Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases
Abstract
1. Introduction
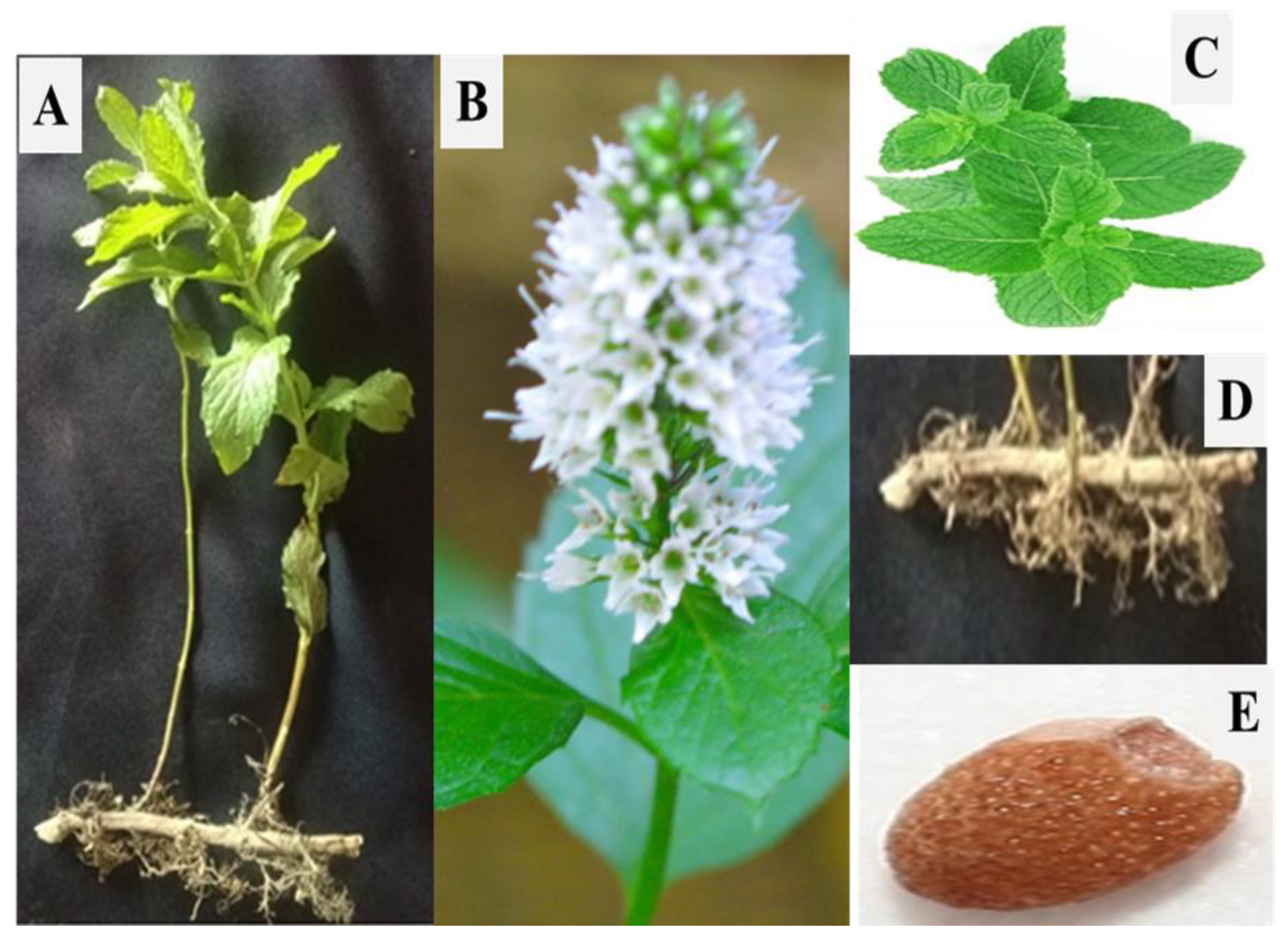
2. Genus Mentha: Morphology and Systematics
2.1. Morphology
2.2. Systematics
3. Essential Oil and the Chemical Composition of the Studied Species of Mentha
4. Health Benefits of Mentha
5. Biological Activities of Mentha
5.1. Antimicrobial Activity of Mentha
5.2. Antioxidant Activity of Mentha
5.3. Antidiabetic Activity of Mentha
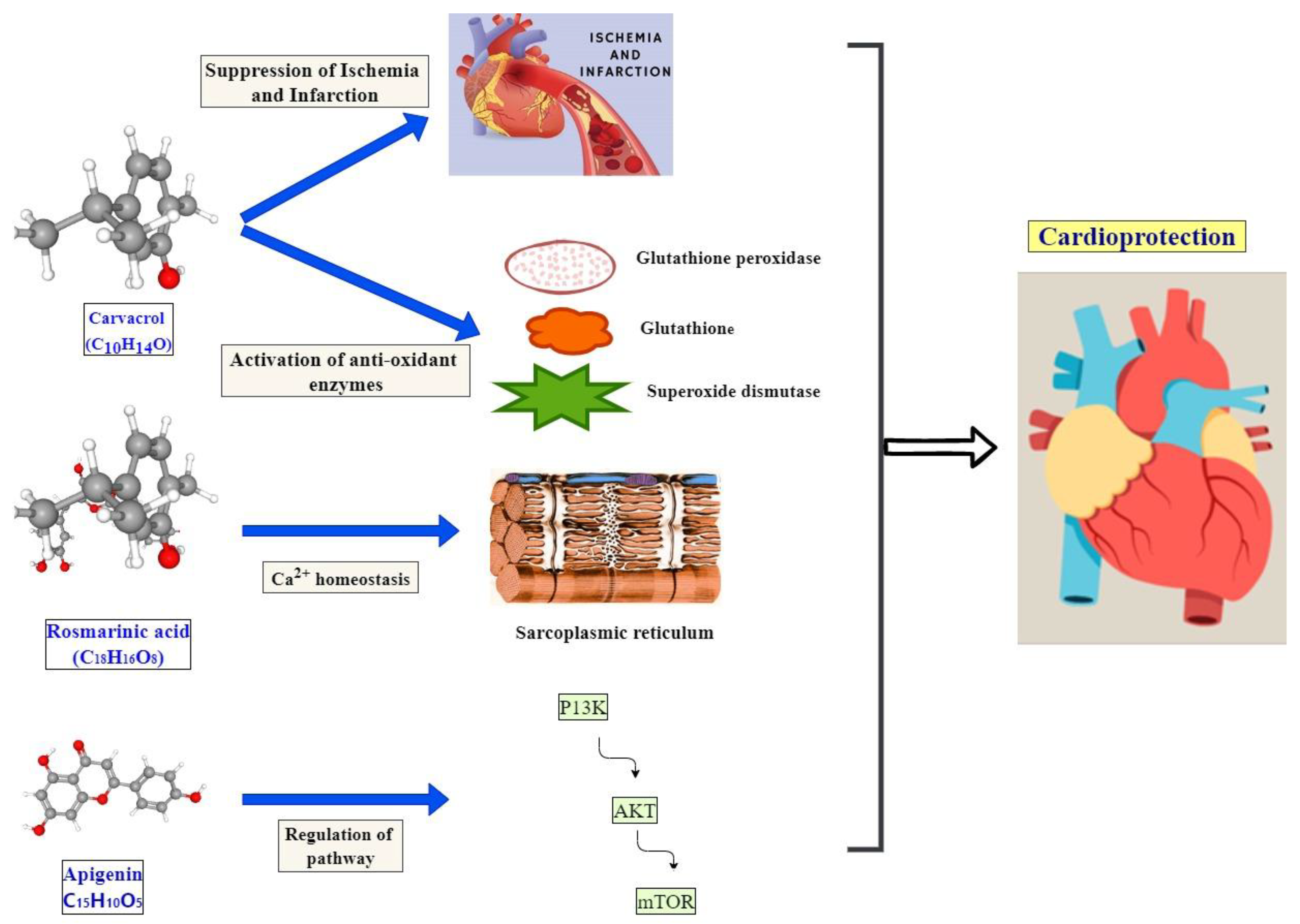
6. Cardioprotective Potential of Mentha by Its Antiinflammatory Effect
Mechanism of Active Compounds with Cardioprotective Effects
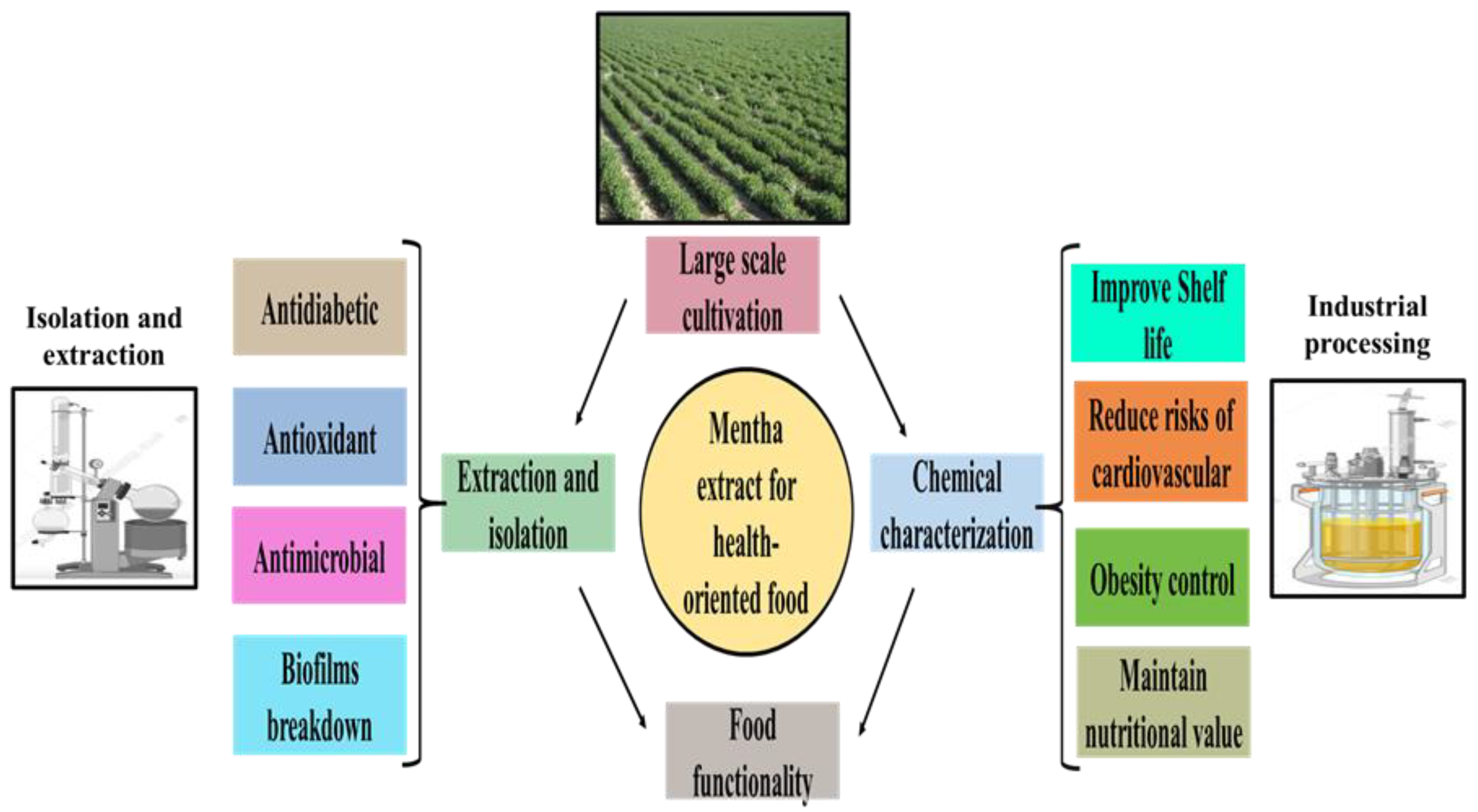
7. Utility of Mentha in Food Industry
8. Current Challenges and Implementations
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R. Plants of genus Mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.Y.; Hameed, I.H.; Ibraheam, I.A. Mentha pulegium: Medicinal uses, Anti-Hepatic, Antibacterial, Antioxidant effect and Analysis of Bioactive Natural Compounds: A Review. Res. J. Pharm. Technol. 2017, 10, 3580–3584. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Zaman, W.; Ye, J.; Ahmad, M.; Saqib, S.; Shinwari, Z.K.; Chen, Z. Phylogenetic exploration of traditional Chinese medicinal plants: A case study on Lamiaceae (angiosperms). Pak. J. Bot. 2022, 54, 1033–1040. [Google Scholar] [CrossRef]
- Tafrihi, M.; Imran, M.; Tufail, T.; Gondal, T.A.; Caruso, G.; Sharma, S.; Sharma, R.; Atanassova, M.; Atanassov, L.; Valere Tsouh Fokou, P. The wonderful activities of the genus Mentha: Not only antioxidant properties. Molecules 2021, 26, 1118. [Google Scholar] [CrossRef] [PubMed]
- Mikaili, P.; Mojaverrostami, S.; Moloudizargari, M.; Aghajanshakeri, S. Pharmacological and therapeutic effects of Mentha Longifolia L. and its main constituent, menthol. Anc. Sci. Life 2013, 33, 131. [Google Scholar] [PubMed]
- Asghar, M.; Younas, M.; Arshad, B.; Zaman, W.; Ayaz, A.; Rasheed, S.; Shah, A.; Ullah, F.; Saqib, S. Bioactive potential of cultivated Mentha arvensis L. for preservation and production of health-oriented food. J. Anim. Plant Sci. 2022, 32, 835–844. [Google Scholar]
- Kalakoti, M.; Kumar, A.; Mehra, S.; Joshi, A. Analysis of phytoconstituents present in Mentha piperita. Indian J. Biotechnol. Pharm. Res. 2014, 2, 25–31. [Google Scholar]
- Jäger, A.K.; Almqvist, J.P.; Vangsøe, S.A.; Stafford, G.I.; Adsersen, A.; Van Staden, J. Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. S. Afr. J. Bot. 2007, 73, 518–521. [Google Scholar] [CrossRef]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical composition and biological activities of Mentha species. Aromat. Med. Plants-Back Nat. 2017, 10, 47–79. [Google Scholar]
- Association, A.D. 6. Glycemic targets: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S55. [Google Scholar] [CrossRef] [PubMed]
- Quintin, D.; Garcia-Gomez, P.; Ayuso, M.; Sanmartin, A. Active biocompounds to improve food nutritional value. Trends Food Sci. Technol. 2019, 84, 19–21. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza Pedrosa, G.T.; da Cruz Almeida, E.T.; Cao, G.; Macarisin, D.; Schaffner, D.W.; de Souza, E.L.; Magnani, M. Successive exposure to Mentha piperita L. essential oil affects the culturability and induces membrane repair in a persister epidemic Salmonella Typhimurium PT4. Microb. Pathog. 2020, 149, 104264. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Banerjee, R.; Biswas, S.; McClements, D.J. Application of nanoemulsion-based approaches for improving the quality and safety of muscle foods: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2677–2700. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Ayad, L.; Khattab, A.R. Sweet and bitter oranges: An updated comparative review of their bioactives, nutrition, food quality, therapeutic merits and biowaste valorization practices. Food Chem. 2020, 331, 127306. [Google Scholar] [CrossRef]
- Bijani, A.; Cumming, R.G.; Hosseini, S.-R.; Yazdanpour, M.; Rahimi, M.; Sahebian, A.; Ghadimi, R. Obesity paradox on the survival of elderly patients with diabetes: An AHAP-based study. J. Diabetes Metab. Disord. 2018, 17, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Tondt, J.; Maguire, E.; Yancy, W.S., Jr. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev. Endocrinol. Metab. 2018, 13, 263–272. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Insulin–PI3K signalling: An evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 2020, 16, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Dutra, K.; Wanderley-Teixeira, V.; Guedes, C.; Cruz, G.; Navarro, D.; Monteiro, A.; Agra, A.; Neto, C.L.; Teixeira, Á. Toxicity of Essential Oils of Leaves of Plants from the Genus Piper with Influence on the Nutritional Parameters of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). J. Essent. Oil Bear. Plants 2020, 23, 213–229. [Google Scholar] [CrossRef]
- Gatti, R.; Lotti, C.; Morigi, R.; Andreani, A. Determination of octopamine and tyramine traces in dietary supplements and phytoextracts by high performance liquid chromatography after derivatization with 2, 5-dimethyl-1H-pyrrole-3, 4-dicarbaldehyde. J. Chromatogr. A 2012, 1220, 92–100. [Google Scholar] [CrossRef]
- Paoli, A.; Cenci, L.; Fancelli, M.; Parmagnani, A.; Fratter, A.; Cucchi, A.; Bianco, A. Ketogenic diet and phytoextracts. Sci. Advis. Board 2010, 21, 24. [Google Scholar]
- Mench, M.; Schwitzguébel, J.-P.; Schroeder, P.; Bert, V.; Gawronski, S.; Gupta, S. Assessment of successful experiments and limitations of phytotechnologies: Contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ. Sci. Pollut. Res. 2009, 16, 876. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Muqarab, R.; Waseem, M. The Solanum melongena COP1 delays fruit ripening and influences Ethylene signaling in tomato. J. Plant Physiol. 2019, 240, 152997. [Google Scholar] [CrossRef]
- Corrêa, R.C.; Peralta, R.M.; Haminiuk, C.W.; Maciel, G.M.; Bracht, A.; Ferreira, I.C. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Niggli, U.; Slabe, A.; Schmid, O.; Halberg, N.; Schlüter, M. Vision for an Organic Food and Farming Research Agenda 2025. Organic Knowledge for the Future; IFOAM Regional Group European Union (IFOAM EU Group): Brussels, Belgium; International Society of Organic Agriculture Research (ISOFAR): Bonn, Germany, 2008. [Google Scholar]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef]
- Hever, J. Plant-based diets: A physician’s guide. Perm. J. 2016, 20, 15–082. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-based diets for cardiovascular safety and performance in endurance sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, G.; Zoppi, G.; Bocchini, S.; Rizzo, A.M.; Chiaramonti, D.; Pirone, R.; Bensaid, S. Aqueous phase reforming of the residual waters derived from lignin-rich hydrothermal liquefaction: Investigation of representative organic compounds and actual biorefinery streams. Catal. Today 2020, 345, 237–250. [Google Scholar] [CrossRef]
- Franco, R.R.; Alves, V.H.M.; Zabisky, L.F.R.; Justino, A.B.; Martins, M.M.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic potential of Bauhinia forficata Link leaves: A non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomed. Pharmacother. 2020, 123, 109798. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
- Caro, D.C.; Rivera, D.E.; Ocampo, Y.; Franco, L.A.; Salas, R.D. Pharmacological evaluation of Mentha spicata L. and Plantago major L., medicinal plants used to treat anxiety and insomnia in Colombian Caribbean coast. Evid. -Based Complement. Altern. Med. 2018, 2018, 5921514. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Shinwari, Z.K.; Sultan, S.; Mehmood, T. Molecular and morphological characterization of selected Mentha species. Pak. J. Bot 2011, 43, 1433–1436. [Google Scholar]
- Akram, M.; Uzair, M.; Malik, N.S.; Mahmood, A.; Sarwer, N.; Madni, A.; Asif, H. Mentha arvensis Linn.: A review article. J. Med. Plants Res. 2011, 5, 4499–4503. [Google Scholar]
- Vining, K.J.; Hummer, K.E.; Bassil, N.V.; Lange, B.M.; Khoury, C.K.; Carver, D. Crop wild relatives as germplasm resource for cultivar improvement in mint (Mentha L.). Front. Plant Sci. 2020, 11, 1217. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.M. Mint: The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zahra, N.B.; Shinwari, Z.K.; Qaiser, M. Dna barcoding: A tool for standardization of Herbal Medicinal Products (HMPS) of Lamiaceae From Pakistan. Pak. J. Bot. 2016, 48, 2167–2174. [Google Scholar]
- Wilson, A.E.; Sparks, D.L.; Knott, K.K.; Willard, S.; Brown, A. Implementing solid phase microextraction (SPME) as a tool to detect volatile compounds produced by giant pandas in the environment. PLoS ONE 2018, 13, e0208618. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Mahdavi, B. Characterization of essential oils and volatiles from the aerial parts of Mentha pulegium L.(Lamiaceae) using microwave-assisted hydrodistillation (MAHD) and headspace solid phase microextraction (HS-SPME) in combination with GC-MS. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sonam, K.A.; Kumar, V.; Guleria, I.; Sharma, M.; Kumar, A.; Alruways, M.; Khan, N.; Raina, R. Antimicrobial Potential and Chemical Profiling of Leaves Essential Oil of Mentha Species Growing under North-West Himalaya Conditions. J. Pure Appl. Microbiol 2021, 15, 2229–2243. [Google Scholar] [CrossRef]
- Fidan, H.; Stankov, S.; Iliev, I.; Gandova, V.; Stoyanova, A.; Dincheva, I. Chemical composition of essential oils from different Mentha ssp. In Proceedings of the 2022 8th International Conference on Energy Efficiency and Agricultural Engineering (EE&AE), Ruse, Bulgaria, 30 June–2 July 2022; pp. 1–4. [Google Scholar]
- Aldogman, B.; Bilel, H.; Moustafa, S.M.N.; Elmassary, K.F.; Ali, H.M.; Alotaibi, F.Q.; Hamza, M.; Abdelgawad, M.A.; El-Ghorab, A.H. Investigation of Chemical Compositions and Biological Activities of Mentha suaveolens L. from Saudi Arabia. Molecules 2022, 27, 2949. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Gruľová, D.; Elshafie, H.S. Chemical composition and antimicrobial properties of Mentha × piperita cv.‘Kristinka’essential oil. Plants 2021, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Cosentino, M.; Bombelli, R.; Conti, A.; Colombo, M.L.; Azzetti, A.; Bergamaschi, A.; Marino, F.; Lecchini, S. Antioxidant properties and in vitro immunomodulatory effects of peppermint (Mentha piperita L.) Essential oils in human leukocytes’. J. Pharm. Sci. Res 2009, 1, 33–43. [Google Scholar]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Mahmoud, E.A.-M.; Al-Askalany, S.A.; Hanafy, E.A. Antioxidant, antibacterial and cytotoxic effect of Cymbopogon citratus, Mentha longifolia, and Artemisia absinthium essential oils. Egypt. J. Chem. 2022, 65, 287–296. [Google Scholar]
- Bai, X.; Aimila, A.; Aidarhan, N.; Duan, X.; Maiwulanjiang, M. Chemical constituents and biological activities of essential oil from Mentha longifolia: Effects of different extraction methods. Int. J. Food Prop. 2020, 23, 1951–1960. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Perotti, M.; Schuff, C.; Catalán, C. Comparative study of chemistry compositions and antimicrobial potentials of essential oils and oleoresins from dried and fresh Mentha longifolia L. J. Coast. Life Med. 2015, 3, 987–991. [Google Scholar] [CrossRef]
- Baali, F.; Boumerfeg, S.; Napoli, E.; Boudjelal, A.; Righi, N.; Deghima, A.; Baghiani, A.; Ruberto, G. Chemical composition and biological activities of essential oils from two wild Algerian medicinal plants: Mentha pulegium L. and Lavandula stoechas L. J. Essent. Oil Bear. Plants 2019, 22, 821–837. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Eryigit, F.; Cengiz, M.; Tepe, B.; Cakir, A.; Mete, E. Screening of the antioxidant activity of the essential oil and methanol extract of Mentha pulegium L. from Turkey. Spectrosc. Lett. 2012, 45, 352–358. [Google Scholar] [CrossRef]
- Batool, I.; Nisar, S.; Hamrouni, L.; Jilani, M.I. Extraction, production and analysis techniques for menthol: A review. Int. J. Chem. Biochem. Sci. 2018, 14, 71–76. [Google Scholar]
- Lampronti, I.; Saab, A.M.; Gambari, R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int. J. Oncol. 2006, 29, 989–995. [Google Scholar] [CrossRef]
- Biswas, N.N.; Saha, S.; Ali, M.K. Antioxidant, antimicrobial, cytotoxic and analgesic activities of ethanolic extract of Mentha arvensis L. Asian Pac. J. Trop. Biomed. 2014, 4, 792–797. [Google Scholar] [CrossRef]
- Diop, S.M.; Guèye, M.T.; Ndiaye, I.; Ndiaye, E.H.B.; Diop, M.B.; Heuskin, S.; Fauconnier, M.-L.; Lognay, G. Chemical composition of essential oils and floral waters of Mentha longifolia (L.) Huds. from Senegal. Am. J. Essent. Oils Nat. Prod. 2016, 4, 46–49. [Google Scholar]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Al-Mijalli, S.H.; Assaggaf, H.; Qasem, A.; El-Shemi, A.G.; Abdallah, E.M.; Mrabti, H.N.; Bouyahya, A. Antioxidant, Antidiabetic, and Antibacterial Potentials and Chemical Composition of Salvia officinalis and Mentha suaveolens Grown Wild in Morocco. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 2844880. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Litaiem, M.; Jelali, N.; Hamdi, N.; Mnif, W. Identification of a new chemotye of the plant Mentha aquatica grown in Tunisia: Chemical composition, antioxidant and biological activities of its essential oil. J. Essent. Oil Bear. Plants 2011, 14, 320–328. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Gallegos-Corona, M.A.; Ramos-Gomez, M.; Reynoso-Camacho, R. Salicylic acid elicitation during cultivation of the peppermint plant improves anti-diabetic effects of its infusions. Food Funct. 2015, 6, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.C.C.; Menezes, A.P.P.; Barbalho, S.M.; Guiguer, É.L. Properties of Mentha piperita: A brief review. World J. Pharm. Med. Res. 2017, 3, 309–313. [Google Scholar]
- Balakrishnan, A. Therapeutic uses of peppermint-a review. J. Pharm. Sci. Res. 2015, 7, 474. [Google Scholar]
- Mackonochie, M.; Heinrich, M. Materia medica chests: Investigating the 19th century use of botanicals by different medical professions. J. Herb. Med. 2019, 16, 100255. [Google Scholar] [CrossRef]
- Harley, R.; Brighton, C. Chromosome numbers in the genus Mentha L. Bot. J. Linn. Soc. 1977, 74, 71–96. [Google Scholar] [CrossRef]
- Pereira, O.R.; Cardoso, S.M. Overview on Mentha and Thymus polyphenols. Curr. Anal. Chem. 2013, 9, 382–396. [Google Scholar] [CrossRef]
- Shai, L.; Magano, S.; Lebelo, S.; Mogale, A. Inhibitory effects of five medicinal plants on rat alpha-glucosidase: Comparison with their effects on yeast alpha-glucosidase. J. Med. Plants Res. 2011, 5, 2863–2867. [Google Scholar]
- Dorman, H.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Dwivedy, A.K.; Prakash, B.; Chanotiya, C.S.; Bisht, D.; Dubey, N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 2017, 106, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Al-Zyadi, Q.A. Effect of planting and harvesting dates on the growth and essential oil content of peppermint (Mentha piperita L.). Plant Arch. 2019, 19, 319–322. [Google Scholar]
- Kozlovich, S.; Chen, G.; Watson, C.J.; Blot, W.J.; Lazarus, P. Role of l-and d-Menthol in the Glucuronidation and Detoxification of the Major Lung Carcinogen, NNAL. Drug Metab. Dispos. 2019, 47, 1388–1396. [Google Scholar] [CrossRef]
- Jeya Shree, T.; Gowri Sree, V.; Poompavai, S.; Sieni, E.; Sgarbossa, P.; Camarillo, I.; Sundararajan, R. Inhibition of Proliferation of HeLa Cells by Pulsed Electric Field Treated Mentha piperita (Mint) Extract. IETE J. Res. 2019, 68, 858–868. [Google Scholar] [CrossRef]
- Sevindik, M. Pharmacological Properties of Mentha Species. J. Tradit. Med. Clin. Naturop. 2018, 7, 2. [Google Scholar] [CrossRef]
- Sheikh, B.Y.; Sarker, M.M.R.; Kamarudin, M.N.A.; Ismail, A. Prophetic medicine as potential functional food elements in the intervention of cancer: A review. Biomed. Pharmacother. 2017, 95, 614–648. [Google Scholar] [CrossRef]
- Roulis, E.; Polkinghorne, A.; Timms, P. Chlamydia pneumoniae: Modern insights into an ancient pathogen. Trends Microbiol. 2013, 21, 120–128. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003, 69, 413–419. [Google Scholar]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.M.; Cavero, R.Y.; Calvo, M.I. In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum. Nutr. 2007, 62, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, S.-g.; Liang, W.; Mei, J.; Di, Y.-y.; Lan, H.-h.; Yang, Y.; Wang, W.-w.; Luo, Y.-y.; Wang, H.-z. Antibacterial activity and mode of action of Mentha arvensis ethanol extract against multidrug-resistant Acinetobacter baumannii. Trop. J. Pharm. Res. 2015, 14, 2099–2106. [Google Scholar] [CrossRef]
- Rodrigues, L.; Duarte, A.; Figueiredo, A.C.; Brito, L.; Teixeira, G.; Moldao, M.; Monteiro, A. Chemical composition and antibacterial activity of the essential oils from the medicinal plant Mentha cervina L. grown in Portugal. Med. Chem. Res. 2012, 21, 3485–3490. [Google Scholar] [CrossRef]
- Wannissorn, B.; Jarikasem, S.; Siriwangchai, T.; Thubthimthed, S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 2005, 76, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Chagas, E.C.; Majolo, C.; Monteiro, P.C.; Oliveira, M.R.d.; Gama, P.E.; Bizzo, H.R.; Chaves, F.C.M. Composition of essential oils of Mentha species and their antimicrobial activity against Aeromonas spp. J. Essent. Oil Res. 2020, 32, 209–215. [Google Scholar] [CrossRef]
- Verma, S.K.; Goswami, P.; Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R.; Darokar, M.P. Chemical composition and antimicrobial activity of bergamot-mint (Mentha citrata Ehrh.) essential oils isolated from the herbage and aqueous distillate using different methods. Ind. Crops Prod. 2016, 91, 152–160. [Google Scholar] [CrossRef]
- Espiritu, A.G.; Doma, B.T., Jr.; Wang, Y.-F.; Masim, F.C.P. Efficacy of methanol extracts of Mentha haplocalyx Briq. and Paeonia suffruticosa Andr. for potential antibacterial activity. Sustain. Environ. Res. 2014, 24, 319–324. [Google Scholar]
- Bekka-Hadji, F.; Bombarda, I.; Touati, A. Antibacterial activity against methicillin-resistant Staphylococcus aureus of five essential oils from Algerian medicinal plants (Lamiaceae). J. EssEntial Oil Res. 2016, 28, 518–527. [Google Scholar] [CrossRef]
- Chessa, M.; Sias, A.; Piana, A.; Mangano, G.S.; Petretto, G.L.; Masia, M.D.; Tirillini, B.; Pintore, G. Chemical composition and antibacterial activity of the essential oil from Mentha requienii Bentham. Nat. Prod. Res. 2013, 27, 93–99. [Google Scholar] [CrossRef]
- Džamić, A.M.; Soković, M.D.; Ristić, M.S.; Novaković, M.; Grujić-Jovanović, S.; Tešević, V.; Marin, P.D. Antifungal and antioxidant activity of Mentha longifolia (L.) Hudson (Lamiaceae) essential oil. Bot. Serbica 2010, 34, 57–61. [Google Scholar]
- Nikavar, B.; Ali, N.A.; Kamalnezhad, M. Evaluation of the antioxidant properties of five Mentha species. Iran. J. Pharm. Sci. 2008, 7, 203–209. [Google Scholar]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef]
- Jain, S.; Jain, D.K.; Balekar, N. In–Vivo Antioxidant activity of ethanolic extract of Mentha pulegium leaf against CCl4 induced toxicity in rats. Asian Pac. J. Trop. Biomed. 2012, 2, S737–S740. [Google Scholar] [CrossRef]
- Agawane, S.B.; Gupta, V.S.; Kulkarni, M.J.; Bhattacharya, A.K.; Koratkar, S.S. Chemo-biological evaluation of antidiabetic activity of Mentha arvensis L. and its role in inhibition of advanced glycation end products. J. Ayurveda Integr. Med. 2019, 10, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bayani, M.; Ahmadi-Hamedani, M.; Javan, A.J. Study of hypoglycemic, hypocholesterolemic and antioxidant activities of Iranian Mentha spicata leaves aqueous extract in diabetic rats. Iran. J. Pharm. Res. IJPR 2017, 16, 75. [Google Scholar]
- Zaidi, S.F.; Muhammad, J.S.; Shahryar, S.; Usmanghani, K.; Gilani, A.-H.; Jafri, W.; Sugiyama, T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012, 141, 403–410. [Google Scholar] [CrossRef]
- Atta, A.; Alkofahi, A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J. Ethnopharmacol. 1998, 60, 117–124. [Google Scholar] [CrossRef]
- Modarresi, M.; Farahpour, M.-R.; Baradaran, B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 2019, 27, 531–537. [Google Scholar] [CrossRef]
- Badal, R.M.; Badal, D.; Badal, P.; Khare, A.; Shrivastava, J.; Kumar, V. Pharmacological action of Mentha piperita on lipid profile in fructose-fed rats. Iran. J. Pharm. Res. IJPR 2011, 10, 843. [Google Scholar]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of antioxidant, anti-inflammatory and cytoprotective properties of ethanolic mint extracts from algeria on 7-ketocholesterol-treated murine RAW 264.7 macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef]
- Caputo, L.; Cornara, L.; Raimondo, F.M.; De Feo, V.; Vanin, S.; Denaro, M.; Trombetta, D.; Smeriglio, A. Mentha pulegium L.: A plant underestimated for its toxicity to be recovered from the perspective of the circular economy. Molecules 2021, 26, 2154. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, Q.; Zhu, S. Carvacrol protects against acute myocardial infarction of rats via anti-oxidative and anti-apoptotic pathways. Biol. Pharm. Bull. 2013, 36, 579–584. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Irina, B.; Maria-Magdalena, Z.; Ioan, B. Chemical composition of essential oils from Mentha aquatica L. at different moments of the ontogenetic cycle. J. Med. Plants Res. 2013, 7, 470–473. [Google Scholar]
- Roy, A.; Park, H.-J.; Abdul, Q.A.; Jung, H.A.; Choi, J.S. Pulegone Exhibits Anti-inflammatory Activities through the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-stimulated RAW 264.7 cells. Nat. Prod. Sci. 2018, 24, 28–35. [Google Scholar] [CrossRef]
- Nascimento, G.A.d.; Souza, D.S.d.; Lima, B.S.; Vasconcelos, C.M.L.d.; Araújo, A.A.d.S.; Durço, A.O.; Quintans-Junior, L.J.; Almeida, J.R.G.d.S.; Oliveira, A.P.; Santana-Filho, V.J.d. Bradycardic and antiarrhythmic effects of the D-limonene in rats. Arq. Bras. De Cardiol. 2019, 113, 925–932. [Google Scholar] [CrossRef]
- Lahlou, S.; Carneiro-Leão, R.F.L.; Leal-Cardoso, J.H.; Toscano, C.F. Cardiovascular effects of the essential oil of Mentha x villosa and its main constituent, piperitenone oxide, in normotensive anaesthetised rats: Role of the autonomic nervous system. Planta Med. 2001, 67, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef]
- Chen, Y.; Ba, L.; Huang, W.; Liu, Y.; Pan, H.; Mingyao, E.; Shi, P.; Wang, Y.; Li, S.; Qi, H. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Javidanpour, S.; Dianat, M.; Badavi, M.; Mard, S.A. The cardioprotective effect of rosmarinic acid on acute myocardial infarction and genes involved in Ca2+ homeostasis. Free Radic. Res. 2017, 51, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Feng, Y.; Liu, D.; Zhang, Z.; Gao, K.; Zhang, W.; Tang, H. Thymoquinone attenuates myocardial ischemia/reperfusion injury through activation of SIRT1 signaling. Cell. Physiol. Biochem. 2018, 47, 1193–1206. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The protective effect of apigenin on myocardial injury in diabetic rats mediating activation of the PPAR-γ pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef]
- Moon, S.-K.; Cho, G.-O.; Jung, S.-Y.; Gal, S.-W.; Kwon, T.K.; Lee, Y.-C.; Madamanchi, N.R.; Kim, C.-H. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: Role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem. Biophys. Res. Commun. 2003, 301, 1069–1078. [Google Scholar] [CrossRef]
- Shi, R.; Wei, Z.; Zhu, D.; Fu, N.; Wang, C.; Yin, S.; Liang, Y.; Xing, J.; Wang, X.; Wang, Y. Baicalein attenuates monocrotaline-induced pulmonary arterial hypertension by inhibiting vascular remodeling in rats. Pulm. Pharmacol. Ther. 2018, 48, 124–135. [Google Scholar] [CrossRef]
- Abbas, M.M.; Kandil, Y.İ.; Abbas, M.A. R-(-)-carvone attenuated doxorubicin induced cardiotoxicity in vivo and potentiated its anticancer toxicity in vitro. Balk. Med. J. 2020, 37, 98. [Google Scholar] [CrossRef] [PubMed]
- Cantanhêde, S.M.; Amado, L.L.; da Costa, B.M.P.A.; Barbas, L.A.L.; Torres, M.F.; Hamoy, A.O.; da Paz, C.A.; da Silva Ferreira, C.B.; Lima, G.O.; de Sousa, J.R. Menthol exposure induces reversible cardiac depression and reduces lipid peroxidation in the heart tissue of tambaqui Colossoma macropomum. Aquaculture 2021, 541, 736847. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, J.; Lv, H.; Wen, T.; Shi, B.; Liu, X.; Zeng, N. Pulegone inhibits inflammation via suppression of NLRP3 inflammasome and reducing cytokine production in mice. Immunopharmacol. Immunotoxicol. 2019, 41, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Pombal, S.; Hernández, Y.; Diez, D.; Mondolis, E.; Mero, A.; Moran-Pinzon, J.; Guerrero, E.I.; Rodilla, J.M. Antioxidant activity of carvone and derivatives against superoxide ion. Nat. Prod. Commun. 2017, 12, 1934578X1701200502. [Google Scholar] [CrossRef]
- de Lima Serafim, C.A.; Araruna, M.E.C.; Júnior, E.B.A.; Silva, L.M.O.; Silva, A.O.; Da Silva, M.S.; Alves, A.F.; Araujo, A.A.; Batista, L.M. (-)-Carveol prevents gastric ulcers via cytoprotective, antioxidant, antisecretory and immunoregulatory mechanisms in animal models. Front. Pharmacol. 2021, 12, 736829. [Google Scholar] [CrossRef]
- Johnson, A.J.; Miles, C. Chewing gum and context-dependent memory: The independent roles of chewing gum and mint flavour. Br. J. Psychol. 2008, 99, 293–306. [Google Scholar] [CrossRef]
- Spence, C.; Ngo, M.K. Assessing the shape symbolism of the taste, flavour, and texture of foods and beverages. Flavour 2012, 1, 12. [Google Scholar] [CrossRef]
- Gracindo, L.; Grisi, M.; Silva, D.; Alves, R.; Bizzo, H.; Vieira, R. Chemical characterization of mint (Mentha spp.) germplasm at Federal District, Brazil. Embrapa Recur. Genéticos Biotecnol. -Artig. Periódico Indexado (ALICE) 2006, 8, 5–9. [Google Scholar]
- Kapp, K.; Orav, A.; Roasto, M.; Raal, A.; Püssa, T.; Vuorela, H.; Tammela, P.; Vuorela, P. Composition and antibacterial effect of mint flavorings in candies and food supplements. Planta Med. 2020, 86, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Bhoora, H.S.G.; Srivastava, N.G.; Gargi, B.; Joshi, M. Comparative study of antibacterial assay of Mentha piperita (in vivo and in vitro cultured) leaves extract on enveloped human pathogenic bacteria and its phytochemical screening. J. Pharmacogn. Phytochem. 2020, 9, 15–19. [Google Scholar]
- Mohamed, L.; Chakraborty, S.; ArulJothi, K.; Mabasa, L.; Sayah, K.; Costa-Lotufo, L.V.; Jardine, A.; Prince, S. Galenia africana plant extract exhibits cytotoxicity in breast cancer cells by inducing multiple programmed cell death pathways. Saudi Pharm. J. 2020, 28, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Eichelbaum, S.; Abood, S.; Acuña, L.; Ahumada, M.; Veisaga, M.-L.; Barbieri, M.A. Challenges and Considerations in the Preparation of Novel Antibiotic Phytochemicals. Eur. J. Med. Plants 2020, 31, 14–28. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]

| Species Name | Essential Oil | Chemical Composition | Composition (%) | Structure | Source | Activities | Reference |
|---|---|---|---|---|---|---|---|
| M. × piperita L. | Monoterpenoids | 1-menthone | 7.32–18.32 | 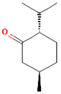 | Aerial parts | Antiinflammatory, antibacterial, neuroprotective, antifatigue, and antioxidant properties | [50] |
| Isomenthone | 0–6.75 | 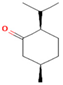 | Aerial parts | Antiviral, scolicidal, immunomodulatory, antitumor, and antioxidant properties | [51] | ||
| Menthol | 18.03–58.42 | 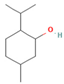 | Aerial parts | Antitumor, neuroprotective, antifatigue, and antioxidant properties | [51] | ||
| Menthyl acetate | 0.72–6.89 | 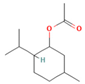 | Aerial parts | Antimicrobial and flavoring agent | [50] | ||
| Sesquiterpenes | Caryophyllene | 0.05–1.54 | 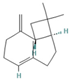 | Aerial parts | Anticancer and analgesic properties | [52] | |
| Germacrene-D | 0.63–1.89 | 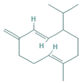 | Aerial parts | Antioxidant and immunomodulatory effects | [53] | ||
| M. longifolia L. | Monoterpenoids | Endo-Borneol | 1.12–6.02 | 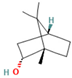 | Aerial parts | Cytotoxicity and anticancer properties | [54] |
| α-Terpineol | 0–0.28 | 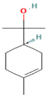 | Aerial parts | Antioxidant and anti-COX-2 activity | [54] | ||
| Isopiperitenone | 0.07–0.36 |  | Aerial parts | Antimicrobial properties | [55] | ||
| Carvacrol | 0–1.06 |  | Aerial parts | Antimicrobial and Cytotoxic properties | [54] | ||
| Cinerolon | 0.08–0.25 | 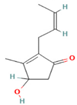 | Aerial parts | Antimicrobial properties | [55] | ||
| Cis-a-Farnescene | 1.03–1.97 | 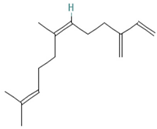 | Aerial parts | Antimicrobial properties | [55] | ||
| Sesquiterpene | Caryophyllene | 2.72–7.03 |  | Aerial parts | Anticancer and analgesic properties | [56] | |
| Germacrene D | 0.98–3.22 | 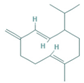 | Aerial parts | Antioxidant and immunomodulatory effects | [56] | ||
| Caryophyllene oxide | 0.12–0.79 | 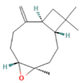 | Aerial parts | Anticancer properties | [56] | ||
| M. pulegium L. | Oxygenated Monoterpenes | Carvone | 56.1 | 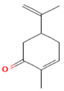 | Aerial parts | Antimicrobial, antioxidant, diuretic, analgesic, and antiseptic properties | [57] |
| Limonene | 15.1 |  | Aerial parts | Antimicrobial, antioxidant, diuretic, analgesic, and antiseptic properties | [57] | ||
| (E)-caryophyllene | 3.6 | 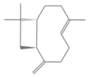 | Aerial parts | Anticancer and analgesic properties | [57] | ||
| Oleic acid | 3.2 |  | Aerial parts | Antioxidant and antimicrobial properties | [58] | ||
| 1,8-cineole | 2.4 | 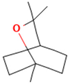 | Aerial parts | Antimicrobial, antioxidant, diuretic, analgesic, and antiseptic properties | [57] | ||
| Monoterpene | Pulegone | 54.3 | 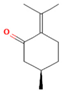 | Aerial parts | Antioxidant and antimicrobial properties | [58] | |
| M. arvensis L. | Menthol | 30.35 | 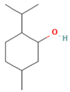 | Leaf | Antiseptic, antibacterial properties, antioxidant, antimicrobial, anticancer, and antiinflammatory activities | [59] | |
| Menthone | 20.50 | 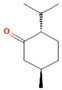 | Leaf | Antiseptic, antibacterial properties, antioxidant, antimicrobial, anticancer, and antiinflammatory activities | [59] | ||
| β-pinene | 7.28 | 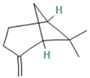 | Leaf | Antimicrobial properties | [53] | ||
| α-terpineol | 7.08 | 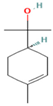 | Leaf | Antiproliferative activity | [60] | ||
| α-pinene | 6.35 | 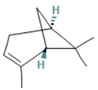 | Leaf | Antiproliferative activity | [60] | ||
| Menthofuran | 5.85 | 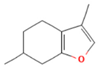 | Leaf | Antioxidant, antimicrobial, cytotoxic, analgesic | [61] | ||
| Iso-menthone | 4.53 | 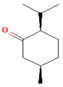 | Leaf | Antiviral, scolicidal, immunomodulatory, antitumor, and antioxidant properties | [51] | ||
| Neo-menthol | 4.36 | 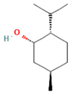 | Leaf | Antioxidant properties and antimicrobial activity | [51] | ||
| Menthyl acetate | 3.26 |  | Leaf | Antimicrobial properties and flavoring agent | [50] | ||
| M. spicata L. | Terpenoids | Carvone | 58.22 | 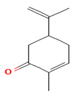 | Leaf | Antimicrobial, antioxidant, diuretic, analgesic, and antiseptic properties | [57] |
| Oxygenated Monoterpenes | Limonene | 19.54 |  | Leaf | Antimicrobial, antioxidant, diuretic, analgesic, and antiseptic properties | [57] | |
| M. suaveolens L. | Terpenoids | Carvone | 64.31 | 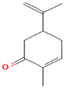 | Leaf | Antimicrobial, antioxidant, diuretic, analgesic, and antiseptic properties | [57] |
| Monoterpenoid | Myrcenol | 5.88 | 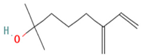 | Leaf | Antioxidants, antifungal, and flavoring agents | [48] | |
| Terpineol | 5.61 |  | Leaf | Antimutagenic potency | [62] | ||
| Pulegone | 3.81 | 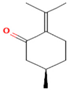 | Whole plant | Antibacterial and antifungal properties | [63] | ||
| Oxygenated Monoterpenes | Limonene | 1.24 | 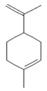 | Leaf | Antidiabetic, antioxidant, and antibacterial properties | [64] | |
| M. aquatica L. | Monoterpene | Pulegone | 39.36 | 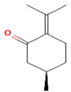 | Leaves | Antioxidant and antibacterial properties | [65] |
| Menthone | 27.69 | 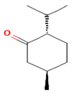 | Leaves | Antioxidant and antibacterial properties | [65] | ||
| M. virdis L. | Oxygenated Monoterpenes | Carvone | 37.26 | 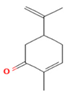 | Leaves | Antioxidant, antidiabetic, dermatoprotective, antidermatophyte, and antibacterial properties | [3] |
| 1.8-Cineole | 11.82 | 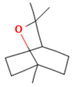 | Leaves | Antioxidant, antidiabetic, dermatoprotective, antidermatophyte, and antibacterial properties | [3] | ||
| Terpinen-4-ol | 08.72 | 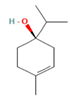 | Leaves | Antioxidant, antidiabetic, dermatoprotective, antidermatophyte, and antibacterial properties | [3] |
| Species Name | Sample Used | Microorganisms | Activities | References |
|---|---|---|---|---|
| M. aquatica L. | Essential oil | Staphylococcus aureus, Escherichia coli, Bacillus sp., and Candida sp. | Showed activity against S. aureus, E. coli, and Bacillus sp., but no result against Candida sp. | [65] |
| M. arvensis L. | Ethanol extract | Acinetobacter baumannii | Results showed 34.5 mm inhibition use at 100 μg/mL | [85] |
| M. ervine L. | Essential oil, pulegone, isomenthone and menthone was used | S. aureus, S. caprae, Enterococcus faecalis, E. faecium, E. hirae, E. coli, Salmonella braenderup, S. typhimurium, S. choleraesuis, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, and Listeria monocytogenes strains | Showed variation in activity against Gram positive and Gram-negative strains, but overall inhibition activity was significant as compared to control | [86] |
| M. arvensis var. piperascens Malinv. ex Holmes | Essential oil | Salmonella enteritidis, E. coli, Clostridium Perfringens, Campylobacter jejuni, and Salmonella species | Significant antibacterial activity against all strains specifically Salmonella species and C. jejuni (inhibition zones more than 40 mm) | [87] |
| M. × piperita L. | Essential oil | Aeromonas spp. | High antibacterial activity was shown by the essential oils, ranging from 1.250 to 16.67 μL/mL | [88] |
| M. longifolia L. | Essential oils, Menthone, carvone Menthol, and piperitenone oxide | S. aureus, E. coli, B. subtilis, Aspergillus flavus, Alternaria solani, Aspergillus niger, Alternaria altarnata, Rhizopus solani, Fusarium solani, and Rhizopus sp. | Among these, menthol showed high antimicrobial activity, with the inhibition zone ranging from 19–33 mm | [82] |
| M. itrate Ehrh. | Essential oils | E. coli, K. pneumonia, Salmonella typhimurium, Staphylococcus epidermidis, Streptococcus mutans, P. aeruginosa, and S. aureus | Average activity was recorded from between 10–15 mm inhibition zone against different strains, but essential oil showed an inhibition zone of more than 15 mm against S. epidermidis | [89] |
| M. haplocalyx Briq. | Methanol extract | E. coli | Potential activity against E. coli | [90] |
| M. pulegium L. | Essential oil | S. aureus | Significant activity was observed as compared to control | [91] |
| M. requienii Bentham | Essential oil | Aspergillus fumigatus, C. albicans, Fusarium spp., and Rhodotorula sp. | Revealed sufficient inhibition against molds and yeasts, ranging from 40 mm to 70 mm | [92] |
| Species Name | Compounds | Cardioprotecting Effect | References |
|---|---|---|---|
| Mentha arvensis L. | Phenolic compounds, Menthol | Ischemic heart disease | [105] |
| Mentha x piperita L. | Aqueous extract | Antiinflammatory | [100] |
| Mentha pulegium L. | Phenolic compounds, Quercitin | Cardioprotective effects | [106] |
| Mentha aquatica L. | Menthofuran | Antiinflammatory | [107] |
| D-carvone | Decrease toxicity | [118] | |
| Mentha canadensis L. | Menthol | Reduce lipid peroxidation | [119] |
| Menthone | Reversible cardiac depression | [119] | |
| Pulegone | Antiinflammatory effect | [108] | |
| Mentha cardiaca J. Gerard ex Baker | Carvone | Decrease toxicity | [118] |
| Limonene | Antiarrhythmic effects | [109] | |
| Mentha cervina L. | Pulegone | Antiinflammatory effect | [108] |
| Limonene | Antiarrhythmic properties | [109] | |
| Mentha diemenica Spreng. | Pulegone | Antiinflammatory | [108] |
| Menthone | Reversible cardiac depression | [119] | |
| Mentha longifolia L. | Menthone | Reduce cardiac depression | [119] |
| Pulegone | Antiinflammatory | [108] | |
| Piperitone | Induce changes in mean aortic pressure and heart rate | [110] | |
| Mentha pulegium L. | Pulegone | Suppress the NLRP3 inflammasome | [120] |
| Piperitone | Normalize heart rate | [110] | |
| Mentha spicata L. | Carvone | Antioxidant | [121] |
| Cis-carveol | Antihypersensitive, Antioxidant | [122] | |
| Piperitenone | Antiinflammatory | [111] | |
| Limonene | Antiarrhythmic properties | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. https://doi.org/10.3390/molecules27196728
Saqib S, Ullah F, Naeem M, Younas M, Ayaz A, Ali S, Zaman W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules. 2022; 27(19):6728. https://doi.org/10.3390/molecules27196728
Chicago/Turabian StyleSaqib, Saddam, Fazal Ullah, Muhammad Naeem, Muhammad Younas, Asma Ayaz, Sajid Ali, and Wajid Zaman. 2022. "Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases" Molecules 27, no. 19: 6728. https://doi.org/10.3390/molecules27196728
APA StyleSaqib, S., Ullah, F., Naeem, M., Younas, M., Ayaz, A., Ali, S., & Zaman, W. (2022). Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules, 27(19), 6728. https://doi.org/10.3390/molecules27196728