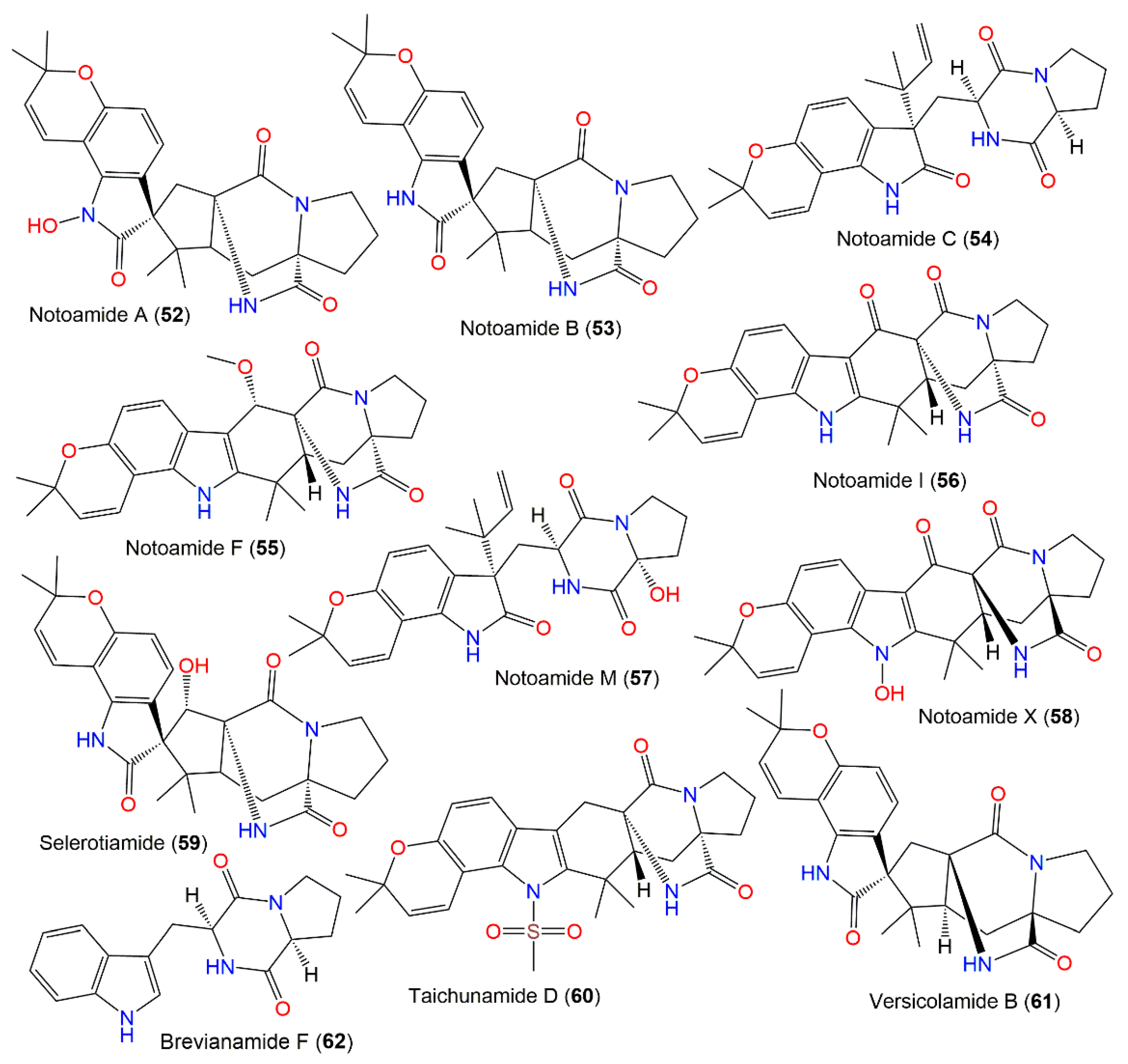
New diketopiperazine alkaloids, asperochramides A–D (48–51), were separated from A. ochraceus EtOAc extract, and the formerly reported 44, 53, 54, 57, 59, 60, and 62 that were elucidated by spectroscopic, ECD, and X-ray analyses (Figure 5). Compounds 50 and 51 are rare indole diketopiperazine alkaloids with a 3-hydroxyl-2-indolone moiety, while 48 and 49 are epimers at C-3, with 3R/11S/17S and 3S/11S/17S, respectively. These metabolites were assessed for their anti-inflammatory potential using the LPS-stimulated murine macrophage RAW 264.7 cells model [90]. Furthermore, 44, 48, 57, 53, and 60 prohibited IL-1β (% inhibition ranged from 36.8% to 49.2%, concentration of 20 μM), while others had lower than 30% at a concentration of 20 μM. Meanwhile, 44, 48, 53, 51, 54, 59, 60, and 62 showed TNF-α inhibition (% inhibition ranged from 32.6% to 41.9%, at a concentration of 20 μM), whereas 50 and 57 exhibited lower TNF-α inhibition ratios than 30% at the same concentration. Hence, 44, 48, 53, and 60 revealed potential anti-inflammatory capacity [90]. Furthermore, notoamides A (52), B (53), I (56), M (57), and X (58) and sclerotiamide (59) were characterized from deep-sea fresh-water A. ochraceus that had no notable anti-H1N1 virus, anti-food allergic, antimicrobial, and anti-inflammatory capacities [80].
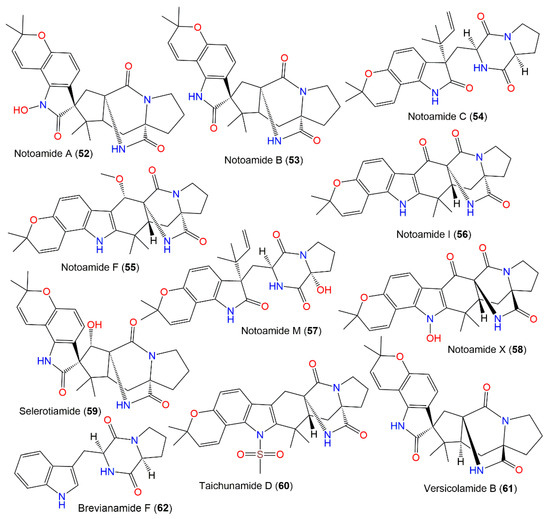
Figure 5.
Diketopiperazines derivatives (52–62) reported from A. ochraceus.
Hu et al. assessed the anti-Parkinson’s disease efficacy of 44, 53–56, and 61. All metabolites were tested for their anti-Parkinson’s disease capacity in MPP+ (dopaminergic selective neurotoxin)-induced SH-SY5Y cells assay. All metabolites revealed anti-Parkinson’s disease potential (EC50s ranged from 2.30 and 7.39 μM), whereas 44 and 56 featured prominent effectiveness (EC50 2.45 and 2.30 μM, respectively) compared to levodopa (EC50 2.06 μM). Their ADMET prediction revealed the appropriate features for CNS drugs, better drug-likeness characteristics, and preferable safety scores of toxicities [24]. Therefore, 56 could be an advantageous lead compound for anti-Parkinson’s therapeutic drugs.
4.4. Benzodiazepine Derivatives
Benzodiazepines are psychoactive metabolites that are naturally reported from Streptomyces, Aspergillus, and Penicillium [91,92]. These metabolites are renowned as a chemotaxonomic marker for this fungus [26,86].
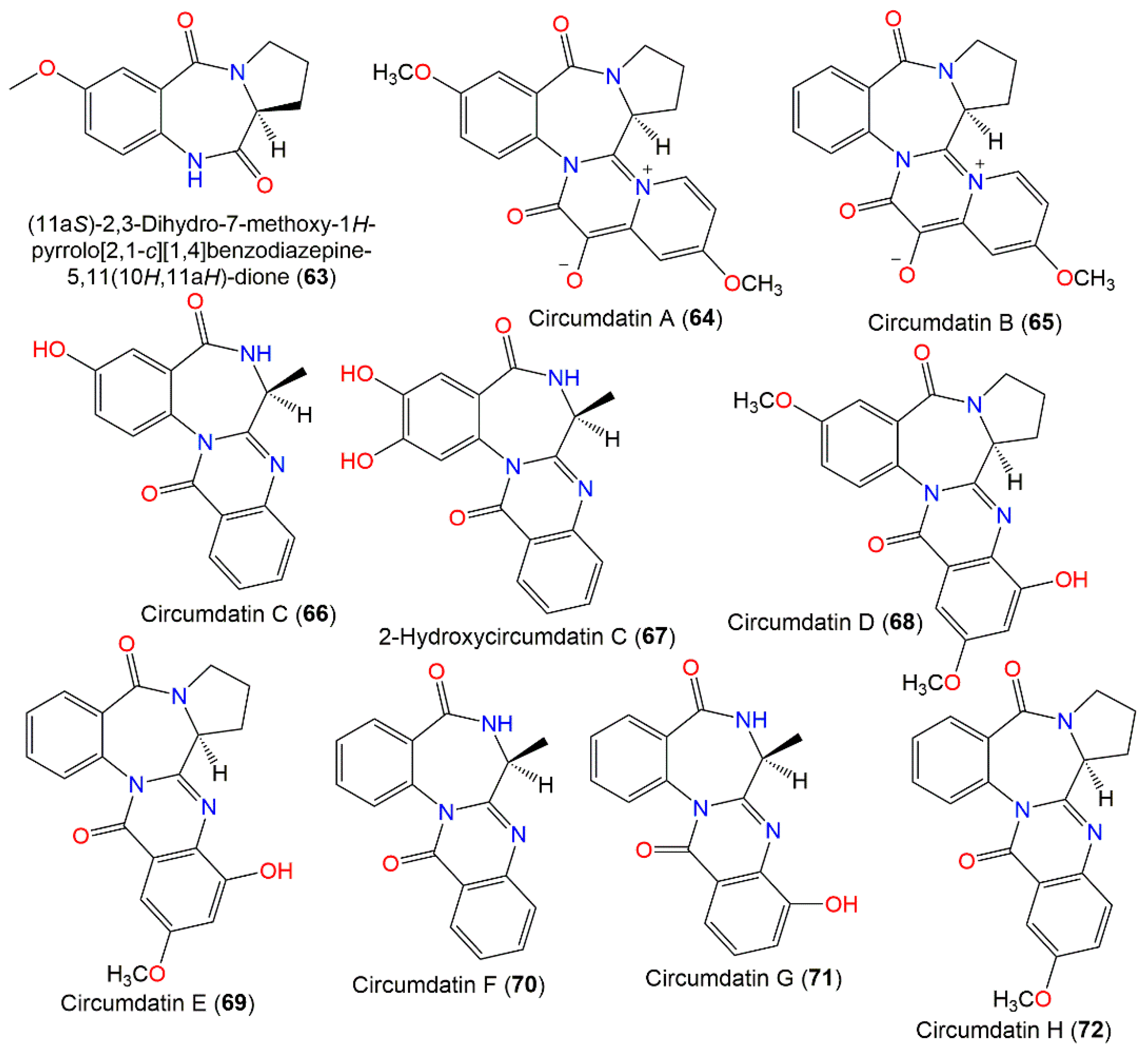
New benzodiazepine-related metabolites, compounds 68–70, in addition to the known 64–66, were separated from an A. ochraceus terrestrial strain obtained from milosorghum seeds using UV-vis/HPLC analysis and characterized using various spectral analyses (Figure 6). Compound 68 is a C-5 methoxy circumdatin E (69), while 70 lacks an OH group at C-5 in circumdatin C (66) [92]. The benzodiazepine core of 66 and 70 was formed from L-proline and substituted anthranilic acid, while that of 64, 65, 68, and 69 has L-alanine instead of L-proline [91].
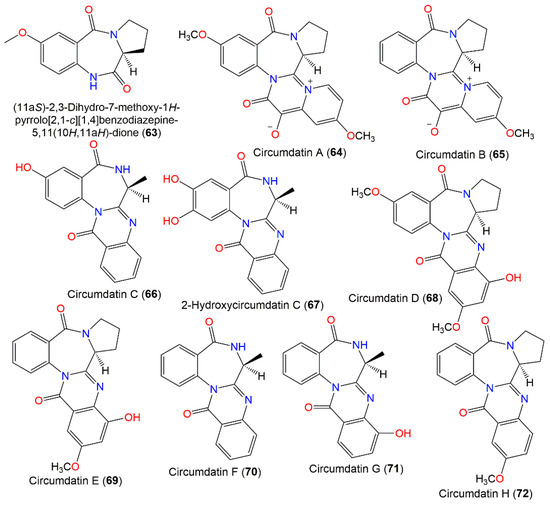
Figure 6.
Benzodiazepine derivatives (63–72) reported from A. ochraceus.
Moreover, a new alkaloid, circumdatin G (71) and 66 and 70 were isolated from a sediment-derived strain by Sephadex LH-20 CC and HPLC. Compound 71 is a 15-OH derivative of 70 based on spectroscopic analysis. They exhibited no HCV-NS3 protease inhibition in the scintillation proximity assay (SPA) [20]. In 2005, circumdatin H (72), a new derivative from the culture broth of soil-derived A. ochraceus, in addition to 69, was isolated by SiO2 CC and HPLC and established by the spectral and chemical method by López-Gresa et al. [86]. Their mitochondrial respiratory chain inhibition capacity was estimated by measuring NADH oxidase potential [86]. These metabolites prohibited (IC50 1.50 and 2.50 μM, respectively) NADH oxidase activity (integrated electron transfer chain), whereas 72 was slightly more potent than 69, suggesting that the C-15 hydroxy group in 69 might lead to a more difficult interaction with the respiratory chain [86]. Thus, these compounds may act as leads in developing new tools for controlling insects [86]. Compound 64 exhibited antioxidant effectiveness (IC50 32 μM) in comparison to ascorbic acid (IC50 20.0 μM) [72].
The marine brown alga Sargassum kjellmanianum-derived A. ochraceus produced a new benzodiazepine analog, 2-hydroxycircumdatin C (67), as well as the known 66, 68, and 70 that were isolated from culture broth and mycelia extract by RP-18, SiO2, and Sephadex LH-20 CC and structurally established by spectroscopic and CD tools. Only 67 revealed marked antioxidant potential (IC50 9.9 μM) that was 8.9-fold more powerful than BHT (butylated hydroxytoluene, IC50 88.2 μM); however, 66 and 68 (IC50 > 100 μg/mL) had weak potential in the DPPH assay. On the other hand, none of them had antibacterial potential in the well diffusion method [26].
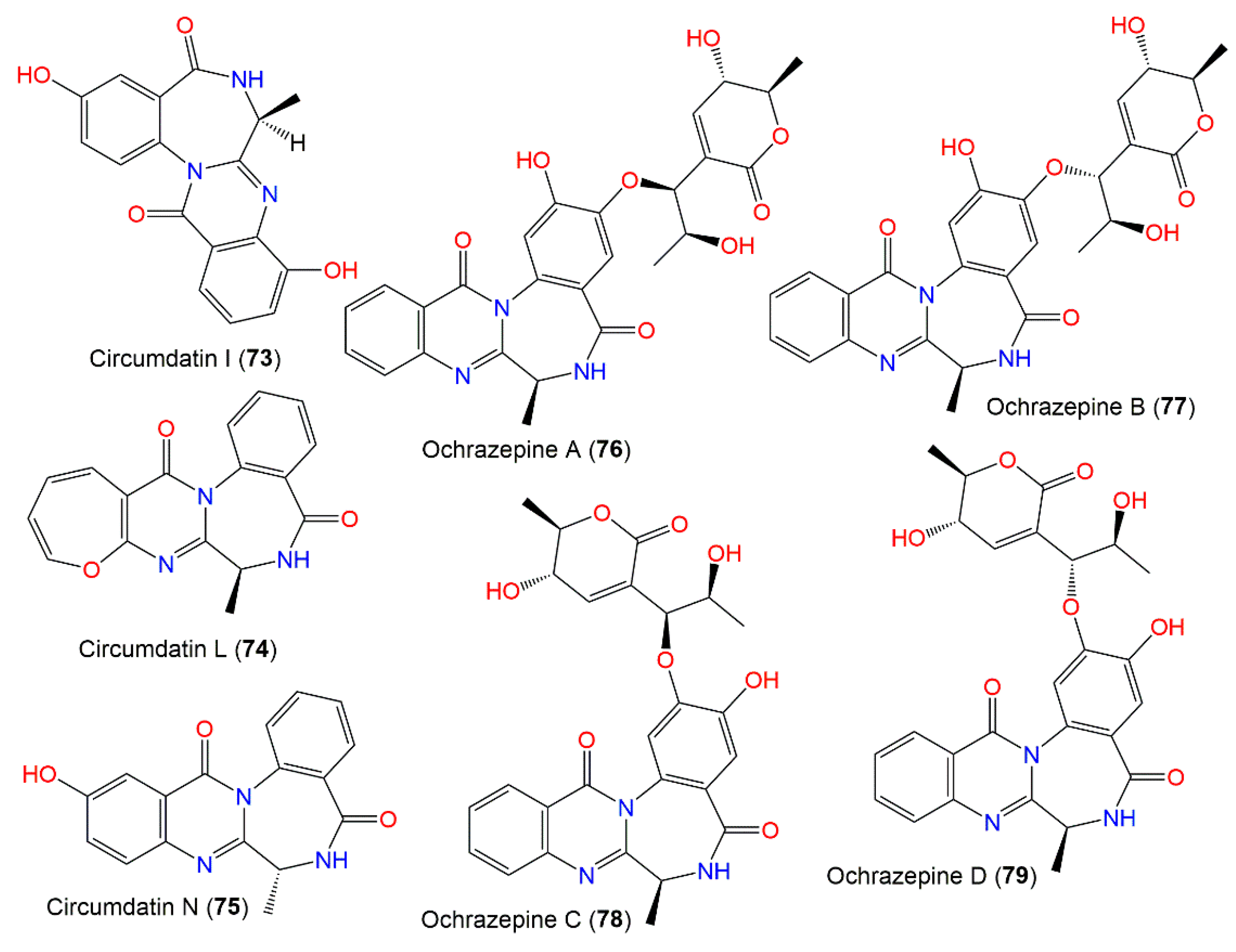
Hu et al. purified a new benzodiazepine derivative, circumdatin N (75), in addition to 70 and 71, from A. ochraceus MCCC3A00521 EtOAc extract by SiO2, RP-18 CC, and HPLC. Compound 75 is structurally related to 70 except for the existence of an additional C13-OH group. Its 19R configuration was established by ECD and X-ray analyses. They exhibited anti-Parkinson’s effectiveness in MPP+ (dopaminergic selective neurotoxin)-induced SH-SY5Y cells assay with EC50s 10.77, 5.44, and 7.39 μM, respectively [24].
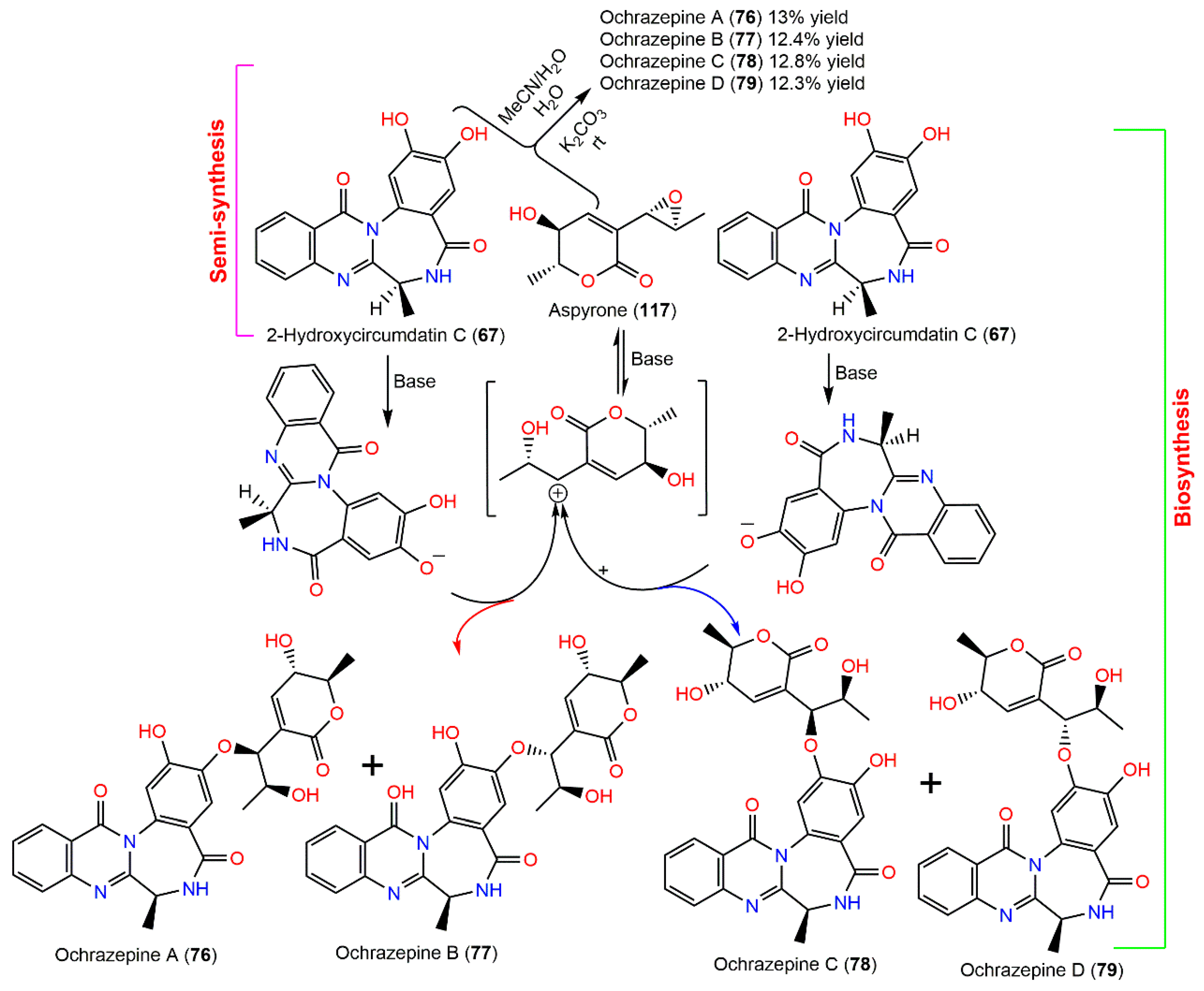
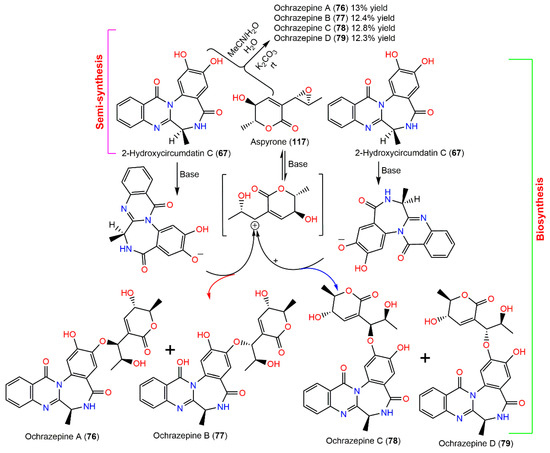
In 2019, Fan et al. purified four new metabolites: ochrazepines A–D (76–79) from coral-associated A. ochraceus LCJ11102 fermentation broth utilizing SiO2, Sephadex LH-20, and HPLC (Figure 7). These metabolites are dimer conjugates derived from 117 and 67 through a nucleophilic addition to epoxide [31] as confirmed by the semisynthesis (Scheme 2).
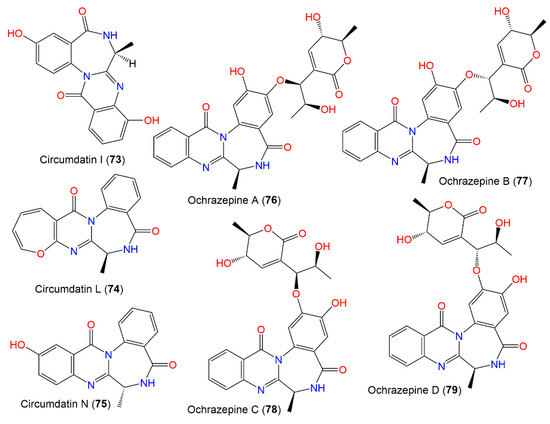
Figure 7.
Benzodiazepine derivatives (73–79) reported from A. ochraceus.
Scheme 2.
Semisynthetic and biosynthetic pathways of ochrazepines A–D (76–79) [31].
Their structures and absolute configuration were assured by spectral and chemical methods. These compounds are epimers, differing only in configurations at C-8′. Their cytotoxic potential versus 2 normal and 26 human cancer cell lines was evaluated in the CTG (Cell Titer Glo) assay. Compound 117 had the most broad-spectrum cytotoxic capacity against 16 cancer cells (IC50 ranged from 2.54 to 9.79 µM) because the epoxide could alkylate DNA by a nucleophilic addition with the sulfhydryl of protein or the base of DNA. Compound 76 also displayed a wide spectrum cytotoxic potential versus 10 cancer cells (IC50 ranged from 3.10 to 11.32 µM). In addition, 67, 77, and 79 exhibited a selective cytotoxic capacity versus U251, while 78 selectively prohibited U87, A673, and Hep3B. Interestingly, the conjugation among 67 and 117 promoted the inhibitory potential on U87 and U251. Compounds 76 and 78 with 8′S configuration revealed cytotoxic potential versus U87 cells; however, 77 and 79 with 8′R configuration were active versus U251 cells. On the other hand, 76–79 exhibited low cytotoxicity on the normal human cells, HEK-293F and L02. Thus, these metabolites could represent leads in anticancer drugs against human glioblastoma cells with non-cytotoxic action to human normal cells [31].
4.5. Indole and Other Alkaloids
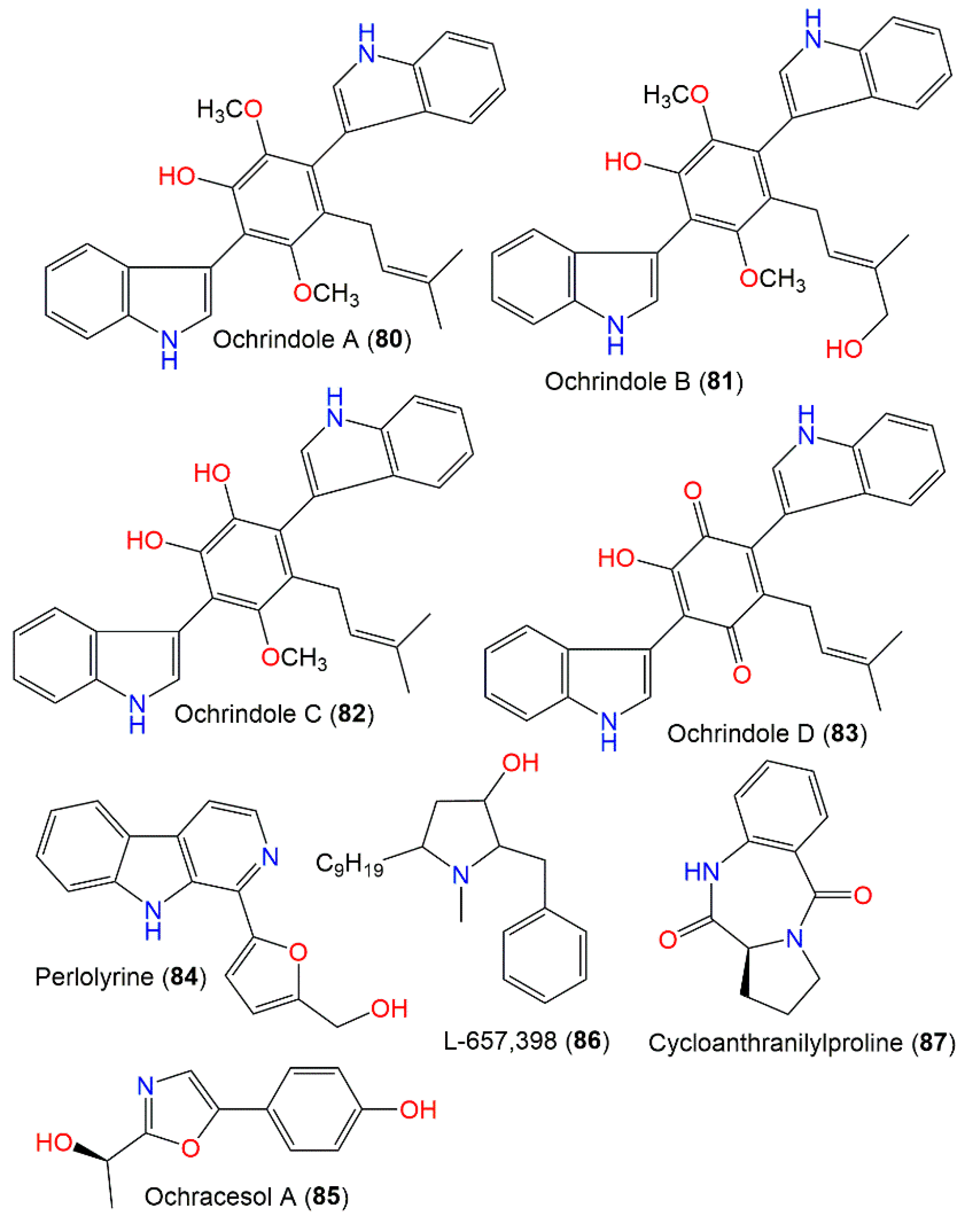
Chromatographic investigation using SiO2 and Sephadex LH-20 CC of anti-insectan A. ochraceus NRRL3519 sclerotia EtOAc extracts yielded new prenylated bis-indolyl benzenoid metabolites, ochrindoles A–D (80–83), and a new bis-indolyl quinone, ochrindole D (83) (Figure 8). These metabolites display a central ring-linked prenyl sidechain. In feeding assays, they had moderate effectiveness (concentration of 200 ppm w/w) towards Carpophilus hemipterus (dried fruit beetle) and H. zea, whereas 80 caused a 20% and 30% weight gain reduction, respectively [29]. These compounds also had potential versus B. subtilis (inhibition zone 15–18 mm, concentration of 100 μg/disk) in the disk assay. It is worth mentioning that these compounds were found to be the main components of A. ochraceus NRRL3519 sclerotia; therefore, they could be accountable for the anti-insectan potential of the A. ochraceus extracts [29]. Perlolyrine (84), a β-carboline alkaloid with a 5-hydroxymethyl substituent in the C-1 linked furan ring, was reported from A. ochraceus MCCC3A00521 culture by Hu et al. [24]. Furthermore, A. ochraceus ATCC22947 yielded a novel pyrrolidine antibiotic, L-657,398 (86), that was separated from mycelia EtOAc extract by SiO2 and Sephadex LH-20 CC and elucidated by various NMR spectroscopic data. This metabolite is related to anisomycin, an anti-protozoal and antifungal metabolite produced by Streptomyces roseochromogenes and S. griseolus [111]. Compound 86 also possessed marked broader efficacy towards yeasts and filamentous fungi than anisomycin using the disk diffusion method [93]. Ochracesol A (85), a new alkaloid, featured an oxazole ring linked to p-OH-disubstituted benzene. Its 17R configuration was assigned by X-ray and ECD analyses. This compound had weak anti-Parkinson’s potential (EC50 17.84 μM) [24].
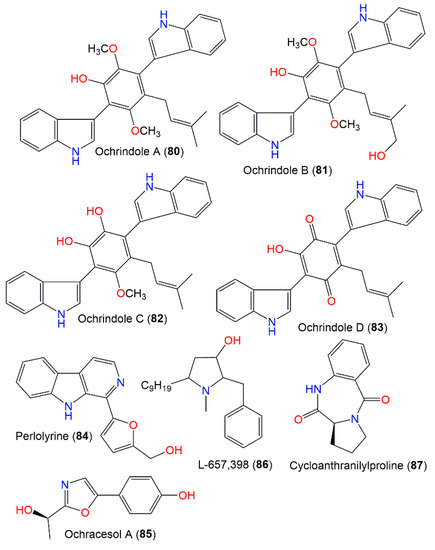
Figure 8.
Indole and other alkaloids (80–87) reported from A. ochraceus.
4.6. Peptides
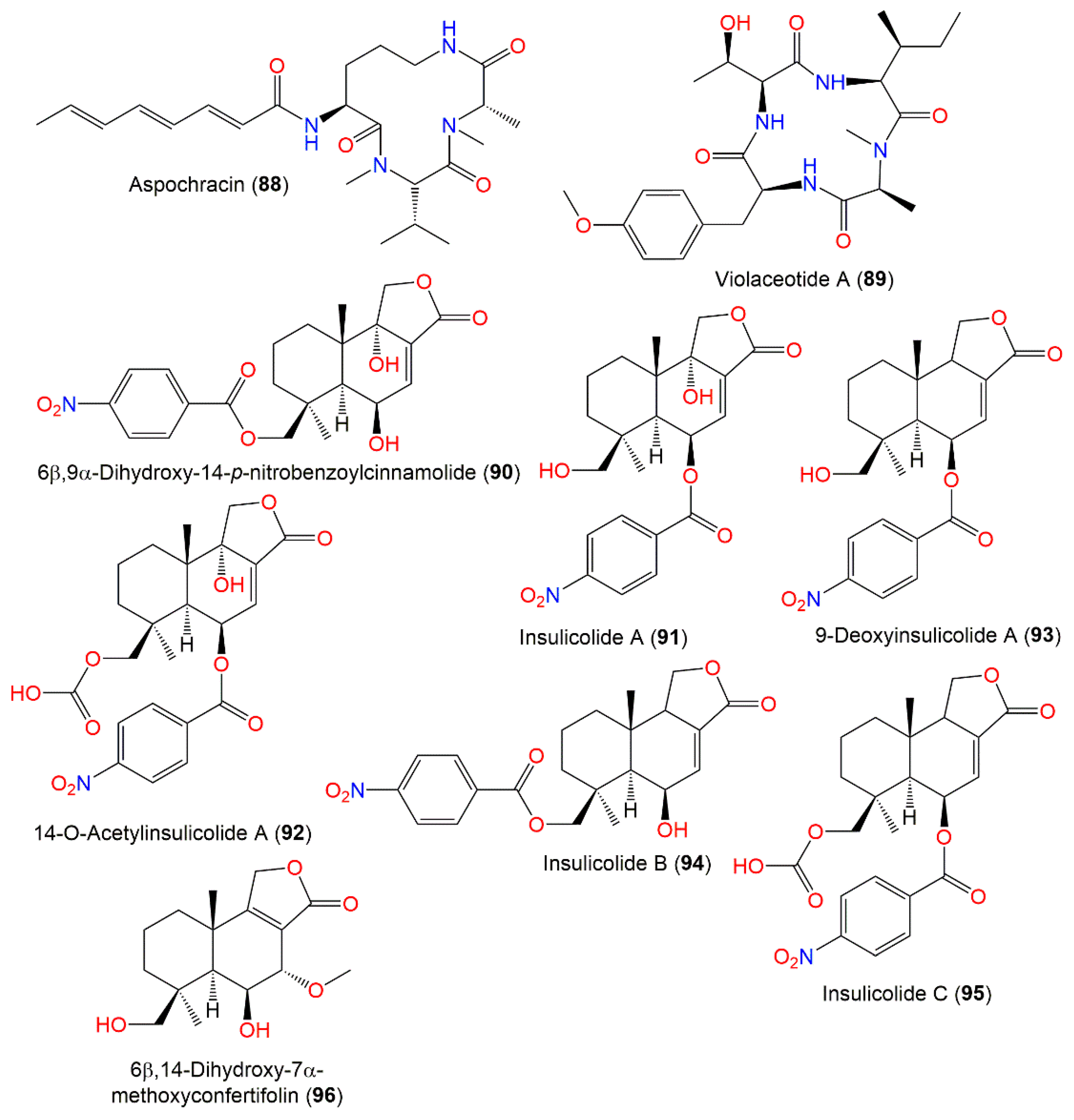
Aspochracin (88), a cyclotripeptide with N-methyl L-valine, N-methyl L-alanine, and L-ornithine residues and an octatrienoic acid side chain, was separated as a pale-yellow powder from A. ochraceus EtOAc extract by SiO2 CC and characterized using NMR and chemical methods. It was tested for its insecticidal, antimicrobial, and cytotoxic activities. It demonstrated insecticidal potential by injection of silkworm and fall webworm, with 17 μ/g minimal concentration, on larvae, causing paralysis followed by death into the final instar larvae of silkworm and 170 μ/g for the final instar larvae of fall webworm. Additionally, it demonstrated the silkworm’s first instar larva and egg contact toxicity using the dipping method. Its hydrogenation completely minified the effect, suggesting the potential of the side chain’s conjugated triene in its action. Its 165 mg/kg IV dose did not kill mice and had no antimicrobial efficacy towards various microbes in agar dilution, paper disc, and liquid dilution methods [95].
4.7. Sesquiterpenoids
Nitrobenzoyl sesquiterpenoids are a rare class of metabolites and were reported mainly from Aspergillus species [25]. A. ochraceus Jcma-1F17 associated with Coelarthrum sp. marine alga collected from Paracel Islands, South China Sea yielded a new nitrobenzoyl sesquiterpenoid, 6β,9α-dihydroxy-14-p-nitrobenzoylcinnamolide (90), and a former analo, 91, that were separated from mycelia EtOAc extract by SiO2 CC and HPLC and characterized by NMR, MS, CD, and optical rotation (Figure 9). They displayed noticeable cytotoxic potential versus HL-60, U937, H1975, MCF-7, BGC-823, Hela, K562, Molt-4, A549, and Huh-7 (IC50 ranged from 1.95 μM to 6.35 μM) in the CCK-8 assay [32]. Additionally, 91 displayed marked in vitro effectiveness versus HCT-116 and moderate selectivity towards a panel of renal tumor cell lines [25]. Moreover, 90 had moderate antiviral activity versus H3N2 and EV71 (IC50 17.0 and 9.4 μM, respectively) while 91 had no obvious potential in the CPE (cytopathic effect) and CCK-8 assays. On the other hand, they did not show any obvious effect versus M. tuberculosis H37Ra in the GFPMA assay [32]. Compounds 94 and 95, new nitrobenzoyl sesquiterpenoids, along with 90–93 and 96, were isolated from culture EtOAc extracts of A. ochraceus Jcma-1F17 derived from marine alga Coelarthrum sp. collected from the South China Sea [97]. Their isolation and elucidation were done using SiO2 CC/Sephadex LH-20/HPLC and NMR/MS/ ECD spectra, respectively. Compound 92 was previously synthesized by treating 91 with acetic anhydride in pyridine [112]. These metabolites were assessed for cytotoxic capacity versus OS-RC-2, ACHN, and 786-O cells in the CCK-8 assay, as well as for NF-κB inhibition using LPS-stimulated RAW264.7 [97]. Compound 91 revealed potent effectiveness versus OSRC-2, ACHN, and 786-O cells (IC50s 1.5, 1.5, and 0.89 μM, respectively) compared to sorafenib (IC50s 7.0, 3.4, and 4.9 μM, respectively), whereas 92 had (IC50 5.3, 4.1, and 2.3 μM) potent potential comparable to sorafenib (Nexavar), the approved drug for advanced renal cell carcinoma treatment, revealing that the C-9-OH group might contribute more cytotoxic capacity towards renal carcinoma cells [97]. Furthermore, 92 induced G0/G1 phase cell cycle arrest and late apoptosis at concentrations of 1 and 2 μM, respectively, after 72 h in 786-O cells. On the other hand, only 94 demonstrated weak inhibition of LPS-induced NF-κB activation (16%, concentration of at 4 μM), while 92 and 94–96 displayed weak toxic influence on RAW264.7 cells [97].
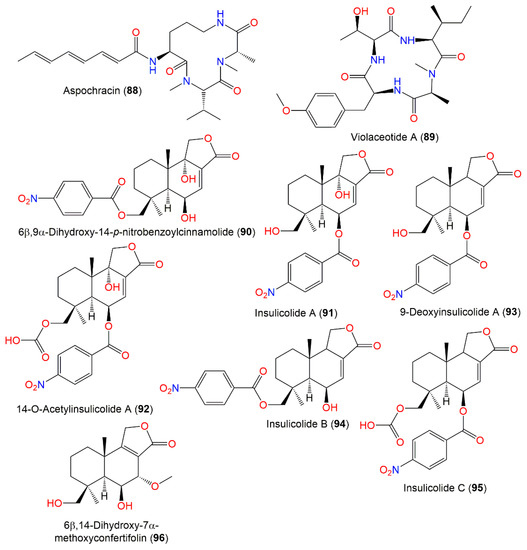
Figure 9.
Peptides (88–89) and sesquiterpenoids (90–96) reported from A. ochraceus.
4.8. Polyketides
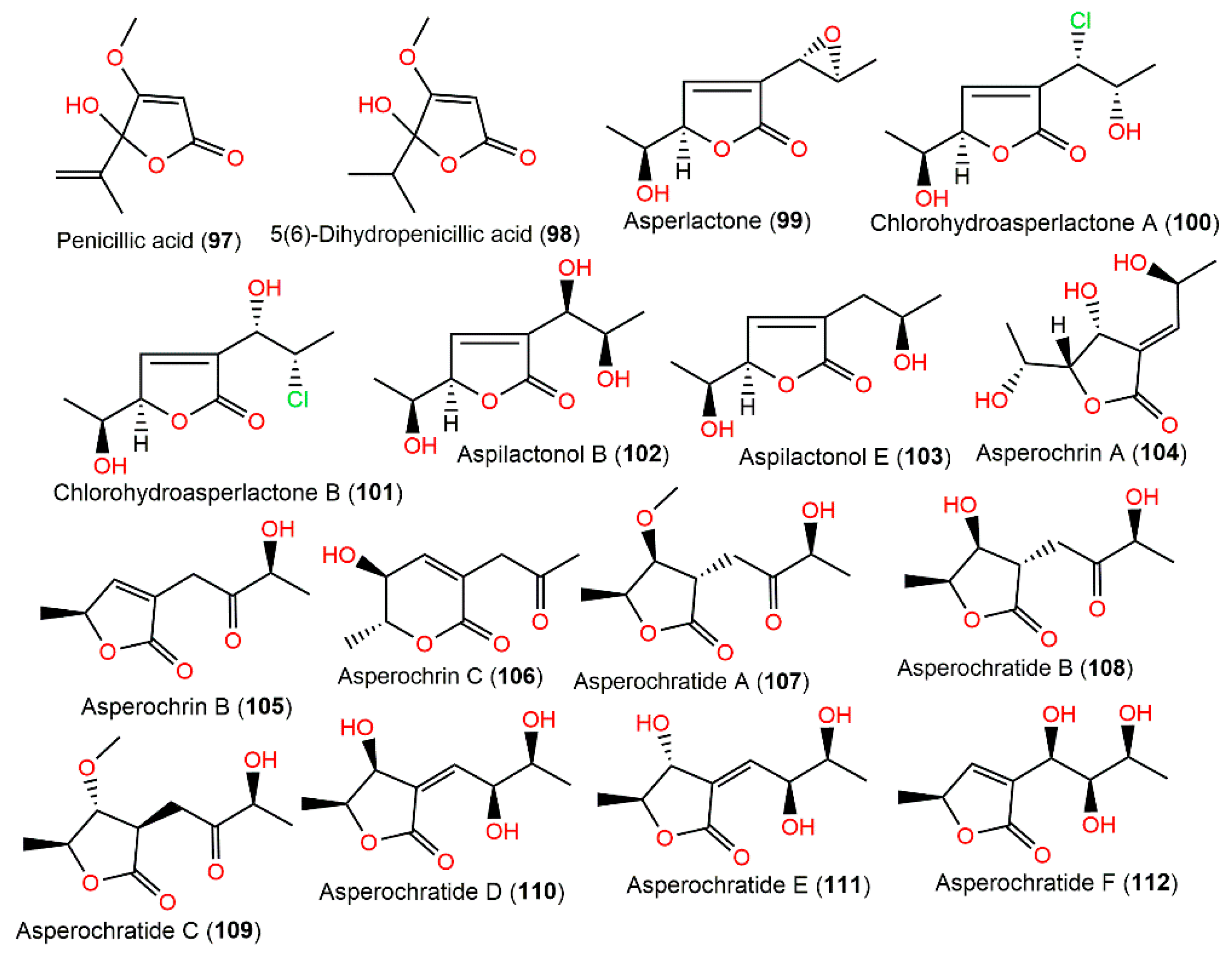
A. ochraceus M#1129-85 EtOAc extract yielded the toxic metabolites penicillic acid (97) and 5(6)-dihydropenicillic acid (98). It was found that 97 was only produced on potato dextrose agar medium; however, A. ochraceus in rice flour yielded 98, suggesting that 97 was transformed into 98 in high-nutritive circumstances (Figure 10). Interestingly, 98 was not mutagenic in the Ames and umu tests [98]. Compounds 99 and 117 were isolated from the mycelia of A. ochraceus collected from an apple-packing house environmental sample. It is noteworthy that 117 displayed better antimicrobial influence than 99 versus 22 bacterial strains and 13 fungal strains in the paper disc assay [99].
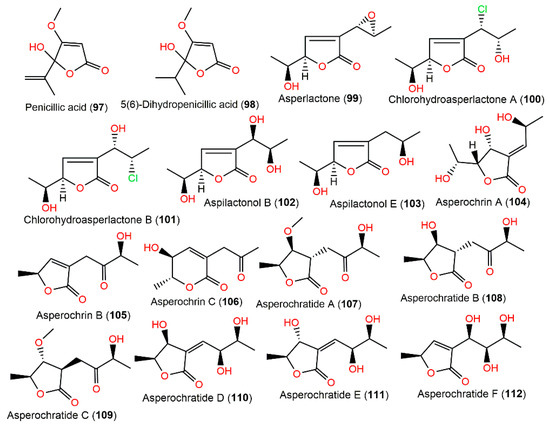
Figure 10.
Polyketides (97–112) reported from A. ochraceus.
The investigation of A. ochraceus MA15 obtained from marine mangrove Bruguiera gymnorrhiza rhizospheric soil resulted in three new metabolites: asperochrins A–C (104–106), along with the formerly separated 97, 98, 100, 101, and 118–121. They were elucidated using NMR, CD, and Mosher’s methods. Compounds 104 and 105 featured α,β-unsaturated γ-lactone, while 106 had α,β-unsaturated δ-lactone moiety. They possessed no activity towards Artemia salina in the brine-shrimp lethality test. Meanwhile, 97, 100, 104, 118, and 119 revealed selective antibacterial potential (MIC ranged from 0.5 to 64.0 μg/mL) versus V. harveyi, V. anguillarum, and A. hydrophilia, whereas 97 and 104 were the most powerful metabolites (MICs ranged from 0.5 to 32.0 μg/mL). It was revealed that increased OH groups elevated activity and the terminal C-6(7) double bond (e.g., 97 vs. 98) was substantial for the activity [71].
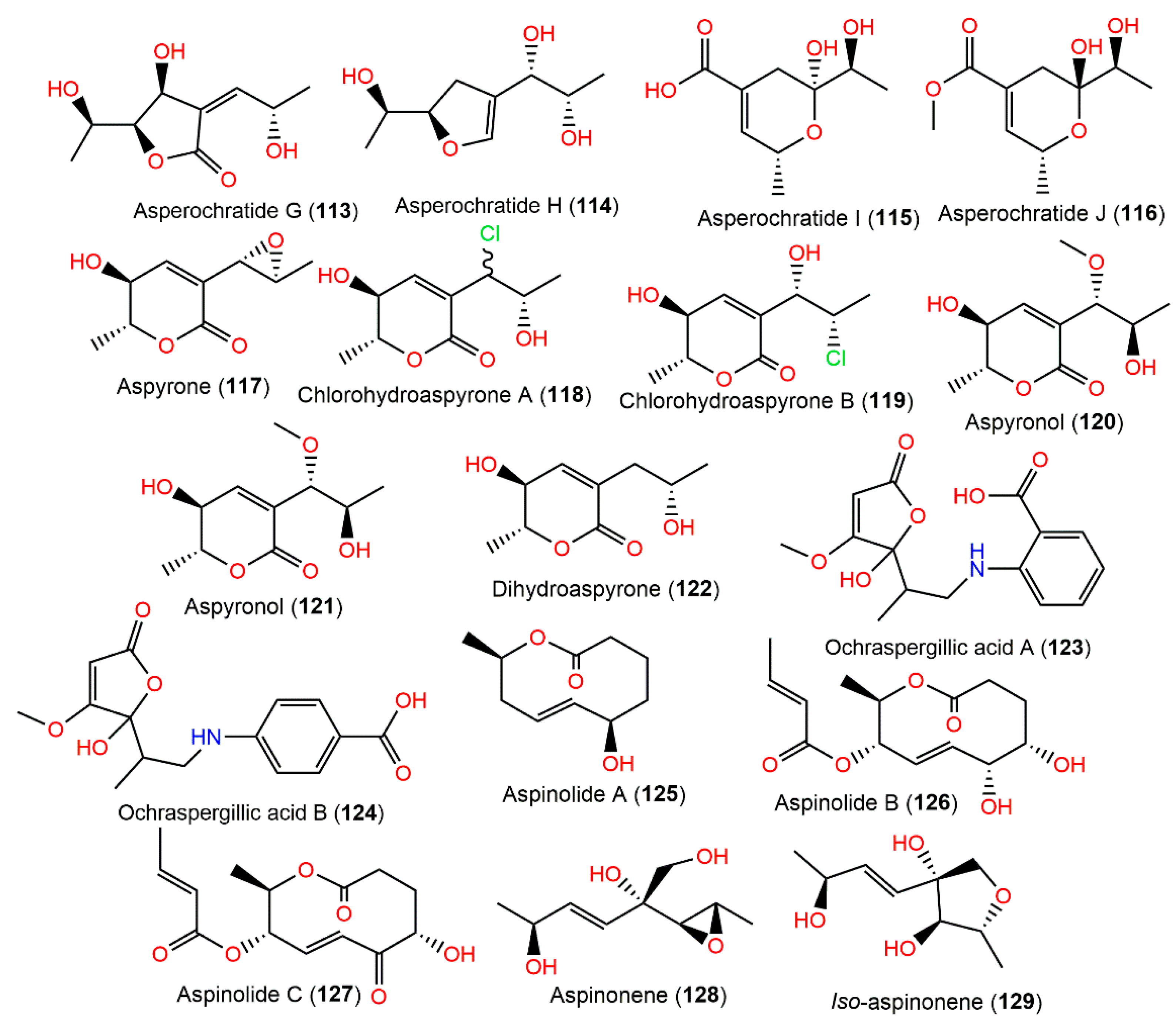
Co-cultivation of A. ochraceus with Streptomyces lividans resulted in an increase in the yields of 97 and 98; however, its co-cultivation with B. subtilis yielded two new penicillic acid derivatives, ochraspergillic acids A (123) and B (124), which are new penicillic acid–aminobenzoic acid hybrids. Interestingly, it was suggested that the aminobenzoic acid moiety of 123 is originated by bacteria [17] (Figure 11).
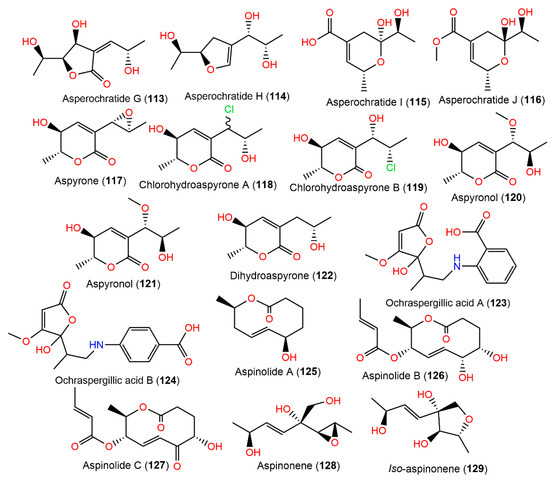
Figure 11.
Polyketides (113–129) reported from A. ochraceus.
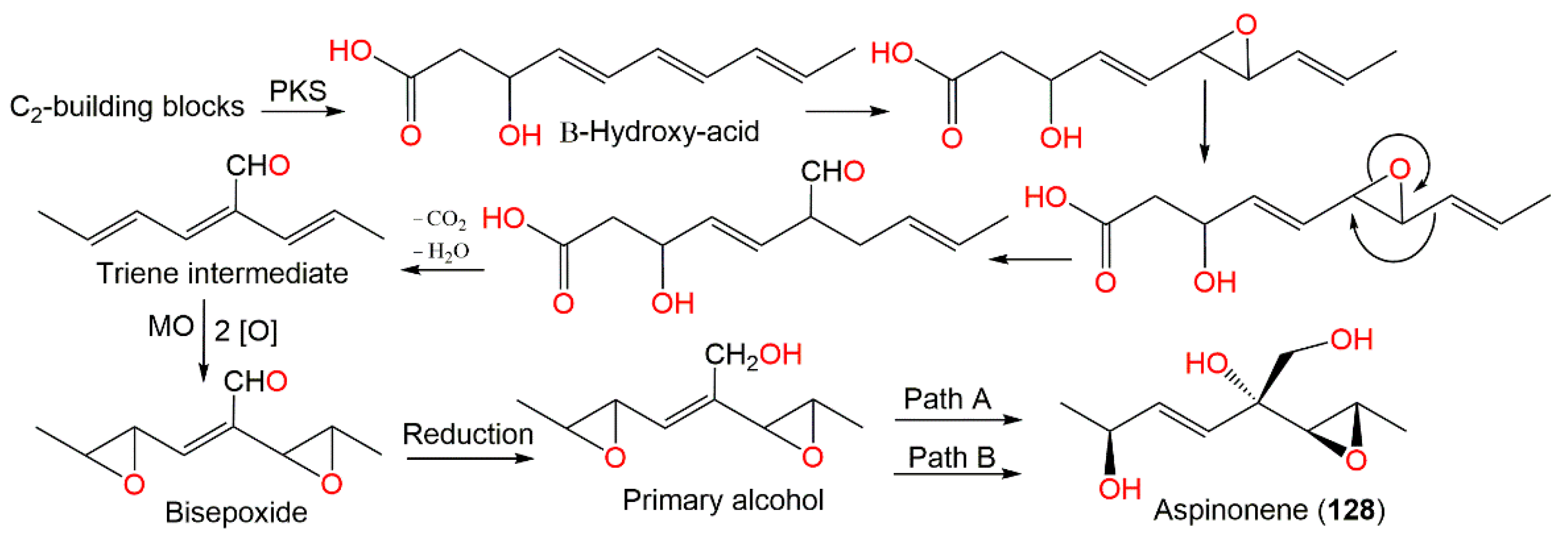
Aspinonene (128), a branched pentaketide with uncommon oxygenation, was separated from A. ochraceus DSM-7428 culture broth using SiO2 and Sephadex LH-20 CC [100]. Its biosynthesis was proposed utilizing [18O2]-rich atmosphere fermentation and [13C]-labelled acetate feeding experiments (Scheme 3). It was proved that 128 was biosynthesized via the polyketide pathway and post-polyketide modification is a key step that directed aspinonene biosynthesis [100].
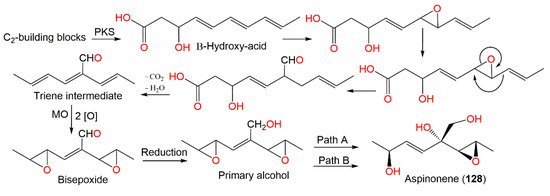
Scheme 3.
Biosynthesis pathway of aspinonene (128) [100]. PKS: Polyketide synthase; MO: monooxygenase; Path A: 1,2-addition + allylic rearrangement; Path B: 1,4-addition.
New C9 polyketides asperochratides A–J (107–116) and 102, 103, 121, and 122 were isolated from EtOAc extract of the fermentation broth of deep-sea water-derived A. ochraceus using SiO2, RP-18, Sephadex LH-20, pTLC, and HPLC and characterized by NMR, MS, Mosher’s method, ICD, ECD, and specific rotation. Structurally, they share the same polyketide skeleton and belong to aspyrone-related metabolites. Compounds 103 and 107–111 exerted a significant cytotoxic effect on the BV-2 cell line with inhibitions ranging from 50.29 to 72.81% in the MTT assay. In the anti-inflammation assay, 103 (1 μM) produced a marked decline (44.87%) of NO concentration in the LPS-stimulated BV-2 cells using Griess reagent. However, none of them exhibited significant anti-H1N1 virus, anti-food allergic, and antimicrobial capacities in vitro [80].
4.9. Xanthine and Quinone Derivatives and Flavonoids
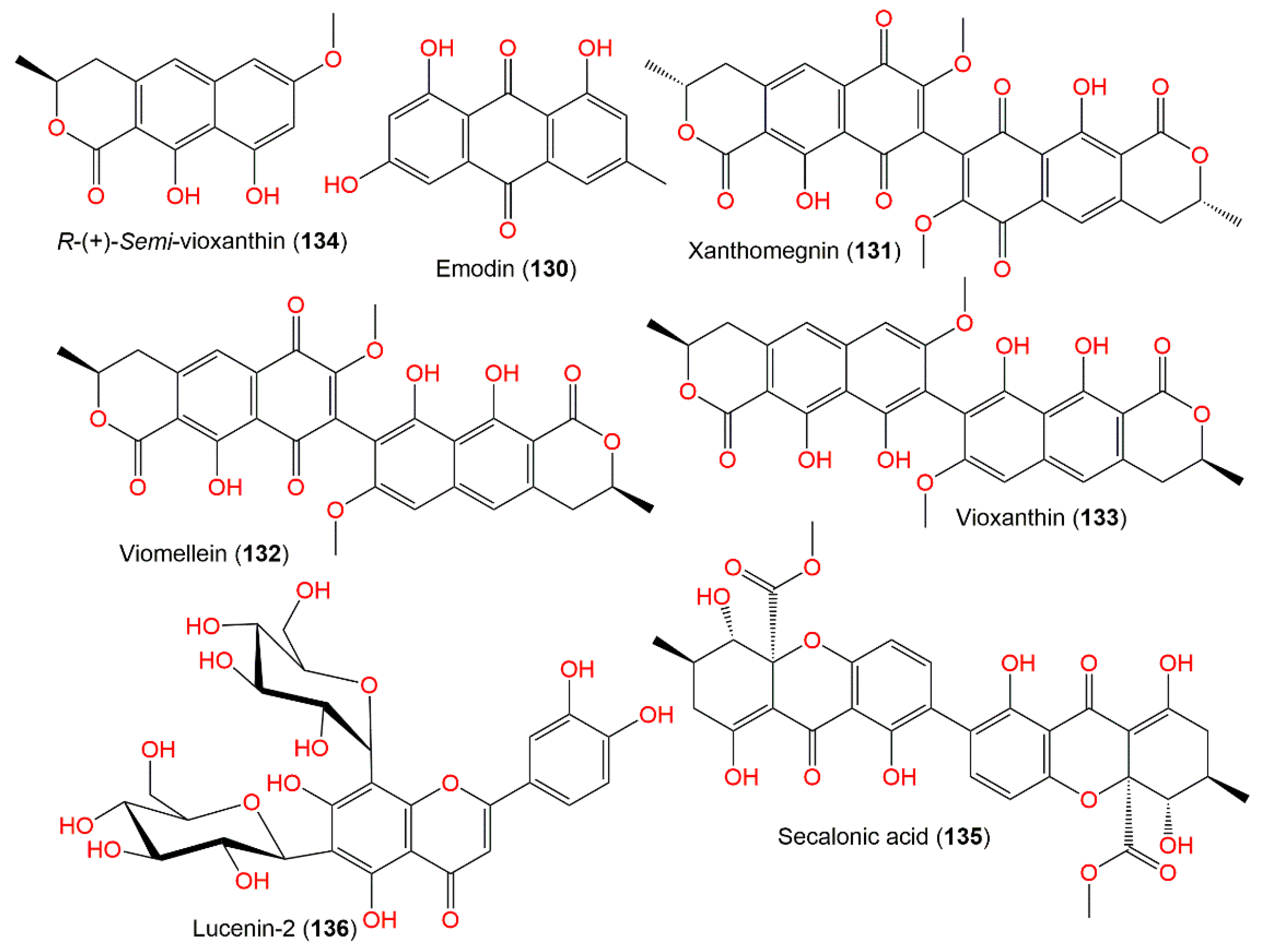
Xanthomegnin (131) and viomellein (132) are structurally related quinones that were produced by the A. ochraceus group (Figure 12). These quinones could induce cholangio-hepatitis and hepatic lesions in mice toxicity assay [105], as well as nephrotoxicity in pigs and swine [107] and mycotoxicosis in mice [17].
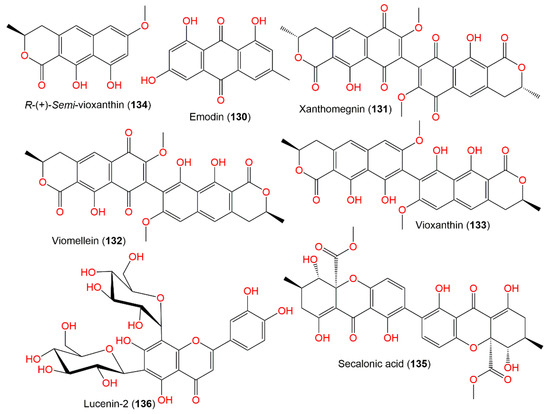
Figure 12.
Quinone (130–132) and xanthine (133–136) derivatives and flavonoids (135) reported from A. ochraceus.
Additionally, 132 exhibited potent cytotoxic capacity versus A2780 and L5178Y (IC50 5.0 and 5.3 μM, respectively) compared to cisplatin (for A2780, IC50 2.2 µM) and kahalalide F (for L5178Y, IC50 4.3 µM), while it was inactive towards human Jurkat T and Ramos B cell lines. Compound 130 was obtained from A. ochraceus isolated from Chinese potato by the solid phase extraction method. The optimal yield of 130 was accomplished when the fermentation was done at pH 7.0 and 32 °C [104]. Secalonic acid A (135), a C-4-C-4′ dimer xanthone, was isolated from the EtOAc fraction of A. ochraceus Wilh IFM4443. This metabolite was formerly reported from Claviceps purpurea [113]. It was not lethal to mice (concentration of 250 mg/kg, orally) and not toxic to the chicken embryos; however, it displayed antibacterial activity towards B. subtitis and piricularia oryzae in the disc assay [103].
4.10. Sterols
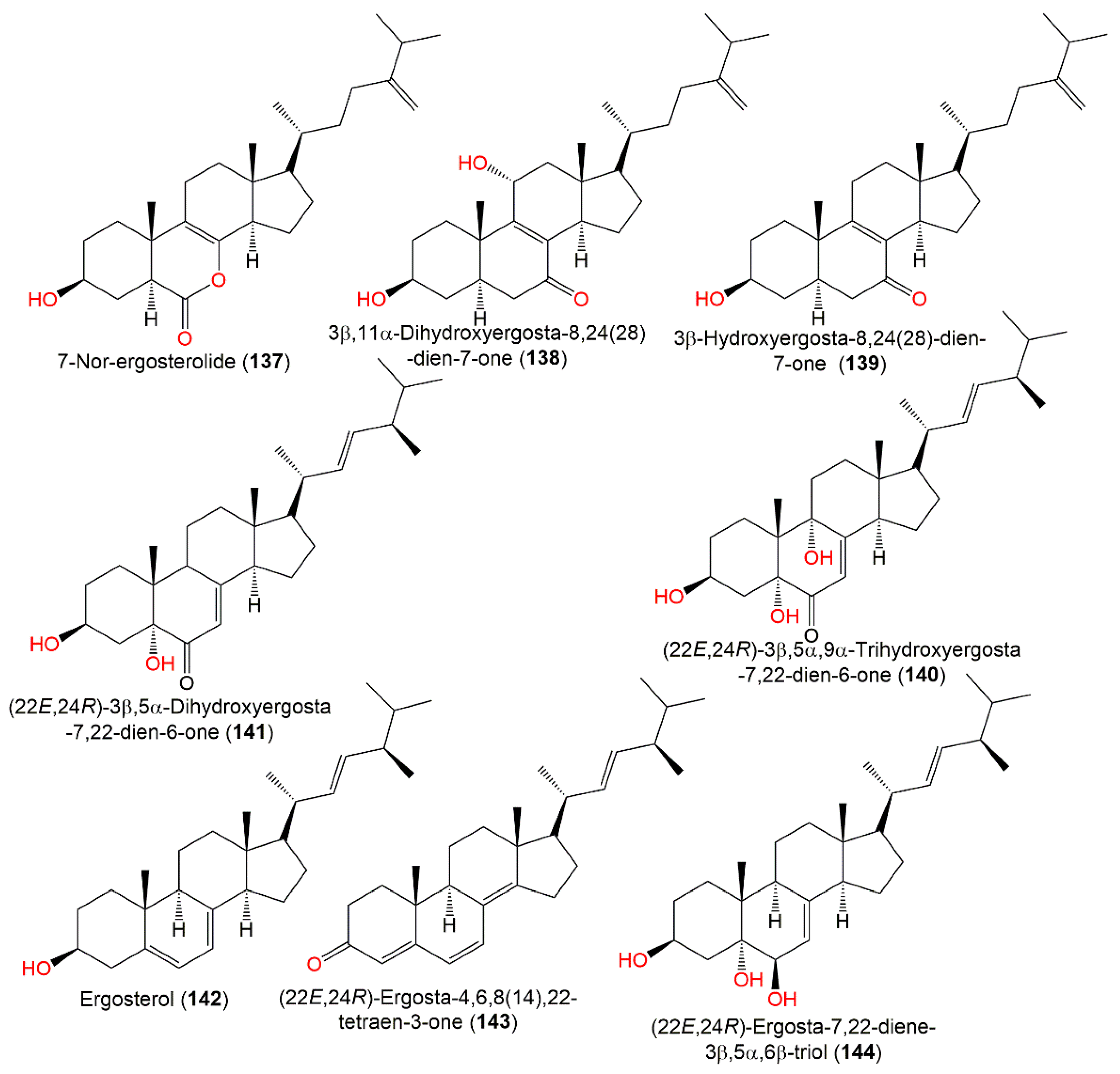
Cui et al. (2010) isolated three new steroids, 137–139, and known steroids, 140–148, from marine brown alga S. kjellmanianum-inhabitant A. ochraceus EN-31 by SiO2 CC. The new compounds were established by spectroscopic analyses and modified Mosher’s method (Figure 13).
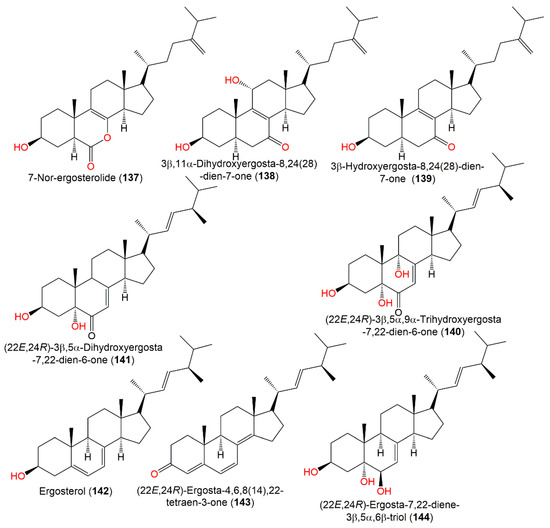
Figure 13.
Sterols (137–144) reported from A. ochraceus.
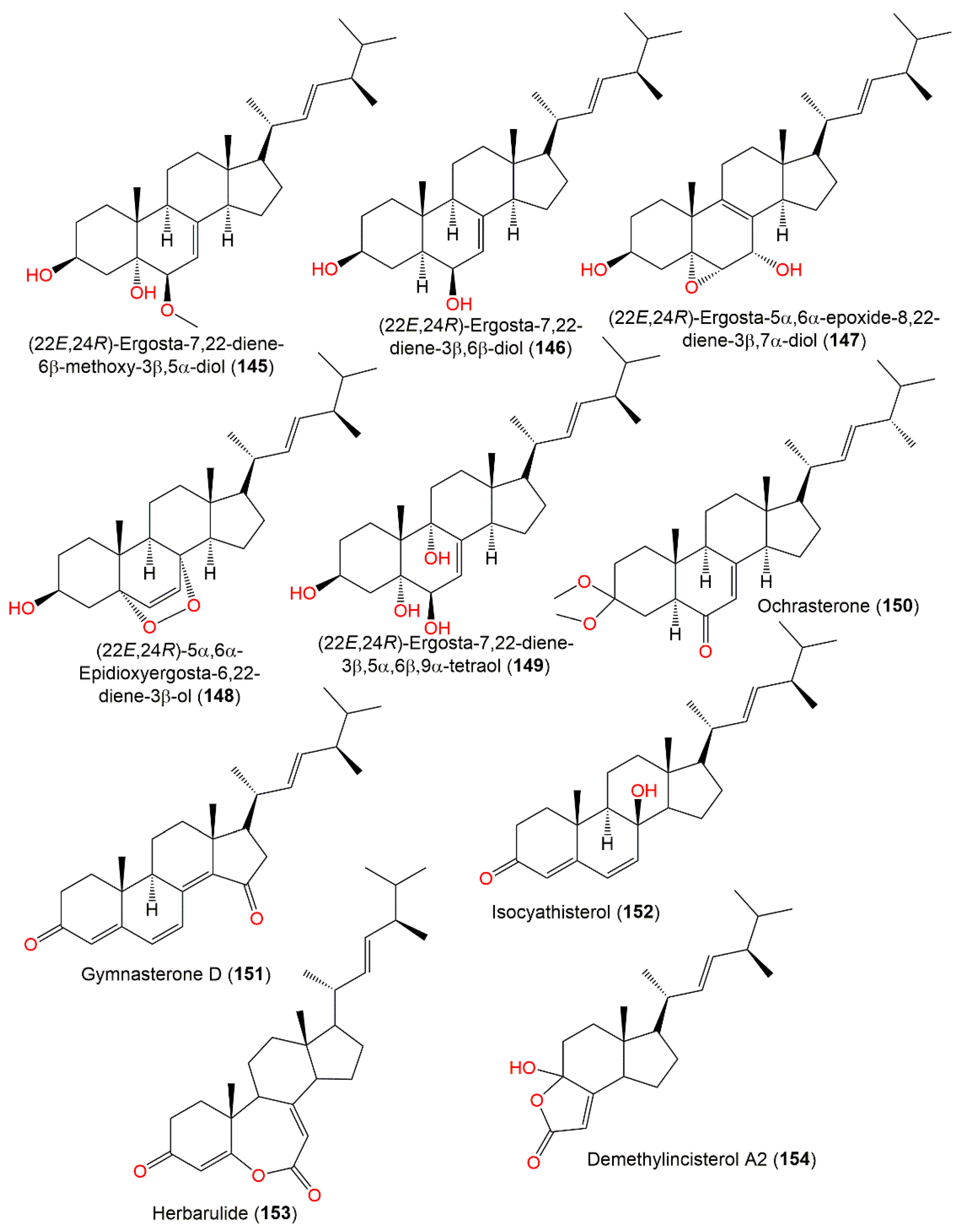
Compound 137 is a rare 7-norsteroid with an uncommon pentalactone B ring system [27]. It demonstrated selective cytotoxic potential towards SW1990, NCI-H460, and SMMC-7721 cell lines (IC50 28.0, 5.0, and 7.0 µg/mL, respectively), while 138 revealed selectivity (IC50 28.0 µg/mL) towards SMMC-7721 in the MTT assay and both had no antimicrobial potential in the agar diffusion test [27]. 22E,24R-Ergosta-7,22-diene-3β,5α,6β,9α-tetraol (149), reported from A. ochraceus derived from fresh sea water, displayed no notable anti-H1N1 virus, anti-food allergic, and antimicrobial activities [80]. On the other hand, 143–154 isolated by Hu et al. from A. ochraceus MCCC3A00521EtOAc extract demonstrated anti-Parkinson’s disease efficacy (EC50 ranged from 35.71 to 49.53 μM) (Figure 14) [24].
Figure 14.
Sterols (145–154) reported from A. ochraceus.
The petroleum ether fraction of A. ochraceus MCCC3A00521 afforded a new sterol, ochrasterone (150), that was characterized by NMR, ECD, and X-ray. Compound 150 has a 5S/9R/10R/13R/14R/17R/20R/24R configuration and 7-en-6-one motif with dimethoxy-substituted C-3 [21]. It displayed no antioxidant, cytotoxic, or neuroprotective effectiveness [21].
4.11. Other Metabolites
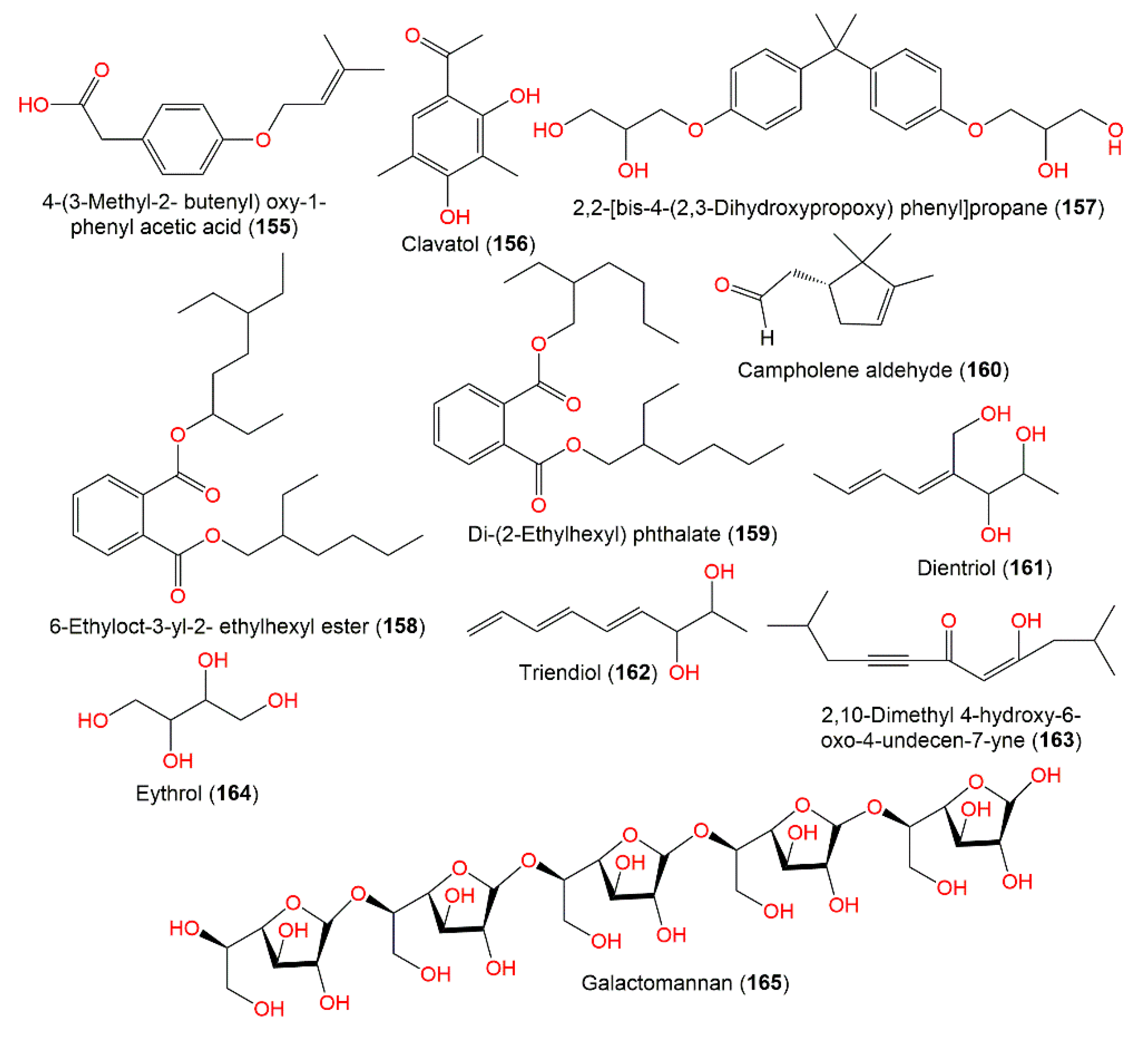
The new compounds 155 and 163 were produced by mutant strain A. ochraceus NRRL3174 due to UV irradiation of its conidia during the active and stationary growth phases, respectively (Figure 15). They were purified by TLC and HPLC. Compound 155 is a phenylacetic acid derivative. They were tested for antimicrobial potential versus M. luteus, B. subtilis, S. aureus, M. smegmatis, L. monocytogenes, K. pneumoniae, P. syringae, and A. tumefaciens, M. ramannianus, and S. cerevisiae in the agar diffusion assay. Compound 163 showed marked effectiveness versus S. aureus and B. subtilis and no effect on the other strains. However, 155 did not display any effects on any strains [109]. Clavatol (156), an acetophenone derivative, had mild antioxidant capacity (IC50 30 μM) in the DPPH assay compared to l-ascorbic acid (IC50, 20.0 μM) [72].
Figure 15.
Other metabolites (155–165) reported from A. ochraceus.
Compounds 158 and 160, purified from sponge-derived A. ochraceus MP2, exhibited antimicrobial capacity versus potential pathogens K. pneumonia ATCC-15380, S. aureus ATCC-25923, and P. aeruginosa ATCC-27853 [108].
Dichotella gemmacea coral-derived A. ochraceus produced AW1 (165), a novel galactomannan extracellular polysaccharide, that was characterized using chemical and spectroscopic methods. AW1 could be a marked source for galacto-furanose oligosaccharides and could have food and pharmacology applications [35].
5. Conclusions
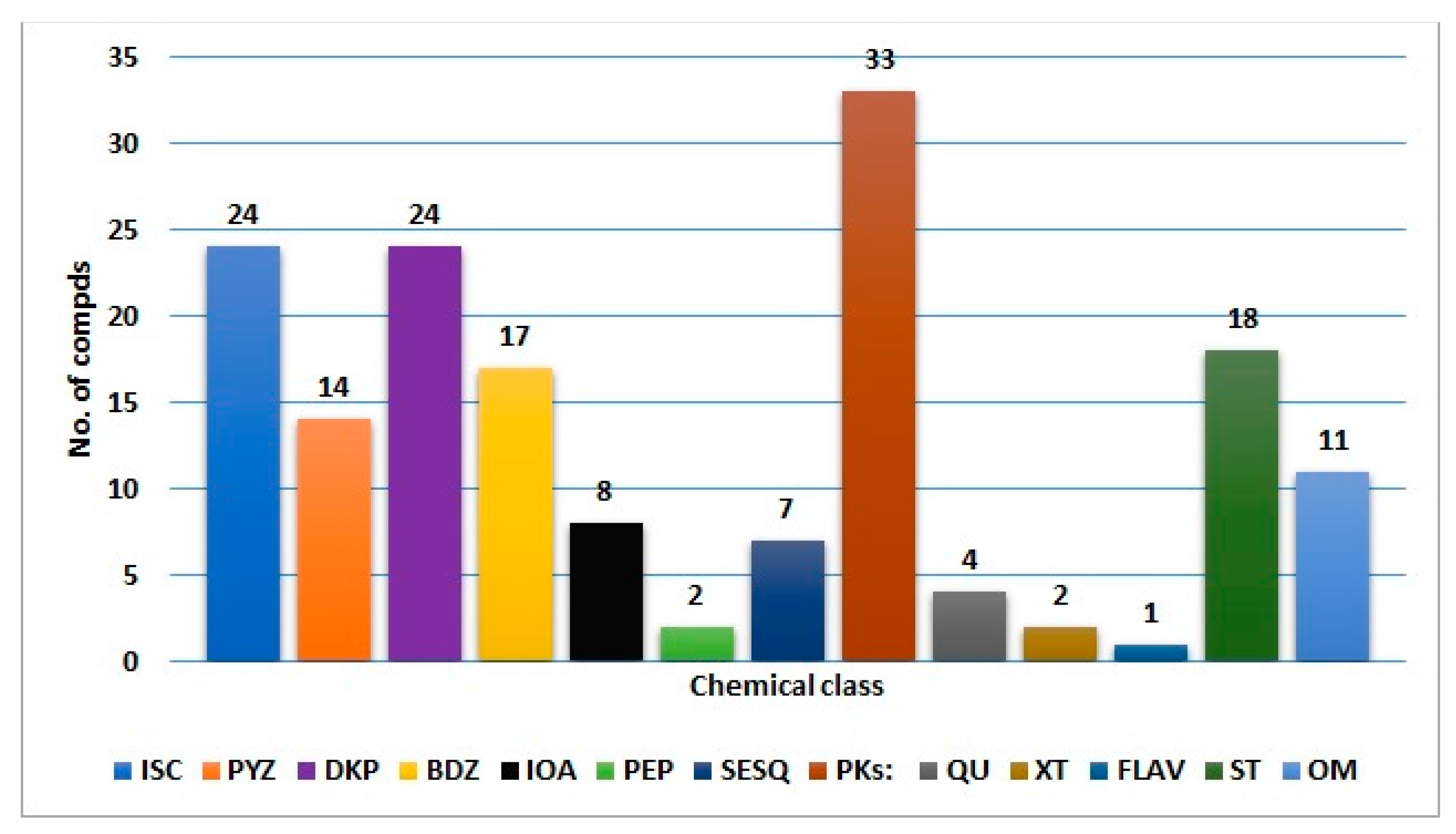
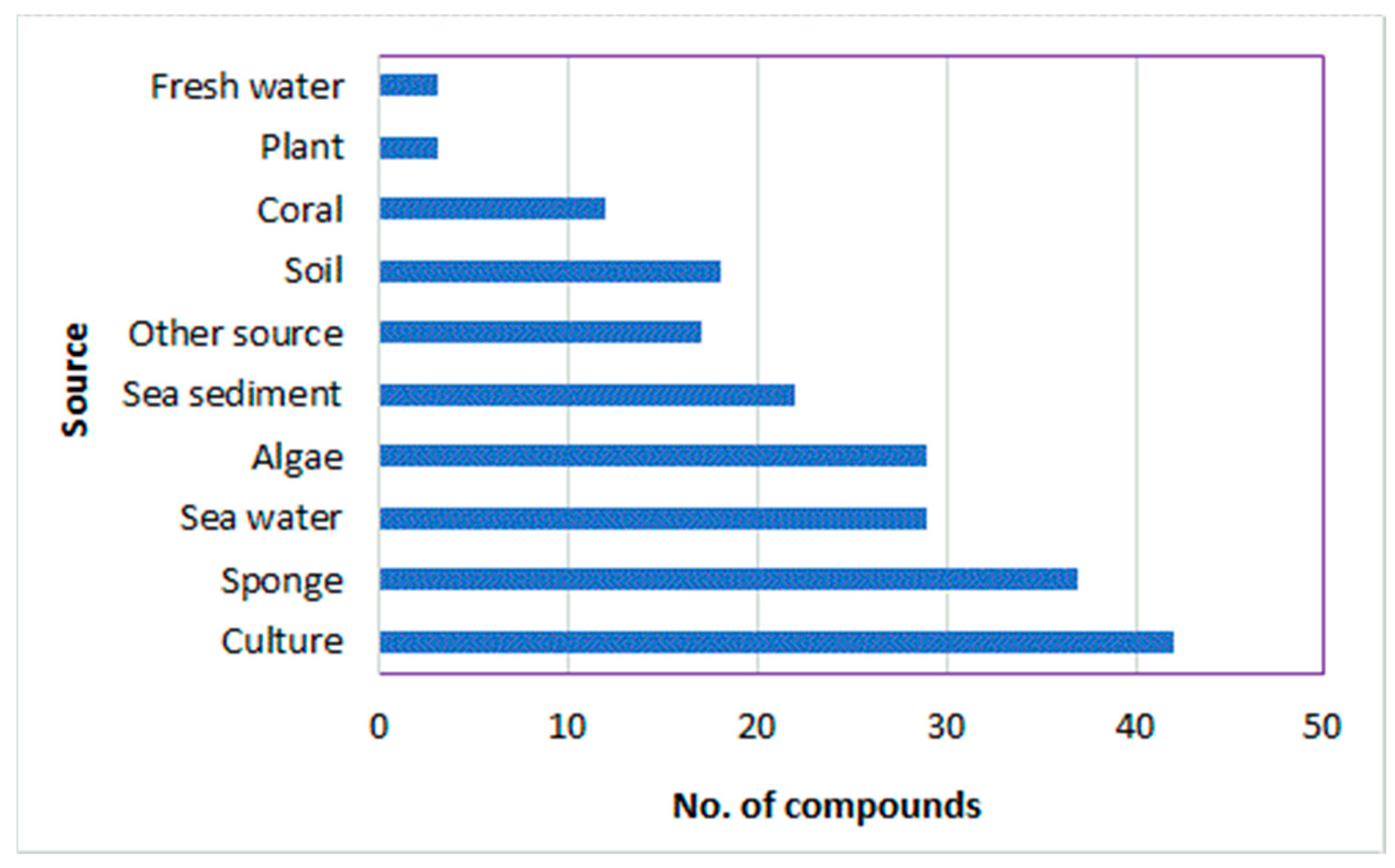
Fungi have been extensively investigated because of their importance as wealth producers for diverse biometabolites and enzymes, in addition to their industrial, agricultural, and pharmaceutical potential. A. ochraceus can biosynthesize diverse biometabolites that may be utilized as leads in the discovery of therapeutic agents for several health concerns. In this review, 165 metabolites belonging to diverse chemical classes (isocoumarins, pyrazines, diketopiperazines, benzodiazepine, indoles, peptides, sesquiterpenoids, polyketides, quinones, xanthines, flavonoids, sterols, and other metabolites) have been reported from A. ochraceus isolated from various source in the period from 1965 to June 2022 (Figure 16 and Figure 17). These sources include soil, culture, marine (sediment, water, sponge, coral, and algae), plants, fresh water, and other sources (Figure 17). In Figure 17, it is clear that the major number of metabolites have been reported from the fungus strains collected from diverse marine sources. Among these metabolites, isocoumarins, diketopiperazines, and polyketides represent the major reported constituents. Additionally, sesquiterpenoids with nitrobenzoyl moiety, a rare class of metabolites, are mainly reported from Aspergillus species, including A. ochraceus. Therefore, these sesquiterpenoids deserve more attention because of their marked antitumor potential.
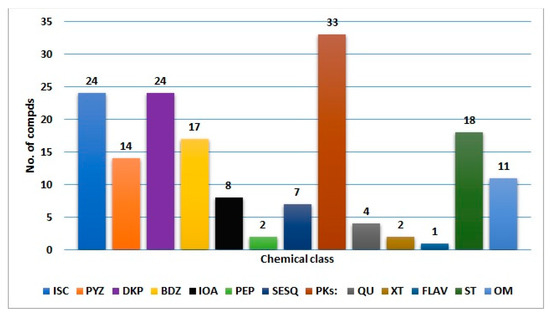
Figure 16.
Different metabolites reported from A. ochraceus. ISC: Isocoumarins; PYZ: pyrazines; DKP: diketopiperazines; BDZ: benzodiazepine: IOA: indole and other alkaloids; PEP: peptides; SESQ: sesquiterpenoids; PKs: polyketides; QU: quinones; XT: xanthes; FLAV: flavonoids; ST: sterols; OM: other metabolites.
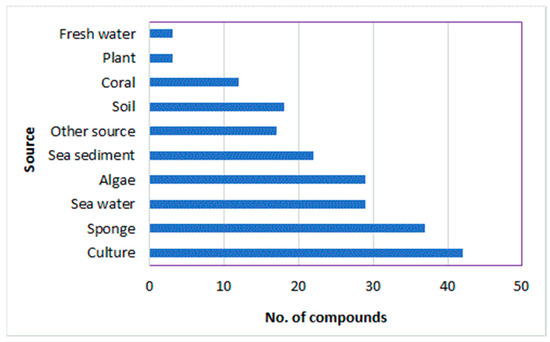
Figure 17.
Number of metabolites reported from A. ochraceus isolated from various sources.
Despite the great number of metabolites reported from this fungus, bioactivities were investigated for a limited number of them. The tested bioactivities were antimicrobial, anti-Parkinson’s, antiviral, antioxidant, cytotoxic, neuroprotective, and insecticidal. Some of the reported metabolites possessed potent effectiveness similar to or higher than the positive control, for example, 4 displayed a selectivity towards V. harveyi compared to that of chloramphenicol. Compounds 6 and 67 demonstrated more potent antioxidant potential than BHT. In addition, 6 had cytoprotective potential H2O2-induced oxidative damage in SHSY5Y cells without toxicity, suggesting its possible protective role in neurodegenerative illnesses with oxidative stress. Additionally, 56 featured prominent anti-Parkinson’s effectiveness compared to levodopa, which could be an advantageous lead compound for anti-Parkinson’s therapeutic drugs. Moreover, some metabolites were reported to show strong cytotoxic capacity (e.g., 18, 76–79, and 91).
In addition, there is a lack of pharmacological studies that focus on exploring the possible mechanisms of the active metabolites. In addition, the untested metabolites should be further explored for their possible bioactivities.
Using the OSMAC technique on the sponge-derived A. ochraceus yielded waspergillamide B (39), a new metabolite with uncommon structural features. In addition, modification of culture media—for example by adding NaBr or NaI—resulted in the production of new metabolites, e.g., (R)-(–)-5-bromomellein (5) and ochramides A–D (31–34), respectively, while mutation of the A. ochraceus strain by UV irradiation produced new metabolites (e.g., 155 and 163). In the same line, the co-cultivation with bacterial strains, such as S. lividans, increased the yield (e.g., 97 and 98); however, B. subtilis yielded new derivatives (e.g., ochraspergillic acids A (123) and B (124)). This suggests further studies on utilizing these techniques could be performed.
Furthermore, the few reports on the biosynthesis of these metabolites promote further study for exploring the biosynthetic pathways of other metabolites and the associated genes that support the potential of A. ochraceus for novel metabolite production using metabolic engineering. Only one study reported the synthesis of NPs using this fungus; further research on synthesizing various types of NPs and its bio-evaluation represents an interesting area for more expected valuable impacts. Additionally, the enzyme production capacity of this fungus has been approved in various studies, suggesting its potential for industrial and biotechnological applications. Thus, A. ochraceus could represent an eco-friendly tool for exchanging hazardous waste into valuable products. Despite the considerable published studies, this fungus still needs more in-depth research from mycologists, biologists, and chemists to shed light on the unexplored potential of this fungus and its metabolites.
Author Contributions
Conceptualization, S.R.M.I., H.M.A. and G.A.M.; resources, R.H.H., M.M.A., A.A.A. and S.G.A.M.; data curation, R.H.H., M.M.A. and A.A.A.; writing—original draft preparation, S.R.M.I., H.M.A., S.G.A.M. and G.A.M.; writing—review and editing, R.H.H., M.M.A., A.A.A., S.G.A.M., S.R.M.I. and G.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
786-O: Human renal carcinoma cell lines; 143B: human bone osteosarcoma cell line; A431: epidermoid carcinoma cell line; A549: human lung adenocarcinoma epithelial cell line; A673: rhabdomyoma cell line; A2780: human ovarian cancer cells line; A2780/DDP: human ovarian mutp53/bc12+ cancer cells line; A2780/Tax: human ovarian cancer taxol-resistant cells line; ABTS: 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate); ACHN: human renal carcinoma cell lines; ADMET: absorption, distribution, metabolism, excretion, and toxicity; B16F10: highly metastatic mouse melanoma cell line; BGC-823: human gastric carcinoma cell line; BHT: butylated hydroxytoluene; BT474: hormone-sensitive breast cancer cell line; BV-2: microglia cells; CCK-8: cell counting kit-8; CD: circular dichroism; CH2Cl2: dichloromethane; CPE: cytopathic effect; CTG: cell Titer Glo; DPPH: 1,1-Diphenyl-2-picrylhydrazyl; DU145: human prostate carcinoma cell line; EC50: half maximal effective concentration; ECD: electronic circular dichroism; ELISA: enzyme-linked immunosorbent assay; EtOAc: ethyl acetate; FRAP: ferric reducing ability of plasma; GFPMA: green fluorescent protein microplate assay; GSH: glutathione; H1299: human non-small cell lung carcinoma cell line; HCC1954: grade 3 invasive ductal carcinoma cell line; H1975: human non-small cell lung carcinoma with L858R and T790M mutation cell line; H2O: water; H2O2: hydrogen peroxide; HCT-116: human colon cancer cell line; HCT-116/mdr+: human colon overexpress mdr+ cancer cell line; HCT-116/topo: human colon resistant to etoposide cancer cell line; HCV: hepatitis C virus; HEK-293F: human embryonic kidney-293F cell line; HepG2: human hepatocellular liver carcinoma cell line; Hep3B: human liver cancer cell line; HeLa: human cervical epithelioid carcinoma cell line; HL-60: human promyelocytic leukemia cell line; HPLC: high-performance liquid chromatography; HUCCT1: bile duct carcinoma cell line; Huh-7: human male hepatoma cell line; IC50: half-maximal inhibitory concentration; IC90: the concentration that will inhibit 90% of the virions; ICD: induced circular dichroism; IL-1β: interleukin-1beta; K562: human erythroleukemic cell line; Karpass299: human T cell lymphoma cell line; L02: human liver cell line; IR: infrared; LPS: lipopolysaccharide; LNCaP: human prostatic-testosterone-sensitive cancer cell line; L5178Y: mouse lymphoma cell line; LX-1: human lung sensitive cancer cell line; MCF-7: human breast adenocarcinoma cell line; MDA-MB-231: human breast cancer cell line; MDA-MB-468: basal breast cancer cell line; MeOH: methanol; MIC: minimum inhibitory concentrations; MKN-45: human gastric cancer cell line; Molt-4: human T lymphoblast cell line; MS: mass spectrometry; MPP+: 1-Methyl-4-phenylpyridinium; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MV-4-11: biphenotypic B myelomonocytic leukemia cell line; N87: gastric carcinoma cell line; NADH: nicotinamide adenine dinucleotide; NCI-H460: human non-small cell lung cancer cell line; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NMR: nuclear magnetic resonance; NO: nitric oxide; OSRC-2: human renal carcinoma cell lines; PC-3: human prostatic-testosterone-independent cell line; ROS: reactive oxygen species; RP-18: reversed phase-18; SH-SY5Y: thrice-cloned subline of the neuroblastoma cell line; SRB: sulforhodamine B; SiO2 CC: silica gel column chromatography; SKBR-3: human breast estradiol-independent cancer cell line; SMMC-7721: human hepatoma cell line; SMP: submitochondrial particles; SPA: scintillation proximity assay; SPC-A1: human lung cancer cell line overexpressing maspin cell line; SW1990: human pancreatic cancer cell line; TLC: thin layer chromatography; TNF-α: rumor necrosis factor alpha; Trolox: 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid; U87: glioblastoma cell line; U251: glioblastoma cell line; U937: pro-monocytic, human myeloid leukaemia cell line; XRD: X-ray diffraction.
References
- Omar, A.M.; Mohamed, G.A.; Ibrahim, S.R.M. Chaetomugilins and Chaetoviridins-Promising Natural Metabolites: Structures, Separation, Characterization, Biosynthesis, Bioactivities, Molecular Docking, and Molecular Dynamics. J. Fungi 2022, 8, 127. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Fungal Depsides-Naturally Inspiring Molecules: Biosynthesis, Structural Characterization, and Biological Activities. Metabolites 2021, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically Active Fungal Depsidones: Chemistry, Biosynthesis, Structural Characterization, and Bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Alolayan, S.O.; Abdallah, H.M.; Mohamed, G.A. Dactylospongia elegans—A Promising Drug Source: Metabolites, Bioactivities, Biosynthesis, Synthesis, and Structural-Activity Relationship. Mar. Drugs 2022, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Choudhry, H.; Asseri, A.H.; Elfaky, M.A.; Mohamed, S.G.; Mohamed, G.A. Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. J. Fungi 2022, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Keller, N.P.; Rokas, A. Unearthing Fungal Chemodiversity and Prospects for Drug Discovery. Curr. Opin. Microbiol. 2019, 51, 22–29. [Google Scholar] [CrossRef]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the Fungus Aspergillus a Threat to Cultural Heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Ibrahim, S.R.; Ahmed, N.; Khoshhal, S.; Abo-Haded, H.M.; Elkablawy, M.A.; Aljuhani, N.; Mohamed, G.A. Aspernolide F, as a New Cardioprotective Butyrolactone Against Doxorubicin-Induced Cardiotoxicity. Int. Immunopharmacol. 2019, 72, 429–436. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.; Mohamed, G.A.; Ross, S.A. Terrenolide S, a New Antileishmanial Butenolide from the Endophytic Fungus Aspergillus terreus. Nat. Prod. Res. 2016, 30, 814–820. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Khedr, A.I. Γ-Butyrolactones from Aspergillus Species: Structures, Biosynthesis, and Biological Activities. Nat. Prod. Commun. 2017, 12, 791–800. [Google Scholar] [CrossRef]
- Ahluwalia, S.K.; Matsui, E.C. Indoor Environmental Interventions for Furry Pet Allergens, Pest Allergens, and Mold: Looking to the Future. J. Allergy Clin. Immunol. Pract. 2018, 6, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.H.; Balbool, B.A.; Abdel-Azeem, A.M. Aspergillus from different habitats and their industrial applications. In Industrially Important Fungi for Sustainable Development; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–106. [Google Scholar]
- Hakamifard, A.; Hashemi, M.; Fakhim, H.; Aboutalebian, S.; Hajiahmadi, S.; Mohammadi, R. Fatal Disseminated Aspergillosis in an Immunocompetent Patient with COVID-19 due to Aspergillus ochraceus. J. Med. Mycol. 2021, 31, 101124. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.; Asfour, H.Z. Antimicrobial Metabolites from the Endophytic Fungus Aspergillus versicolor. Phytochem. Lett. 2020, 35, 152–155. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Gao, J. Chemistry and Biology of Secondary Metabolites from Aspergillus Genus. Nat. Prod. J. 2018, 8, 275–304. [Google Scholar] [CrossRef]
- Cole, R.J.; Moore, J.H.; Davis, N.D.; Kirksey, J.W.; Diener, U.L. 4-Hydroxymellein. New Metabolite of Aspergillus ochraceus. J. Agric. Food Chem. 1971, 19, 909–911. [Google Scholar] [CrossRef]
- Frank, M.; Özkaya, F.C.; Müller, W.E.; Hamacher, A.; Kassack, M.U.; Lin, W.; Liu, Z.; Proksch, P. Cryptic Secondary Metabolites from the Sponge-Associated Fungus Aspergillus ochraceus. Mar. Drugs 2019, 17, 99. [Google Scholar] [CrossRef]
- Stoev, S.D.; Vitanov, S.; Anguelov, G.; Petkova-Bocharova, T.; Creppy, E.E. Experimental Mycotoxic Nephropathy in Pigs Provoked by a Diet Containing Ochratoxin A and Penicillic Acid. Vet. Res. Commun. 2001, 25, 205–223. [Google Scholar] [CrossRef]
- Stoev, S.D. Balkan Endemic Nephropathy–Still Continuing Enigma, Risk Assessment and Underestimated Hazard of Joint Mycotoxin Exposure of Animals Or Humans. Chem. Biol. Interact. 2017, 261, 63–79. [Google Scholar] [CrossRef]
- Dai, J.; Carté, B.K.; Sidebottom, P.J.; Sek Yew, A.L.; Ng, S.; Huang, Y.; Butler, M.S. Circumdatin G, a New Alkaloid from the Fungus Aspergillus ochraceus. J. Nat. Prod. 2001, 64, 125–126. [Google Scholar] [CrossRef]
- Tong, Z.; Xiao, X.; Lu, Y.; Zhang, Y.; Hu, P.; Jiang, W.; Zhou, H.; Pan, S.; Huang, Z.; Hu, L. New Metabolites from Aspergillus ochraceus with Antioxidative Activity and Neuroprotective Potential on H2O2 Insult SH-SY5Y Cells. Molecules 2022, 27, 52. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Zhu, T.; Zhu, W. Pyrazinone Derivatives from the Coral-Derived Aspergillus ochraceus LCJ11-102 under High Iodide Salt. Arch. Pharm. Res. 2018, 41, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yuan, C.; Zhang, J.; Liu, S.; Cao, P.; Hua, H.; Di, Y.; Hao, X. Speramides A–B, Two New Prenylated Indole Alkaloids from the Freshwater-Derived Fungus Aspergillus ochraceus KM007. Tetrahedron. Lett. 2016, 57, 4952–4955. [Google Scholar] [CrossRef]
- Hu, L.; Tian, S.; Wu, R.; Tong, Z.; Jiang, W.; Hu, P.; Xiao, X.; Zhang, X.; Zhou, H.; Tong, Q. Identification of Anti-parkinson’s Disease Lead Compounds from Aspergillus ochraceus Targeting Adenosin Receptors A2A. ChemistryOpen 2021, 10, 630–638. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Jensen, P.R.; Renner, M.K.; Fenical, W. New Cytotoxic Sesquiterpenoid Nitrobenzoyl Esters from a Marine Isolate of the Fungus Aspergillus versicolor. Tetrahedron 1998, 54, 1715–1724. [Google Scholar] [CrossRef]
- Cui, C.; Li, X.; Li, C.; Sun, H.; Gao, S.; Wang, B. Benzodiazepine Alkaloids from Marine-derived Endophytic Fungus Aspergillus ochraceus. Helv. Chim. Acta 2009, 92, 1366–1370. [Google Scholar] [CrossRef]
- Cui, C.; Li, X.; Meng, L.; Li, C.; Huang, C.; Wang, B. 7-nor-Ergosterolide, a Pentalactone-Containing Norsteroid and Related Steroids from the Marine-Derived Endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, F.S.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. New Diketopiperazine Metabolites from the Sclerotia of Aspergillus ochraceus. J. Nat. Prod. 1992, 55, 931–939. [Google Scholar] [CrossRef]
- De Guzman, F.S.; Bruss, D.R.; Rippentrop, J.M.; Gloer, K.B.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Ochrindoles A-D: New Bis-Indolyl Benzenoids from the Sclerotia of Aspergillus ochraceus NRRL 3519. J. Nat. Prod. 1994, 57, 634–639. [Google Scholar] [CrossRef]
- Delgadillo, I. Isolation of Secondary Metabolites of Aspergillus ochraceus by HPLC. Mycotoxin Res. 1986, 2, 9–17. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Y.; Du, Y.; Wang, Y.; Fu, P.; Zhu, W. Circumdatin-Aspyrone Conjugates from the Coral-Associated Aspergillus ochraceus LCJ11-102. Mar. Drugs 2019, 17, 400. [Google Scholar] [CrossRef]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z. Cytotoxic and Antiviral Nitrobenzoyl Sesquiterpenoids from the Marine-Derived Fungus Aspergillus ochraceus Jcma1F17. MedChemComm 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Fuchser, J.; Zeeck, A. Aspinolides and Aspinonene/Aspyrone Co-Metabolites, New Pentaketides Produced by Aspergillus ochraceus. Liebigs Ann./Recl. 1997, 1, 87–95. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Botana, L.M. UPLC–MS–IT–TOF Identification of Circumdatins Produced by Aspergillus ochraceus. J. Agric. Food Chem. 2017, 65, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Mao, W.; Yan, M.; Zhao, C.; Li, N.; Shan, J.; Lin, C.; Liu, X.; Guo, T.; Guo, T. Galactomannan with Novel Structure Produced by the Coral Endophytic Fungus Aspergillus ochraceus. Carbohydr. Polym. 2014, 105, 325–333. [Google Scholar] [CrossRef]
- Aracri, F.M.; Cavalcanti, R.M.; Guimaraes, L.H.S. Extracellular Tannase from Aspergillus ochraceus: Influence of the Culture Conditions on Biofilm Formation, Enzyme Production, and Application. J. Microbiol. Biotechnol. 2019, 29, 1749–1759. [Google Scholar] [CrossRef]
- Asha, P.; Divya, J.; Singh, I.B. Purification and Characterisation of Processive-Type Endoglucanase and Β-Glucosidase from Aspergillus ochraceus MTCC 1810 through Saccharification of Delignified Coir Pith to Glucose. Bioresour. Technol. 2016, 213, 245–248. [Google Scholar] [CrossRef]
- Batomunkueva, B.P.; Egorov, N.S. Isolation, Purification, and Resolution of the Extracellular Proteinase Complex of Aspergillus ochraceus 513 with Fibrinolytic and Anticoagulant Activities. Microbiology 2001, 70, 519–522. [Google Scholar] [CrossRef]
- Betini, J.H.A.; Michelin, M.; Peixoto-Nogueira, S.D.C.; Jorge, J.A.; Terenzi, H.F.; Polizeli, M.L.T.M. Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus Produced under Solid-State Fermentation and their Application in Cellulose Pulp Bleaching. Bioprocess Biosyst. Eng. 2009, 32, 819–824. [Google Scholar] [CrossRef]
- Biswas, S.R.; Jana, S.C.; Mishra, A.K.; Nanda, G. Production, Purification, and Characterization of Xylanase from a Hyperxylanolytic Mutant of Aspergillus ochraceus. Biotechnol. Bioeng. 1990, 35, 244–251. [Google Scholar] [CrossRef]
- Ghosh, K.; Dhar, A.; Samanta, T.B. Purification and Characterization of an Invertase Produced by Aspergillus ochraceus TS. Indian J. Biochem. Biophys 2001, 38, 180–185. [Google Scholar]
- Gonçalves, H.B.; Riul, A.J.; Quiapim, A.C.; Jorge, J.A.; Guimarães, L.H.S. Characterization of a Thermostable Extracellular Tannase Produced Under Submerged Fermentation by Aspergillus ochraceus. EJB 2012, 15, 4. [Google Scholar]
- Ibrahim, S.R.; Mohamed, S.G.; Altyar, A.E.; Mohamed, G.A. Natural Products of the Fungal Genus Humicola: Diversity, Biological Activity, and Industrial Importance. Curr. Microbiol. 2021, 78, 2488–2509. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Altyar, A.E.; Mohamed, S.G.; Mohamed, G.A. Genus Thielavia: Phytochemicals, Industrial Importance and Biological Relevance. Nat. Prod. Res. 2021, 36, 5108–5123. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chowdhury, R.; Bhattacharya, P. Sustainability of Cereal Straws for the Fermentative Production of Second Generation Biofuels: A Review of the Efficiency and Economics of Biochemical Pretreatment Processes. Appl. Energy 2017, 198, 284–298. [Google Scholar] [CrossRef]
- Sharma, N.; Bhardwaj, N.K.; Singh, R.B.P. Environmental Issues of Pulp Bleaching and Prospects of Peracetic Acid Pulp Bleaching: A Review. J. Clean. Prod. 2020, 256, 120338. [Google Scholar] [CrossRef]
- Haile, A.; Gelebo, G.G.; Tesfaye, T.; Mengie, W.; Mebrate, M.A.; Abuhay, A.; Limeneh, D.Y. Pulp and Paper Mill Wastes: Utilizations and Prospects for High Value-Added Biomaterials. Bioresour. Bioprocess. 2021, 8, 1–22. [Google Scholar] [CrossRef]
- Michelin, M.; Peixoto-Nogueira, S.C.; Betini, J.; Da Silva, T.M.; Jorge, J.A.; Terenzi, H.F.; Polizeli, M. Production and Properties of Xylanases from Aspergillus terricola Marchal and Aspergillus ochraceus and their use in Cellulose Pulp Bleaching. Bioprocess Biosyst. Eng. 2010, 33, 813–821. [Google Scholar] [CrossRef]
- Michelin, M.; Polizeli, M.D.L.; Ruzene, D.S.; Silva, D.P.; Vicente, A.A.; Jorge, J.A.; Terenzi, H.F.; Teixeira, J.A. Xylanase and Β-Xylosidase Production by Aspergillus ochraceus: New Perspectives for the Application of Wheat Straw Autohydrolysis Liquor. Appl. Biochem. Biotechnol. 2012, 166, 336–347. [Google Scholar] [CrossRef]
- Veana, F.; Flores-Gallegos, A.C.; Gonzalez-Montemayor, A.M.; Michel-Michel, M.; Lopez-Lopez, L.; Aguilar-Zarate, P.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Invertase: An enzyme with importance in confectionery food industry. In Enzymes in Food Technology; Springer: Cham, Switzerland, 2018; pp. 187–212. [Google Scholar]
- Guimarães, L.H.S.; Terenzi, H.F.; de Moraes, M.D.L.T.; Jorge, J.A. Production and Characterization of a Thermostable Extracellular Β-D-Fructofuranosidase Produced by Aspergillus ochraceus with Agroindustrial Residues as Carbon Sources. Enzym. Microb. Technol. 2007, 42, 52–57. [Google Scholar] [CrossRef]
- Kotb, E. The Biotechnological Potential of Fibrinolytic Enzymes in the Dissolution of Endogenous Blood Thrombi. Biotechnol. Prog. 2014, 30, 656–672. [Google Scholar] [CrossRef]
- Pascreau, T.; de la Morena-Barrio, M.E.; Lasne, D.; Serrano, M.; Bianchini, E.; Kossorotoff, M.; Boddaert, N.; Bruneel, A.; Seta, N.; Vicente, V. Elevated Thrombin Generation in Patients with Congenital Disorder of Glycosylation and Combined Coagulation Factor Deficiencies. J. Thromb. Haemost. 2019, 17, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Marlar, R.A.; Kleiss, A.J.; Griffin, J.H. Mechanism of Action of Human Activated Protein C, a Thrombin-Dependent Anticoagulant Enzyme. Blood 1982, 59, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Osmolovskiy, A.A.; Rukavitsyna, E.D.; Kreier, V.G.; Baranova, N.A.; Egorov, N.S. Production of Proteinases with Fibrinolytic and Fibrinogenolytic Activity by a Micromycete Aspergillus ochraceus. Microbiology 2017, 86, 512–516. [Google Scholar] [CrossRef]
- Chávez-González, M.; Rodríguez-Durán, L.V.; Balagurusamy, N.; Prado-Barragán, A.; Rodríguez, R.; Contreras, J.C.; Aguilar, C.N. Biotechnological Advances and Challenges of Tannase: An Overview. Food Bioproc. Tech. 2012, 5, 445–459. [Google Scholar] [CrossRef]
- Belmares, R.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Coronel, A.R.; Aguilar, C.N. Microbial Production of Tannase: An Enzyme with Potential use in Food Industry. LWT-Food Sci. Technol. 2004, 37, 857–864. [Google Scholar] [CrossRef]
- Banerjee, D.; Mahapatra, S. Fungal Tannase: A Journey from Strain Isolation to Enzyme Applications. Dyn. Biochem. Proc. Biotechnol. Mol. Biol. 2012, 6, 49–60. [Google Scholar]
- Goswami, P.; Chinnadayyala, S.S.R.; Chakraborty, M.; Kumar, A.K.; Kakoti, A. An Overview on Alcohol Oxidases and their Potential Applications. Appl. Microbiol. Biotechnol. 2013, 97, 4259–4275. [Google Scholar] [CrossRef]
- Isobe, K.; Kato, A.; Ogawa, J.; Kataoka, M.; Iwasaki, A.; Hasegawa, J.; Shimizu, S. Characterization of Alcohol Oxidase from Aspergillus ochraceus AIU 031. J. Gen. Appl. Microbiol. 2007, 53, 177–183. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable Bio-Ethanol Production from Agro-Residues: A Review. Renew. Sust. Energ. Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Rathna, G.S.; Saranya, R.; Kalaiselvam, M. Bioethanol from Sawdust using Cellulase Hydrolysis of Aspergillus ochraceus and Fermentation by Saccharomyces cerevisiae. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 733–742. [Google Scholar]
- Kadam, A.A.; Telke, A.A.; Jagtap, S.S.; Govindwar, S.P. Decolorization of Adsorbed Textile Dyes by Developed Consortium of Pseudomonas sp. SUK1 and Aspergillus ochraceus NCIM-1146 under Solid State Fermentation. J. Hazard. Mater. 2011, 189, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Kalme, S.D.; Govindwar, S.P. Decolorisation of Textile Dyes by Aspergillus ochraceus (NCIM-1146). J. Basic Microbiol. 2006, 5, 407–410. [Google Scholar]
- Telke, A.A.; Kadam, A.A.; Jagtap, S.S.; Jadhav, J.P.; Govindwar, S.P. Biochemical Characterization and Potential for Textile Dye Degradation of Blue Laccase from Aspergillus ochraceus NCIM-1146. Biotechnol. Bioprocess Eng. 2010, 15, 696–703. [Google Scholar] [CrossRef]
- Abha, S.; Singh, C.S. Hydrocarbon Pollution: Effects on Living Organisms, Remediation of Contaminated Environments, and Effects of Heavy Metals Co-Contamination on Bioremediation. In Introduction to Enhanced Oil Recovery (EOR) Processes and Bioremediation of Oil-Contaminated Sites; Romero-Zerón, L., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 186–206. [Google Scholar]
- Darsa, K.V.; Thatheyus, A.J.; Ramya, D. Biodegradation of Petroleum Compound using the Bacterium Bacillus subtilis. Sci. Int. 2014, 2, 20–25. [Google Scholar] [CrossRef]
- Saratale, G.; Kalme, S.; Bhosale, S.; Govindwar, S. Biodegradation of Kerosene by Aspergillus ochraceus NCIM-1146. J. Basic Microbiol. 2007, 47, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sebiomo, A.; Awosanya, A.O.; Awofodu, A.D. Utilization of Crude Oil and Gasoline by Ten Bacterial and Five Fungal Isolates. J. Microbiol. Antimicrob. 2011, 3, 55–63. [Google Scholar]
- Vijayakumar, P.S.; Prasad, B. Intracellular Biogenic Silver Nanoparticles for the Generation of Carbon Supported Antiviral and Sustained Bactericidal Agents. Langmuir 2009, 25, 11741–11747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Meng, L.; Wang, B. Polyketides from the Marine Mangrove-Derived Fungus Aspergillus ochraceus MA-15 and their Activity Against Aquatic Pathogenic Bacteria. Phytochem. Lett. 2015, 12, 232–236. [Google Scholar] [CrossRef]
- Yun, K.; Feng, Z.; Choi, H.D.; Kang, J.S.; Son, B.W. New Production of (R)-(–)-5-Bromomellein, a Dihydroisocoumarin Derivative from the Marine-Derived Fungus Aspergillus ochraceus. Chem. Nat. Compd. 2013, 49, 24–26. [Google Scholar] [CrossRef]
- Yamazaki, M.; Maebayashi, Y.; Miyaki, K. Isolation of a New Metabolite, 6-Methoxy-8-Hydroxyisocoumarin-3-Carboxylic Acid from Aspergillus ochraceus WILH. Chem. Pharm. Bull. 1972, 20, 2276–2278. [Google Scholar] [CrossRef]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a Toxic Metabolite Produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.D.; Searcy, J.W.; Diener, U.L. Production of Ochratoxin A by Aspergillus ochraceus in a Semisynthetic Medium. Appl. Microbiol. 1969, 17, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Marquardt, R.R.; Abramson, D.; Frohlich, A.A. Metabolites of Ochratoxins in Rat Urine and in a Culture of Aspergillus ochraceus. Appl. Environ. Microbiol. 1996, 62, 648–655. [Google Scholar] [CrossRef]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L. Mycotoxins. Part II. the Constitution of Ochratoxins A, B, and C, Metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. 1965, 1965, 7083–7088. [Google Scholar] [CrossRef]
- Moore, J.H.; Davis, N.D.; Diener, U.L. Mellein and 4-Hydroxymellein Production by Aspergillus ochraceus Wilhelm. Appl. Microbiol. 1972, 23, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Liu, P.; Wang, Z.; Zhu, W. Effects of Environmental Stress on Secondary Metabolites of Aspergillus ochraceus LCJ11-102 Associated with the Coral Dichotella gemmacea. Acta Microbiol. Sin. 2010, 50, 1023–1029. [Google Scholar]
- Zou, Z.; Zhang, G.; Li, S.; He, Z.; Yan, Q.; Lin, Y.; Xie, C.; Xia, J.; Luo, Z.; Luo, L. Asperochratides A–J, Ten New Polyketides from the Deep-Sea-Derived Aspergillus ochraceus. Bioorg. Chem. 2020, 105, 104349. [Google Scholar] [CrossRef]
- Harris, J.P.; Mantle, P.G. Biosynthesis of Diaporthin and Orthosporin by Aspergillus ochraceus. Phytochemistry 2001, 57, 165–169. [Google Scholar] [CrossRef]
- Stander, M.A.; Steyn, P.S.; Lübben, A.; Miljkovic, A.; Mantle, P.G.; Marais, G.J. Influence of Halogen Salts on the Production of the Ochratoxins by Aspergillus ochraceus Wilh. J. Agric. Food Chem. 2000, 48, 1865–1871. [Google Scholar] [CrossRef]
- Hadidane, R.; Bacha, H.; Creppy, E.E.; Hammami, M.; Ellouze, F.; Dirheimer, G. Isolation and Structure Determination of Natural Analogues of the Mycotoxin Ochratoxin A Produced by Aspergillus ochraceus. Toxicology 1992, 76, 233–243. [Google Scholar] [CrossRef]
- Yamazaki, M.; Maebayashi, Y.; Miyaki, K. Isolation of a New Type of Pyrazine Metabolite from Aspergillus ochraceus WILH. Chem. Pharm. Bull. 1972, 20, 2274–2276. [Google Scholar] [CrossRef]
- Maebayashi, Y.; Sumita, M.; Fukushima, K.; Yamasaki, M. Isolation and Structure of Red Pigment from Aspergillus ochraceus Wilh. Chem. Pharm. Bull. 1978, 26, 1320–1322. [Google Scholar] [CrossRef][Green Version]
- López-Gresa, M.P.; González, M.C.; Primo, J.; Moya, P.; Romero, V.; Estornell, E. Circumdatin H, a New Inhibitor of Mitochondrial NADH Oxidase, from Aspergillus ochraceus. J. Antibiot. 2005, 58, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.K.; Størmer, F.C.; Petersen, D.; Aasen, A.J. Cycloechinulin, a Trp-Ala-Derived Alkaloid from the Fungus Aspergillus ochraceus. Acta Crystallogr. Sect. E Struct. Rep. Online 2001, 57, o909–o912. [Google Scholar] [CrossRef]
- Sugie, Y.; Hirai, H.; Inagaki, T.; Ishiguro, M.; Kim, Y.; Kojima, Y.; Sakakibara, T.; Sakemi, S.; Sugiura, A.; Suzuki, Y. A New Antibiotic CJ-17, 665 from Aspergillus ochraceus. J. Antibiot. 2001, 54, 911–916. [Google Scholar] [CrossRef]
- Qian-Cutrone, J.; Huang, S.; Shu, Y.; Vyas, D.; Fairchild, C.; Menendez, A.; Krampitz, K.; Dalterio, R.; Klohr, S.E.; Gao, Q. Stephacidin A and B: Two Structurally Novel, Selective Inhibitors of the Testosterone-Dependent Prostate LNCaP Cells. J. Am. Chem. Soc. 2002, 124, 14556–14557. [Google Scholar] [CrossRef]
- Wen, H.; Liu, X.; Zhang, Q.; Deng, Y.; Zang, Y.; Wang, J.; Liu, J.; Zhou, Q.; Hu, L.; Zhu, H. Three New Indole Diketopiperazine Alkaloids from Aspergillus ochraceus. Chem. Biodivers. 2018, 15, e1700550. [Google Scholar] [CrossRef]
- Rahbaek, L.; Breinholt, J.; Frisvad, J.C.; Christophersen, C. Circumdatin A, B, and C: Three New Benzodiazepine Alkaloids Isolated from a Culture of the Fungus Aspergillus ochraceus. J. Org. Chem. 1999, 64, 1689–1692. [Google Scholar] [CrossRef]
- Rahbæk, L.; Breinholt, J. Circumdatins D, E, and F: Further Fungal Benzodiazepine Analogues from Aspergillus ochraceus. J. Nat. Prod. 1999, 62, 904–905. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Liesch, J.; Hensens, O.; Zitano, L.; Honeycutt, S.; Garrity, G.; Fromtling, R.A.; Onishi, J.; Monaghan, R. L-657, 398, a Novel Antifungal Agent: Fermentation, Isolation, Structural Elucidation and Biological Properties. J. Antibiot. 1988, 41, 1774–1779. [Google Scholar] [CrossRef]
- Chang, C.; Myokei, R.; Sakurai, A.; Takahashi, N.; Tamura, S. Aspochracin, a New Insecticidal Metabolite of Aspergillus ochraceus Part II. Synthesis of Hexahydroaspochracin. Agric. Biol. Chem. 1969, 33, 1501–1506. [Google Scholar] [CrossRef]
- Myokei, R.; Sakurai, A.; Chang, C.; Kodaira, Y.; Takahashi, N.; Tamura, S. Aspochracin, a New Insecticidal Metabolite of Aspergillus ochraceus: Part I. Isolation, Structure and Biological Activities. Agric. Biol. Chem. 1969, 33, 1491–1500. [Google Scholar]
- Myokei, R.; Sakurai, A.; Chang, C.; Kodaira, Y.; Takahashi, N.; Tamura, S. Structure of Aspochracin, an Insecticidal Metabolite of Aspergillus ochraceus. Tetrahedron Lett. 1969, 10, 695–698. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, B.; Lin, X.; Luo, X.; Pang, X.; Tang, L.; Liu, Y.; Li, X.; Zhou, X. Nitrobenzoyl Sesquiterpenoids with Cytotoxic Activities from a Marine-Derived Aspergillus ochraceus Fungus. J. Nat. Prod. 2018, 81, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Obana, H.; Kumeda, Y.; Nishimune, T. Aspergillus ochraceus Production of 5, 6-Dihydropenicillic Acid in Culture and Foods. J. Food Prot. 1995, 58, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Balcells, M.; Sala, N.; Sanchis, V.; Canela, R. Bactericidal and Fungicidal Activity of Aspergillus ochraceus Metabolites and some Derivatives. Pestic. Sci. 1998, 53, 9–14. [Google Scholar] [CrossRef]
- Fuchser, J.; Thiericke, R.; Zeeck, A. Biosynthesis of Aspinonene, a Branched Pentaketide Produced by Aspergillus ochraceus, Related to Aspyrone. J. Chem. Soc. Perkin Trans. 1 1995, 13, 1663–1666. [Google Scholar] [CrossRef]
- Simpson, T.J.; Holker, J.S. The Biosynthesis of a Pyrone Metabolite of Aspergillus melleus an Application of Long-Range 13C-13C Coupling Constants. Tetrahedron Lett. 1975, 16, 4693–4696. [Google Scholar] [CrossRef]
- Moore, J.H.; Murray, T.P.; Marks, M.E. Production of 3-(1,2-Epoxypropyl)-5, 6-Dihydro-5-Hydroxy-6-Methylpyran-2-One by Aspergillus ochraceus. J. Agric. Food Chem. 1974, 22, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Maebayashi, Y.; Miyaki, K. The Isolation of Secalonic Acid A from Aspergillus ochraceus Cultured on Rice. Chem. Pharm. Bull. 1971, 19, 199–201. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, X.; Cui, T. Production of Emodin from Aspergillus ochraceus at Preparative Scale. Afr. J. Biotechnol. 2010, 9, 512–517. [Google Scholar]
- Robbers, J.E.; Hong, S.; Tuite, J.; Carlton, W.W. Production of Xanthomegnin and Viomellein by Species of Aspergillus Correlated with Mycotoxicosis Produced in Mice. Appl. Environ. Microbiol. 1978, 36, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Stack, M.E.; Mislivec, P.B. Production of Xanthomegnin and Viomellein by Isolates of Aspergillus ochraceus, Penicillium cyclopium, and Penicillium viridicatum. Appl. Environ. Microbiol. 1978, 36, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Stack, M.E.; Mislivec, P.B.; Denizel, T.; Gibson, R.; Pohland, A.E. Ochratoxins A and B, Xanthomegnin, Viomellein and Vioxanthin Production by Isolates of Aspergillus ochraceus from Green Coffee Beans. J. Food Prot. 1983, 46, 965–968. [Google Scholar] [CrossRef]
- Meenupriya, J.; Thangaraj, M. Analytical Characterization and Structure Elucidation of Metabolites from Aspergillus ochraceus MP2 Fungi. Asian Pac. J. Trop. Biomed. 2011, 1, 376–380. [Google Scholar]
- Awad, G.; Mathieu, F.; Coppel, Y.; Lebrihi, A. Characterization and Regulation of New Secondary Metabolites from Aspergillus ochraceus M18 obtained by UV Mutagenesis. Can. J. Microbiol. 2005, 51, 59–67. [Google Scholar] [CrossRef]
- Takase, S.; Kawai, Y.; Uchida, I.; Tanaka, H.; Aoki, H. Structure of Amauromine, a New Hypotensive Vasodilator Produced by Amauroascus sp. Tetrahedron 1985, 41, 3037–3048. [Google Scholar] [CrossRef]
- Tanner, F.W., Jr.; Sobin, B.A.; Gardocki, J.F. Some Chemical and Biologic Properties of Anisomycin. Antibiot. Ann. 1954, 1955, 809–812. [Google Scholar]
- Zhao, H.; Anbuchezhian, R.; Sun, W.; Shao, C.; Zhang, F.; Yin, Y.; Yu, Z.; Li, Z.; Wang, C. Cytotoxic Nitrobenzoyloxy-Substituted Sesquiterpenes from Sponge Derived Endozoic Fungus Aspergillus insulicola md10-2. Curr. Pharm. Biotechnol. 2016, 17, 271–274. [Google Scholar] [CrossRef]
- Franck, B.; Baumann, G. Mutterkorn-farbstoffe, XIV. Isolierung, Struktur Und Absolute Konfiguration Der Ergochrome AD, BD, CD Und DD. Chem. Ber. 1966, 99, 3875–3883. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).