Exploratory Quality Control Study for Polygonum multiflorum Thunb. Using Dinuclear Anthraquinones with Potential Hepatotoxicity
Abstract
1. Introduction
2. Results and Discussion
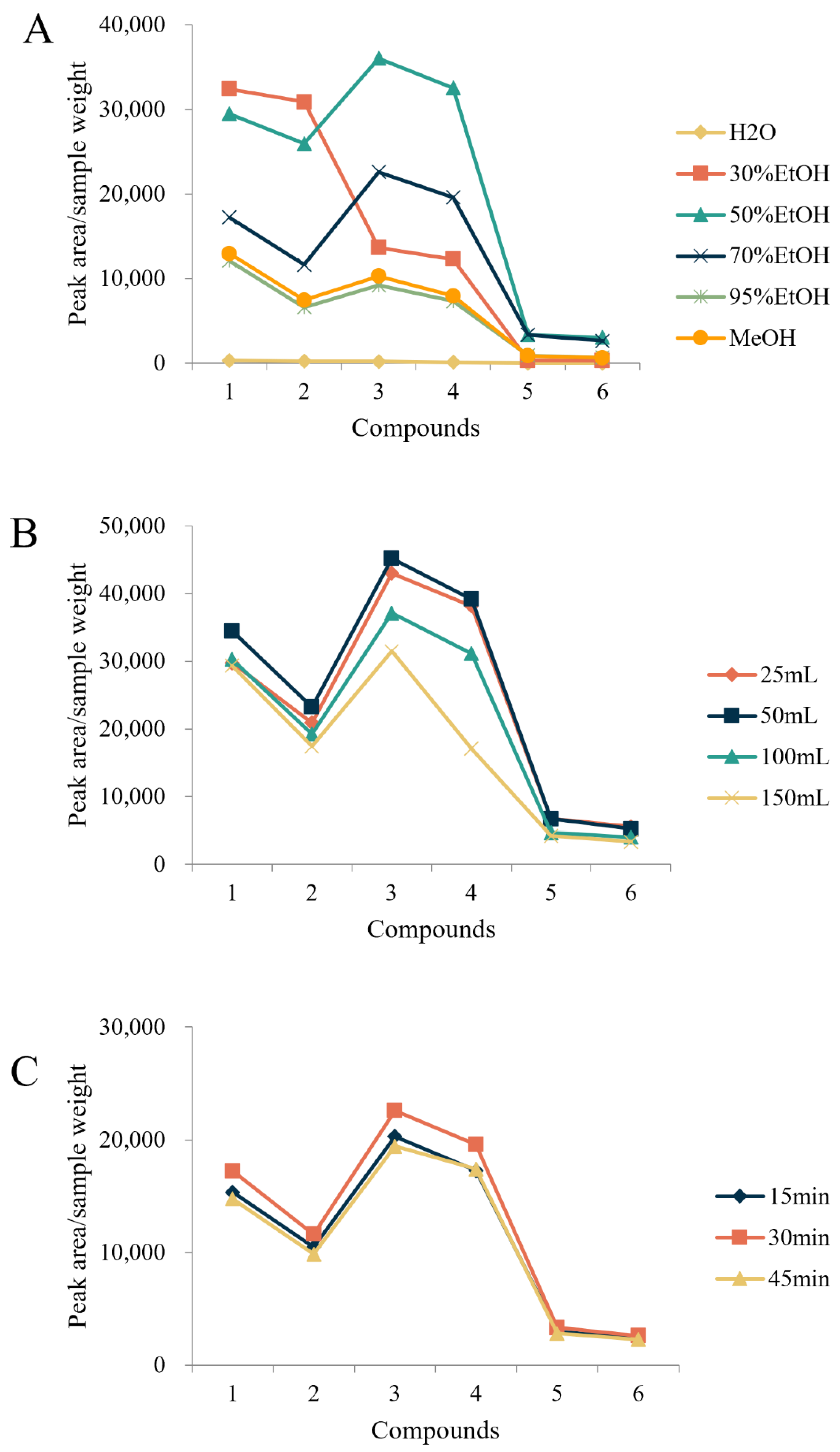
2.1. Optimization of the Extraction Method
2.2. Optimization of UHPLC-QQQ-MS/MS Conditions
2.3. Method Validation
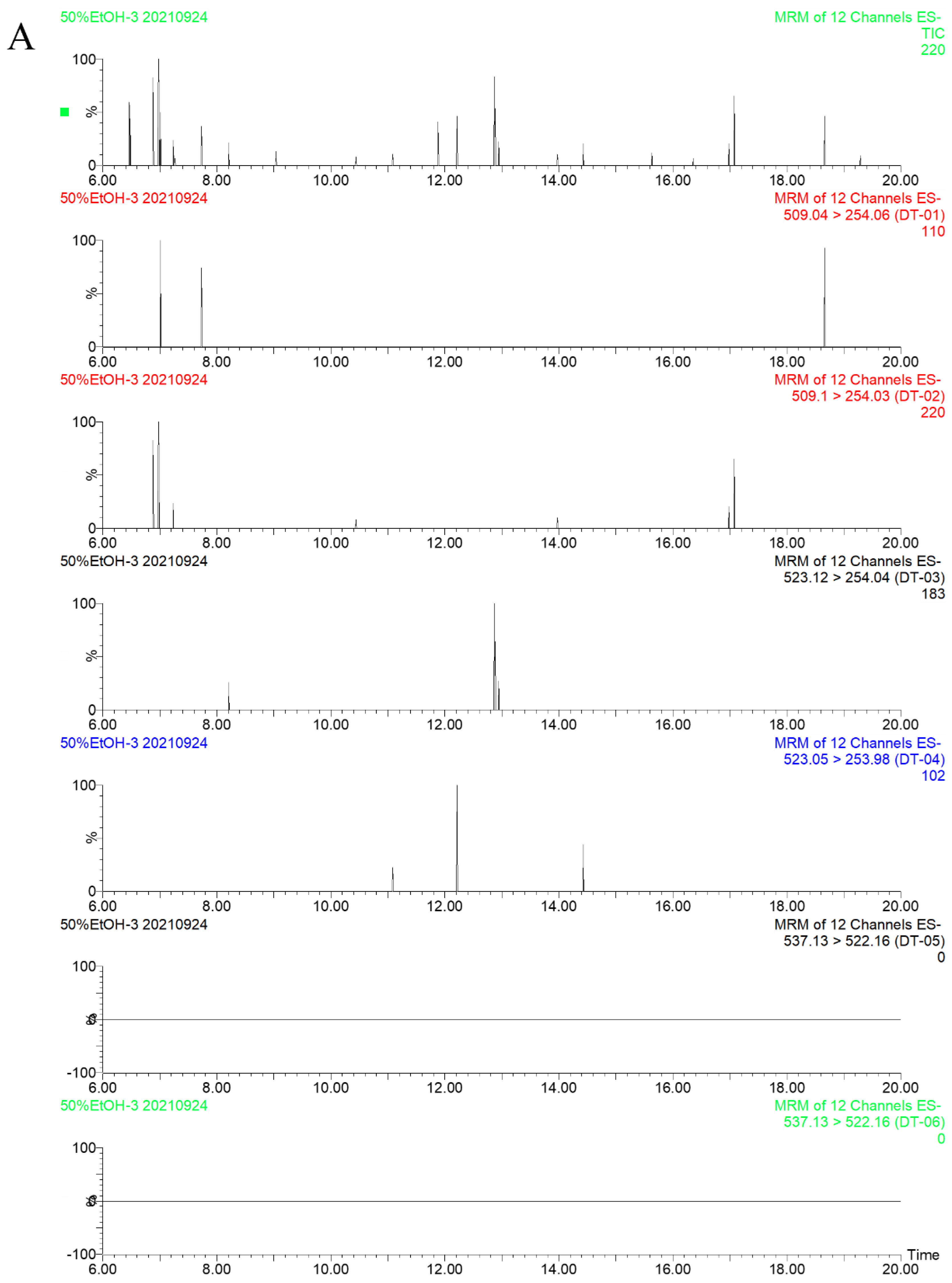
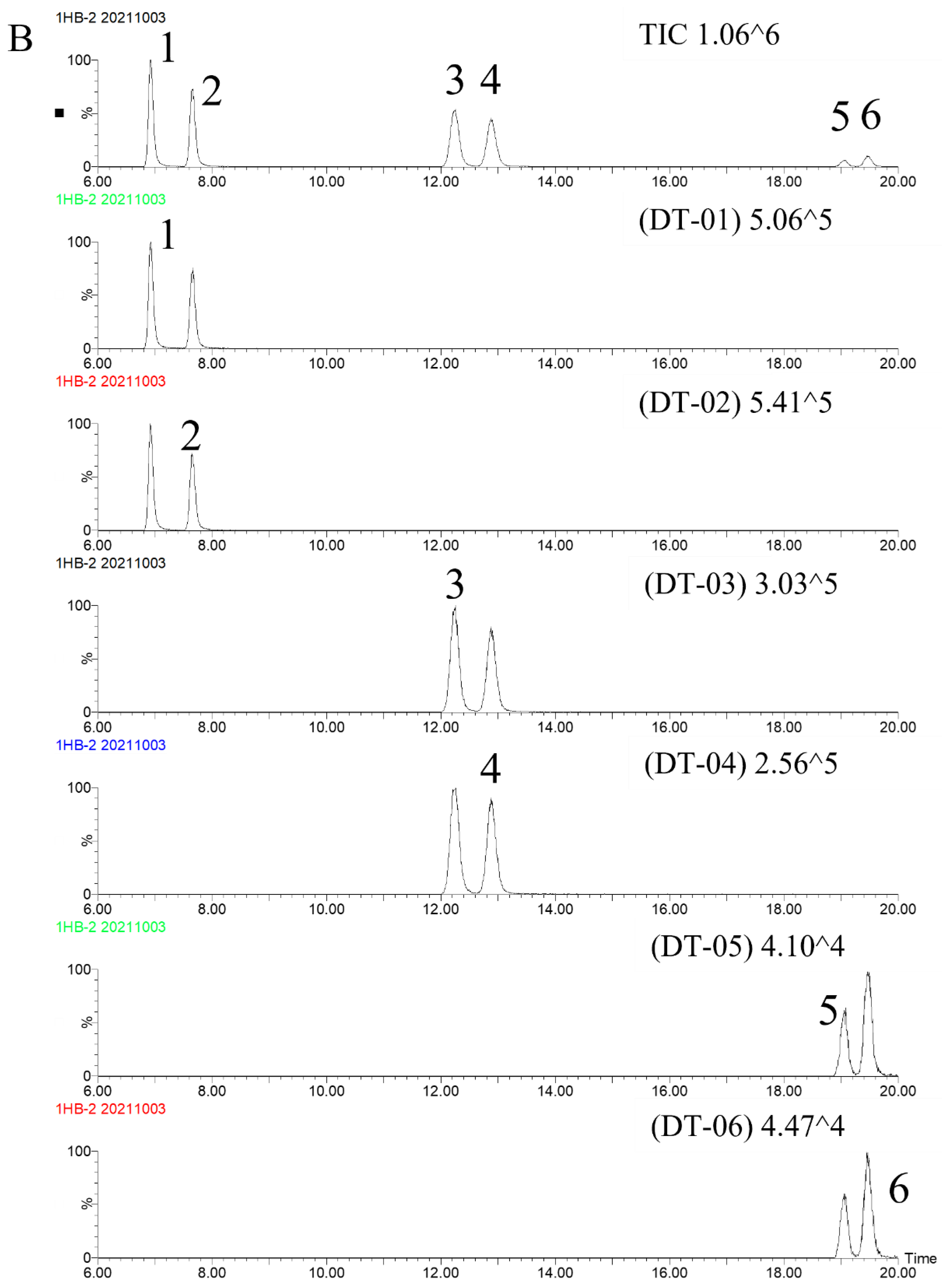
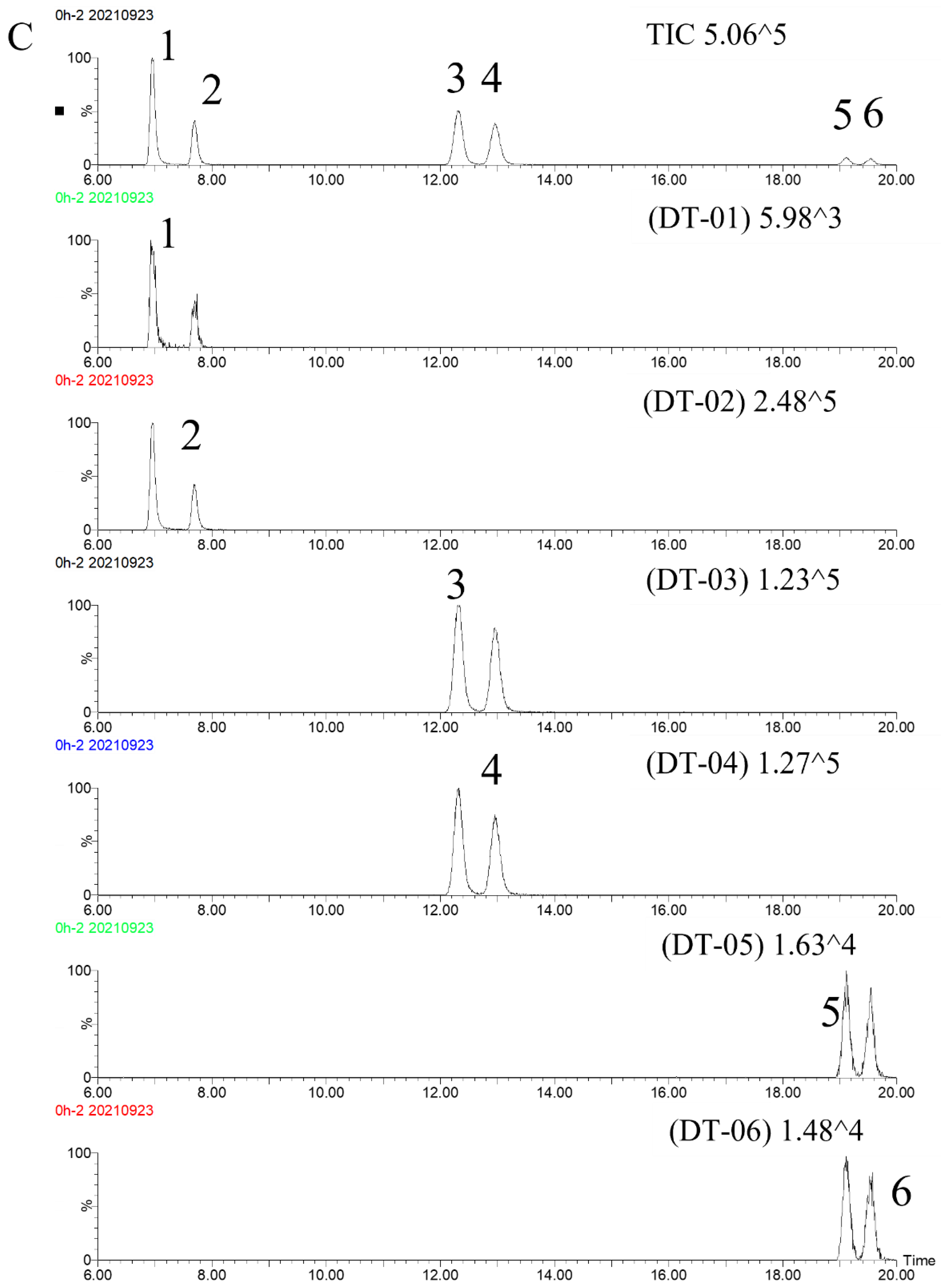
2.3.1. Specificity
2.3.2. Precision
2.3.3. Stability and Repeatability
2.3.4. Linearity Range, Limits of Detection (LODs), and Limits of Quantification (LOQs)
2.3.5. Recovery
2.4. Results of Quantitative Analysis
2.4.1. Quantification of the Six Free Dianthrones in PMR
2.4.2. Quantification of the Six Free and Total Dianthrones in 90 Batches of PMRP
2.4.3. Quantification of the Six Dianthrones in PMRP, Processed by Different Methods
2.5. Cytotoxic Effects of Dianthrone Exposure in HepG2 Cells
2.6. Discussion
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Instruments and Conditions
3.3. Preparation of Solutions
3.3.1. Preparation of Standard Solutions
3.3.2. Preparation of Sample Solution (Free Dianthrones)
3.3.3. Preparation of Sample Solution (Total Dianthrones)
3.4. Cytotoxic Effects of Dianthrone Exposure in HepG2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Feng, Y.; Bounda, G.-A. Review of Clinical Studies of Polygonum Multiflorum Thunb. and Its Isolated Bioactive Compounds. Pharmacogn. Res. 2015, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional Usages, Botany, Phytochemistry, Pharmacology and Toxicology of Polygonum Multiflorum Thunb.: A Review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.-Y.; Lee, H.-I.; Ham, J.R.; Yee, S.-T.; Kang, K.-Y.; Lee, M.-K. Heshouwu (Polygonum Multiflorum Thunb.) Ethanol Extract Suppresses Pre-Adipocytes Differentiation in 3T3-L1 Cells and Adiposity in Obese Mice. Biomed. Pharmacother. 2018, 106, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.-Y.; Lee, M.-K. Polygonum Multiflorum Thunb. Hot Water Extract Reverses High-Fat Diet-Induced Lipid Metabolism of White and Brown Adipose Tissues in Obese Mice. Plants 2021, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lin, H.; Zhang, M.; Ni, B.; Yin, X.; Qu, C.; Ni, J. A Novel Method to Analyze Hepatotoxic Components in Polygonum Multiflorum Using Ultra-Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. J. Hazard. Mater. 2015, 299, 249–259. [Google Scholar] [CrossRef] [PubMed]
- López-Terrada, D.; Cheung, S.W.; Finegold, M.J.; Knowles, B.B. Hep G2 Is a Hepatoblastoma-Derived Cell Line. Hum. Pathol. 2009, 40, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Grafakou, M.-E.; Barda, C.; Heilmann, J.; Skaltsa, H. In Vitro Cytotoxic and Anti-Inflammatory Activities of Sesquiterpene Lactones from Centaurea papposa (Coss.) Greuter. Nat. Prod. Res. 2022, 36, 3211–3215. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Jekő, J.; Cziáky, Z.; Pereira, C.G.; Custódio, L. The Medicinal Halophyte Frankenia Laevis L. (Sea Heath) Has In Vitro Antioxidant Activity, α-Glucosidase Inhibition, and Cytotoxicity towards Hepatocarcinoma Cells. Plants 2022, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Yang, J.-B.; Li, W.-F.; Qiu, C.-X.; Hu, G.; Wang, S.-T.; Song, Y.-F.; Gao, H.-Y.; Liu, Y.; Wang, Q.; et al. In Vivo Hepatotoxicity Screening of Different Extracts, Components, and Constituents of Polygoni Multiflori Thunb. in Zebrafish (Danio Rerio) Larvae. Biomed. Pharmacother. 2020, 131, 110524. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-B.; Song, Y.-F.; Liu, Y.; Gao, H.-Y.; Wang, Q.; Wang, Y.; Cheng, X.-L.; Zuo, T.-T.; Hu, X.-W.; Wei, F.; et al. UHPLC-QQQ-MS/MS Assay for the Quantification of Dianthrones as Potential Toxic Markers of Polygonum Multiflorum Thunb: Applications for the Standardization of Traditional Chinese Medicines (TCMs) with Endogenous Toxicity. Chin. Med. 2021, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, M.; Ma, S.-C.; Wen, R.-H. Predicting Toxic Monomer Components in Polygonum multiflorum Based on Quantitative Structureactivity Relationship. Chin. J. Pharm. 2021, 18, 352–355+360. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Yang, J.-B.; Sun, H.; Song, Y.-F.; Cheng, X.-L.; Wang, X.-T.; Wei, F.; Wang, Y.; Wang, Q.; Jin, H.-T.; et al. Determination of anthraquinones in polygoni multiflori radix form different origins and processed by different methods by HPLC. Chin. J. Pharm. 2022, 19, 522–526. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Wang, Y.; Zhao, Q.; Zheng, F.; Dou, Z.; Yang, W.; Hu, L.; Liu, C. Rapid Discovery of the Potential Toxic Compounds in Polygonum Multiflorum by UHPLC/Q-Orbitrap-MS-Based Metabolomics and Correlation Analysis. Front. Pharmacol. 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, P.; Chen, H.; Xing, Y.; Liu, C.; Pan, G.; Dou, Z.; Han, L. The Composition Differences between Small Black Beans and Big Black Beans from Different Habitats and Its Effects on the Processing of Polygonum multiflorum. Phytochem. Anal. 2021, 32, 767–779. [Google Scholar] [CrossRef] [PubMed]


| No. * | Stability RSD (%) | Repeatability RSD (%) (n = 6) | Precision | Recovery (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day RSD (%) (n = 6) | Inter-Day RSD (%) (n = 3) | Sample (g) | Original (μg) △ | Spiked (μg) 1 | Found (μg) 2 | Recovery (%) | Average Recovery (%) | RSD (%) | |||
| 1 | 6.39 | 2.58 | 4.25 | 17.69 | 0.5002 | 2.0403 | 1.4060 | 3.4049 | 97.06 | 91.12 | 5.12 |
| 0.5004 | 2.0411 | 1.4060 | 3.3355 | 92.06 | |||||||
| 0.5005 | 2.0415 | 1.4060 | 3.3790 | 95.13 | |||||||
| 0.5001 | 2.0399 | 1.4060 | 3.3147 | 90.68 | |||||||
| 0.5004 | 2.0411 | 1.4060 | 3.2627 | 86.89 | |||||||
| 0.5005 | 2.0415 | 1.4060 | 3.2353 | 84.91 | |||||||
| 2 | 4.70 | 4.27 | 2.83 | 15.17 | 0.5002 | 0.8368 | 0.5192 | 1.4506 | 118.23 | 110.08 | 5.43 |
| 0.5004 | 0.8371 | 0.5192 | 1.4078 | 109.92 | |||||||
| 0.5005 | 0.8373 | 0.5192 | 1.4376 | 115.62 | |||||||
| 0.5001 | 0.8366 | 0.5192 | 1.3929 | 107.15 | |||||||
| 0.5004 | 0.8371 | 0.5192 | 1.3660 | 101.87 | |||||||
| 0.5005 | 0.8373 | 0.5192 | 1.3965 | 107.71 | |||||||
| 3 | 7.94 | 2.66 | 4.81 | 22.13 | 0.5002 | 1.4312 | 0.9562 | 2.4394 | 105.44 | 93.54 | 8.55 |
| 0.5004 | 1.4318 | 0.9562 | 2.3309 | 94.04 | |||||||
| 0.5005 | 1.4321 | 0.9562 | 2.3881 | 99.99 | |||||||
| 0.5001 | 1.4309 | 0.9562 | 2.2962 | 90.50 | |||||||
| 0.5004 | 1.4318 | 0.9562 | 2.2406 | 84.59 | |||||||
| 0.5005 | 1.4321 | 0.9562 | 2.2608 | 86.67 | |||||||
| 4 | 6.42 | 4.26 | 4.06 | 18.72 | 0.5002 | 1.3005 | 0.7841 | 2.1913 | 113.61 | 102.11 | 8.02 |
| 0.5004 | 1.3010 | 0.7841 | 2.0904 | 100.68 | |||||||
| 0.5005 | 1.3013 | 0.7841 | 2.1634 | 109.95 | |||||||
| 0.5001 | 1.3003 | 0.7841 | 2.0859 | 100.20 | |||||||
| 0.5004 | 1.3010 | 0.7841 | 2.0231 | 92.08 | |||||||
| 0.5005 | 1.3013 | 0.7841 | 2.0551 | 96.14 | |||||||
| 5 | 5.60 | 5.06 | 5.17 | 15.28 | 0.5005 | 1.8202 | 0.9862 | 2.6891 | 88.11 | 85.93 | 7.20 |
| 0.5002 | 1.8191 | 0.9862 | 2.7313 | 92.50 | |||||||
| 0.5004 | 1.8198 | 0.9862 | 2.6423 | 83.40 | |||||||
| 0.5005 | 1.8202 | 0.9862 | 2.7235 | 91.60 | |||||||
| 0.5001 | 1.8187 | 0.9862 | 2.5667 | 75.84 | |||||||
| 0.5005 | 1.8202 | 0.9862 | 2.6497 | 84.12 | |||||||
| 6 | 7.94 | 4.66 | 5.99 | 16.80 | 0.5002 | 1.3005 | 0.7841 | 2.1913 | 113.61 | 102.11 | 8.02 |
| 0.5004 | 1.3010 | 0.7841 | 2.0904 | 100.68 | |||||||
| 0.5005 | 1.3013 | 0.7841 | 2.1634 | 109.95 | |||||||
| 0.5001 | 1.3003 | 0.7841 | 2.0859 | 100.20 | |||||||
| 0.5004 | 1.3010 | 0.7841 | 2.0231 | 92.08 | |||||||
| 0.5005 | 1.3013 | 0.7841 | 2.0551 | 96.14 | |||||||
| No. * | Stability RSD (%) | Repeatability RSD (%) (n = 6) | Precision | Recovery (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day RSD (%) (n = 6) | Inter-Day RSD (%) (n = 3) | Sample (g) | Original (μg) △ | Spiked (μg) 1 | Found (μg) 2 | Recovery (%) | Average Recovery (%) | RSD (%) | |||
| 1 | 6.05 | 4.44 | 1.56 | 8.96 | 0.5004 | 9.1247 | 8.0449 | 16.2095 | 88.07 | 81.10 | 7.37 |
| 0.5000 | 9.1174 | 8.0449 | 16.1979 | 88.01 | |||||||
| 0.5000 | 9.1174 | 8.0449 | 15.6875 | 81.67 | |||||||
| 0.5003 | 9.1229 | 8.0449 | 15.4832 | 79.06 | |||||||
| 0.5004 | 9.1247 | 8.0449 | 15.1956 | 75.46 | |||||||
| 0.5004 | 9.1247 | 8.0449 | 15.1027 | 74.31 | |||||||
| 2 | 6.98 | 5.43 | 1.36 | 9.86 | 0.5004 | 8.7546 | 6.7196 | 15.1793 | 95.61 | 85.40 | 11.08 |
| 0.5000 | 8.7476 | 6.7196 | 15.1753 | 95.66 | |||||||
| 0.5000 | 8.7476 | 6.7196 | 14.6383 | 87.67 | |||||||
| 0.5003 | 8.7528 | 6.7196 | 14.4006 | 84.05 | |||||||
| 0.5004 | 8.7546 | 6.7196 | 13.8468 | 75.78 | |||||||
| 0.5004 | 8.7546 | 6.7196 | 13.7023 | 73.63 | |||||||
| 3 | 6.13 | 4.82 | 1.20 | 9.44 | 0.5004 | 7.5397 | 6.6519 | 12.9933 | 81.99 | 76.73 | 6.29 |
| 0.5000 | 7.5337 | 6.6519 | 12.9775 | 81.84 | |||||||
| 0.5000 | 7.5337 | 6.6519 | 12.5910 | 76.03 | |||||||
| 0.5003 | 7.5382 | 6.6519 | 12.7026 | 77.64 | |||||||
| 0.5004 | 7.5397 | 6.6519 | 12.1783 | 69.73 | |||||||
| 0.5004 | 7.5397 | 6.6519 | 12.4074 | 73.18 | |||||||
| 4 | 5.32 | 4.46 | 1.04 | 8.59 | 0.5004 | 9.6078 | 8.7507 | 16.8310 | 82.54 | 77.37 | 6.78 |
| 0.5000 | 9.6001 | 8.7507 | 16.9669 | 84.19 | |||||||
| 0.5000 | 9.6001 | 8.7507 | 16.2378 | 75.85 | |||||||
| 0.5003 | 9.6058 | 8.7507 | 16.4300 | 77.98 | |||||||
| 0.5004 | 9.6078 | 8.7507 | 15.8746 | 71.62 | |||||||
| 0.5004 | 9.6078 | 8.7507 | 15.9094 | 72.01 | |||||||
| 5 | 6.78 | 3.41 | 1.49 | 10.41 | 0.5004 | 8.2760 | 10.6095 | 19.6722 | 107.41 | 105.99 | 7.45 |
| 0.5000 | 8.2694 | 10.6095 | 20.6117 | 116.33 | |||||||
| 0.5000 | 8.2694 | 10.6095 | 20.0914 | 111.43 | |||||||
| 0.5003 | 8.2744 | 10.6095 | 19.3897 | 104.77 | |||||||
| 0.5004 | 8.2760 | 10.6095 | 18.1766 | 93.32 | |||||||
| 0.5004 | 8.2760 | 10.6095 | 18.5247 | 102.70 | |||||||
| 6 | 6.56 | 6.12 | 1.62 | 11.05 | 0.5004 | 6.1007 | 5.8564 | 11.3627 | 89.85 | 83.24 | 8.06 |
| 0.5000 | 6.0958 | 5.8564 | 11.4982 | 92.25 | |||||||
| 0.5000 | 6.0958 | 5.8564 | 10.6751 | 78.19 | |||||||
| 0.5003 | 6.0995 | 5.8564 | 11.0595 | 84.69 | |||||||
| 0.5004 | 6.1007 | 5.8564 | 10.6269 | 77.29 | |||||||
| 0.5004 | 6.1007 | 5.8564 | 10.6198 | 77.16 | |||||||
| No. | Compounds | Regression Equation | R2 | Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | trans-emodin-emodin dianthrones | y = 524.18x + 3553.5 | 0.9941 | 1.20–306.04 | 0.60 | 1.20 |
| 2 | cis-emodin-emodin dianthrones | y = 470.23x + 2282.8 | 0.9949 | 0.55–281.16 | 0.27 | 0.55 |
| 3 | trans-emodin-physcion dianthrones | y = 699.51x + 4752.4 | 0.9939 | 0.93–357.22 | 0.47 | 0.93 |
| 4 | cis-emodin-physcion dianthrones | y = 531.68x + 4300.9 | 0.9939 | 0.85–432.80 | 0.42 | 0.85 |
| 5 | trans-physcion-physcion dianthrones | y = 44.934x + 37.402 | 0.9981 | 0.93–475.60 | 0.46 | 0.93 |
| 6 | cis-physcion-physcion dianthrones | y = 72.981x + 121.73 | 0.9983 | 0.39–200.93 | 0.20 | 0.39 |
| No. | Compounds | Regression Equation | R2 | Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | trans-emodin-emodin dianthrones | y = 437.66x + 3967.5 | 0.9911 | 0.83–423.36 | 0.41 | 0.83 |
| 2 | cis-emodin-emodin dianthrones | y = 363.94x + 1279.5 | 0.9971 | 0.66–337.39 | 0.33 | 0.66 |
| 3 | trans-emodin-physcion dianthrones | y = 642.03x + 3708.2 | 0.9926 | 0.69–350.65 | 0.34 | 0.69 |
| 4 | cis-emodin-physcion dianthrones | y = 477.46x + 3564 | 0.9948 | 0.90–458.26 | 0.45 | 0.9 |
| 5 | trans-physcion-physcion dianthrones | y = 37.473x – 2.0684 | 0.9997 | 0.84–429.20 | 0.42 | 0.84 |
| 6 | cis-physcion-physcion dianthrones | y = 45.271x + 8.0834 | 0.9999 | 0.45–230.69 | 0.23 | 0.45 |
| No. | Compounds | Concentration (μM) | OD Value | Cell Survival Rate (%) |
|---|---|---|---|---|
| 0 | Control | - | 1.91 ± 0.10 | 100.00 |
| 1 | trans-emodin-physcion dianthrones | 10 | 2.01 ± 0.08 | 105.0 |
| 20 | 2.40 ± 0.05 | 125.56 | ||
| 40 | 1.87 ± 0.04 | 97.80 | ||
| 80 | 1.30 ± 0.05 *** | 67.95 | ||
| 2 | cis-emodin-physcion dianthrones | 10 | 1.13 ± 0.06 *** | 59.41 |
| 20 | 1.09 ± 0.08 *** | 57.02 | ||
| 40 | 0.72 ± 0.05 *** | 37.68 | ||
| 80 | 1.14 ± 0.04 *** | 59.96 | ||
| 3 | trans-physcion-physcion dianthrones | 10 | 1.77 ± 0.05 | 92.71 |
| 20 | 1.72 ± 0.04 | 90.28 | ||
| 40 | 1.71 ± 0.07 * | 89.82 | ||
| 80 | 1.81 ± 0.07 | 95.13 | ||
| 4 | cis-physcion-physcion dianthrones | 10 | 1.64 ± 0.05 * | 86.09 |
| 20 | 1.71 ± 0.02 * | 89.46 | ||
| 40 | 1.63 ± 0.03 ** | 85.58 | ||
| 80 | 1.83 ± 0.10 | 95.68 |
| No. | Compounds | Retention Times (RT, min) | Precursor Ion (m/z) | Quantitative Ion (m/z) | Qualitative Ion (m/z) | Cone (V) | Collision Energy (CE, V) | Ion Mode |
|---|---|---|---|---|---|---|---|---|
| 1 | trans-emodin-emodin dianthrones | 6.96 | 509.0 | 254.1 | 225.1 | 24 | 20, 70 | (–)ESI |
| 2 | cis-emodin-modin dianthrones | 7.70 | 509.1 | 254.0 | 225.1 | 24 | 22, 68 | (–)ESI |
| 3 | trans-emodin-physcion dianthrones | 12.34 | 523.1 | 254.0 | 225.1 | 60 | 20, 60 | (–)ESI |
| 4 | cis-emodin-physcion dianthrones | 12.96 | 523.05 | 254.0 | 225.1 | 60 | 20, 76 | (–)ESI |
| 5 | trans-physcion-physcion dianthrones | 19.13 | 537.1 | 522.2 | 268.1 | 54 | 32, 28 | (–)ESI |
| 6 | cis-physcion-physcion dianthrones | 19.56 | 537.1 | 522.2 | 268.1 | 54 | 32, 28 | (–)ESI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Yang, J.; Wang, X.; Song, Y.; Cheng, X.; Wei, F.; Wang, Y.; Gu, D.; Sun, H.; Ma, S. Exploratory Quality Control Study for Polygonum multiflorum Thunb. Using Dinuclear Anthraquinones with Potential Hepatotoxicity. Molecules 2022, 27, 6760. https://doi.org/10.3390/molecules27196760
Gao H, Yang J, Wang X, Song Y, Cheng X, Wei F, Wang Y, Gu D, Sun H, Ma S. Exploratory Quality Control Study for Polygonum multiflorum Thunb. Using Dinuclear Anthraquinones with Potential Hepatotoxicity. Molecules. 2022; 27(19):6760. https://doi.org/10.3390/molecules27196760
Chicago/Turabian StyleGao, Huiyu, Jianbo Yang, Xueting Wang, Yunfei Song, Xianlong Cheng, Feng Wei, Ying Wang, Donglin Gu, Hua Sun, and Shuangcheng Ma. 2022. "Exploratory Quality Control Study for Polygonum multiflorum Thunb. Using Dinuclear Anthraquinones with Potential Hepatotoxicity" Molecules 27, no. 19: 6760. https://doi.org/10.3390/molecules27196760
APA StyleGao, H., Yang, J., Wang, X., Song, Y., Cheng, X., Wei, F., Wang, Y., Gu, D., Sun, H., & Ma, S. (2022). Exploratory Quality Control Study for Polygonum multiflorum Thunb. Using Dinuclear Anthraquinones with Potential Hepatotoxicity. Molecules, 27(19), 6760. https://doi.org/10.3390/molecules27196760








