Phytochemistry, Bioactivities of Metabolites, and Traditional Uses of Fagopyrum tataricum
Abstract
1. Introduction
2. Flavonoids
3. Phenolic Acids
4. Tartary Buckwheat Flour Products
5. Tartary Buckwheat Groats
6. Green Parts of Tartary Buckwheat Plants
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohnishi, O. Search for the wild ancestor of buckwheat—III. The wild ancestor of cultivated common buckwheat and of Tatary buckwheat. Econ. Bot. 1998, 52, 123–133. [Google Scholar] [CrossRef]
- Ohnishi, O. Buckwheat in the Himalayan Hills. In Ethnobotany of Buckwheat; Kreft, I., Chang, K.J., Choi, Y.S., Park, C.H., Eds.; Jinsol Publishing Co.: Seoul, South Korea, 2003; pp. 21–33. [Google Scholar]
- Ohnishi, O. The origin of cultivated buckwheat in Mankang district of the Sanjiang area of Eastern Tibet and its diffusion to India and the Himalayan hills. Folia Biol. Geol. 2020, 61, 7–15. [Google Scholar] [CrossRef]
- Smrkolj, P.; Stibilj, V.; Kreft, I.; Germ, M. Selenium species in buckwheat cultivated with foliar addition of Se (VI) and various levels of UV-B radiation. Food Chem. 2006, 96, 675–681. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Košir, I.J.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercetin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B radiation affects flavonoids and fungal colonisation in Fagopyrum esculentum and F. tataricum. Open Life Sci. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Couch, J.F.; Naghski, J.; Krewson, C.F. Buckwheat as a source of rutin. Science 1946, 103, 197–198. [Google Scholar] [CrossRef]
- Vombergar, B.; Luthar, Z. The concentration of flavonoids, tannins and crude proteins in grain fractions of common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum Gaertn.). Folia Biol. Geol. 2018, 59, 101–157. [Google Scholar] [CrossRef]
- Tavčar Benković, E.; Kreft, S. Fagopyrins and protofagopyrins: Detection, analysis and potential phototoxicity in buckwheat. J. Agric. Food Chem. 2015, 63, 5715–5724. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Rai, M. Possible ways of fagopyrin biosynthesis and production in buckwheat plants. Fitoterapia 2013, 84, 72–79. [Google Scholar] [CrossRef]
- Kočevar Glavač, N.; Stojilkovski, K.; Kreft, S.; Park, C.H.; Kreft, I. Determination of fagopyrins, rutin and quercetin in Tartary buckwheat products. LWT—Food Sci. Technol. 2017, 79, 423–427. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, Z.; Ding, M.; Logacheva, M.D.; Kreft, I.; Wang, D.; Yan, M.; Shao, J.; Tang, Y.; Wu, Y.; et al. FtSAD2 and FtJAZ1 regulate activity of the FtMYB11 transcription repressor of the phenylpropanoid pathway in Fagopyrum tataricum. New Phytol. 2017, 216, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Bonafaccia, F.; Luthar, Z. Impact of rutin and other phenolic substances on the digestibility of buckwheat grain metabolites. Int. J. Mol. Sci. 2022, 23, 3923. [Google Scholar] [CrossRef]
- Kreft, I. Bitter Seed Tartary Buckwheat; Slovenian Academy of Sciences and Arts: Ljubljana, Slovenia; Fagopyrum—Slovenian Association for Buckwheat Promotion: Maribor, Slovenia, 2022; p. 119. [Google Scholar]
- Janeš, D.; Prosen, H.; Kreft, S. Identification and quantification of aroma compounds of tartary buckwheat (Fagopyrum tataricum Gaertn.) and some of its milling fractions. J. Food Sci. 2012, 77, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, P.; Zhou, J.; He, J.; Cai, J. The improvement of sensory and bioactive properties of yogurt with the introduction of Tartary buckwheat. Foods 2022, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Lukšič, L.; Árvay, J.; Vollmannová, A.; Tóth, T.; Škrabanja, V.; Trček, J.; Germ, M.; Kreft, I. Hydrothermal treatment of Tartary buckwheat grain hinders the transformation of rutin to quercetin. J. Cereal Sci. 2016, 72, 131–134. [Google Scholar] [CrossRef]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef]
- Kreft, I.; Pongrac, P.; Zhou, M.; Vogel-Mikuš, K.; Pelicon, P.; Vavpetič, P.; Nečemer, M.; Elteren, J.T.V.; Regvar, M.; Likar, M.; et al. New insights into structures and composition of plant food materials. J. Microbiol. Biotech. Food Sci. 2017, 7, 57–61. [Google Scholar] [CrossRef]
- Janovská, D.; Jágr, M.; Svoboda, P.; Dvořáček, V.; Meglič, V.; Hlásná Čepková, P. Breeding buckwheat for nutritional quality in the Czech Republic. Plants 2021, 10, 1262. [Google Scholar] [CrossRef]
- Hornyák, M.; Dziurka, M.; Kula-Maximenko, M.; Pastuszak, J.; Szczerba, A.; Szklarczyk, M.; Płazek, A. Photosynthetic efficiency, growth and secondary metabolism of common buckwheat (Fagopyrum esculentum Moench) in different controlled-environment production systems. Sci. Rep. 2022, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Jeromel, L.; Siketič, Z.; Ogrinc-Potočnik, N.; Vavpetič, P.; Rupnik, Z.; Bučar, K.; Pelicon, P. Development of mass spectrometry by high energy focused heavy ion: MeV-SIMS with 8 MeV Cl7+ beam. Nucl. Instrum. Methods Phys. Res. Sect. B 2014, 332, 22–27. [Google Scholar] [CrossRef]
- Jenčič, B.; Jeromel, L.; Ogrinc-Potočnik, N.; Vogel-Mikuš, K.; Kovačec, E.; Regvar, M.; Siketič, Z.; Vavpetič, P.; Rupnik, Z.; Bučar, K.; et al. Molecular imaging of cannabis leaf tissue with MeV-SIMS method. Nucl. Instrum. Methods Phys. Res. Sect. B 2016, 371, 205–210. [Google Scholar] [CrossRef]
- Jenčič, B.; Vavpetič, P.; Kelemen, M.; Vencelj, M.; Vogel-Mikuš, K.; Kavčič, A.; Pelicon, P. MeV-SIMS TOF imaging of organic tissue with continuous primary beam. J. Am. Soc. Mass Spectrom. 2019, 30, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Hwang, K.T. Determination and photochemical conversion of protofagopyrins and fagopyrins in buckwheat plants. J. Food Compos. Anal. 2021, 100, 103894. [Google Scholar] [CrossRef]
- Shi, Y.; Chao, L.; Mei, L.Z.; Chen, Z.; Li, X.; Miaoa, M. Soluble tetraaminophthalocyanines indium functionalized graphene platforms for rapid and ultra-sensitive determination of rutin in Tartary buckwheat tea. Food Control 2022, 132, 108550. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Rolta, R.; Yadav, R.; Salaria, D.; Sourirajan, A.; Dev, K. In silico screening of hundred phytocompounds of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: An approach to prevent virus assembly. J. Biomol. Struct. Dyn. 2020, 39, 7017–7034. [Google Scholar] [CrossRef]
- Qu, X.B.; Su, Z.M.; Hu, D.H.; Bao, Y.L.; Meng, X.Y.; Wu, Y.; Li, Y.X. Studies on molecular structure of hypericin and its interactions with HIV-1 protease by molecular modeling. Chem. J. Chin. Univ. 2009, 30, 1402–1405. [Google Scholar]
- Vogrinčič, M.; Kreft, I.; Filipič, M.; Žegura, B. Antigenotoxic effect of tartary (Fagopyrum tataricum) and common (Fagopyrum esculentum) buckwheat flour. J. Med. Food 2013, 16, 944–952. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Q.; Ma, S.; Zhou, X. Effect of quercetin on the in vitro Tartary buckwheat starch digestibility. Int. J. Biol. Macromol. 2021, 183, 818–830. [Google Scholar] [CrossRef]
- Wu, X.; Fu, G.; Xu, Z.; Dong, B.; Li, R.; Wan, Y.; Jiang, G.; Liu, C. In vitro nutrition properties of whole Tartary buckwheat straight noodles and its amelioration on type 2 diabetic rats. Food Biosci. 2022, 46, 101525. [Google Scholar] [CrossRef]
- Hosaka, T.; Nii, Y.; Tomotake, H.; Ito, T.; Tamanaha, A.; Yamasaka, Y.; Sasaga, S.; Edazawa, K.; Tsutsumi, R.; Shuto, E. Extracts of common buckwheat bran prevent sucrose digestion. J. Nutr. Sci. Vitaminol. 2011, 57, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, J.; Liu, X.; Geng, F.; Huang, Q.; Zhao, J.; Xiang, D.; Zhao, G. Analysis of tartary buckwheat (Fagopyrum tataricum) seed proteome using offline two-dimensional liquid chromatography and tandem mass spectrometry. J. Food Biochem. 2019, 43, e12863. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Wieslander, G. A Review on epidemiological and clinical studies on buckwheat Allergy. Plants 2021, 10, 607. [Google Scholar] [CrossRef]
- Wieslander, G.; Fabjan, N.; Vogrincic, M.; Kreft, I.; Janson, C.; Spetz-Nyström, U.; Vombergar, B.; Tagesson, C.; Leanderson, P.; Norbäck, D. Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day-care centre staffs. Tohoku J. Exp. Med. 2011, 225, 123–130. [Google Scholar] [CrossRef]
- Wieslander, G.; Fabjan, N.; Vogrinčič, M.; Kreft, I.; Vombergar, B.; Norbäck, D. Effects of common and Tartary buckwheat consumption on mucosal symptoms, headache and tiredness: A double-blind crossover intervention study. J. Food Agric. Environ. 2012, 10, 107–110. [Google Scholar]
- He, J.; Klag, M.J.; Whelton, P.K.; Mo, J.P.; Chen, J.Y.; Qian, M.C.; Mo, P.S.; He, G.Q. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am. J. Clin. Nutr. 1995, 61, 366–372. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, Z.; Liang, C.; Liao, K.; Xiang, D.; Huang, J.; Wei, C.; Shi, T.; Chen, Q. Agronomic and metabolomics analysis of rice Tartary buckwheat (Fagopyrum tataricum Gaertn) bred by hybridization. Sci. Rep. 2022, 12, 11986. [Google Scholar] [CrossRef] [PubMed]
- Aubert, L.; Quinet, M. Comparison of heat and drought stress responses among twelve Tartary buckwheat (Fagopyrum tataricum) varieties. Plants 2022, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Borovaya, S.A.; Klykov, A.G. Some aspects of flavonoid biosynthesis and accumulation in buckwheat plants. Plant Biotechnol. Rep. 2020, 14, 213–225. [Google Scholar] [CrossRef]
- Matsui, K.; Walker, A.R. Biosynthesis and regulation of flavonoids in buckwheat. Breed. Sci. 2019, 70, 74–84. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.A.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Maccati, F.; Galli, F. Dietary Fiber and Phenolic Compounds in Common and Tartary Buckwheat. In Proceedings of the International Symposium of Buckwheat Sprouts—Developement and Utilization of Buckwheat as Medicinal Natural Products, Bongpyoung, South Korea, 7–9 September 2009; pp. 16–19. [Google Scholar]
- Ghimeray, A.K.; Sharma, P.; Briatia, X. Phenolic Content and Free Radical Scavenging Activity of Seed, Seedling and Sprout of Buckwheat. In Proceedings of the International Symposium of Buckwheat Sprouts—Developement and Utilization of Buckwheat as Medicinal Natural Products, Bongpyoung, South Korea, 7–9 September 2009; pp. 41–45. [Google Scholar]
- Lahanov, A.P.; Muzalevskaja, R.S.; Shelepina, N.V.; Gorkova, I.V. Biochemical Characteristics of Some Species of Genus Fagopyrum Mill. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2022; Faberová, I., Dvořáček, V., Čepková, P., Hon, I., Holubec, V., Stehno, Z., Eds.; Research Institute of Crop Production: Prague, Czech Republic, 2004; pp. 604–611. [Google Scholar]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.A.; Briggs, C.J. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum and F. homotropicum and their protective effects against lipid peroxidation. Food. Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Zielińska, D.; Turemko, M.; Kwiatkowski, J.; Zieliński, H. Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and Tartary buckwheat plants. Molecules 2012, 17, 9668–9682. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, C.; Zhao, H.; Zhao, J.; Chen, H.; Bu, T.; Anhu, W.; Wu, Q. Deep sequencing of the transcriptome reveals distinct flavonoid metabolism features of black tartary buckwheat (Fagopyrum tataricum Garetn.). Prog. Biophys. Mol. Biol. 2017, 124, 49–60. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Yang, Q.; Gong, X.; Ma, H.; Dang, K.; Chen, G.; Gao, X.; Fenf, B. Analysis of flavonoid metabolites in buckwheat leaves using UPLC-ESI-MS/MS. Molecules 2019, 24, 1310. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Kim, S.-J.; Park, S.U.; Woo, S.H.; Noda, T.; Takigawa, S. Physiological roles of rutin in the buckwheat plant. Jpn. Agric. Res. 2015, 49, 37–43. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Characterization of rutin-rich bread made with ‘Manten-Kirari’, a trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Sci. Technol. Res. 2015, 21, 733–738. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K. Acute and subacute toxicity studies on rutin-rich Tartary buckwheat dough in experimental animals. J. Nutr. Sci. Vitaminol. 2015, 61, 175–181. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K.; Otsuka, S.; Katsu, K. Breeding of buckwheat to reduce bitterness and rutin hydrolysis. Plants 2021, 10, 791. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K.; Otsuka, S. Development of Novel Detection Method for Rutinosidase in Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Plants 2022, 11, 320. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Golob, A.; Luthar, Z.; Kreft, I. The temperature threshold for the transformation of rutin to quercetin in Tartary buckwheat dough. Food Chem. 2019, 283, 28–31. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Mehany, A.B.M.; Belal, A.; Santali, E.Y.; Shaaban, S.; Abourehab, M.A.S.; El-Feky, O.A.; Diab, M.; Galala, F.M.A.A.; Elkaeed, E.B.; Abdelhamid, G. Biological effect of quercetin in repairing brain damage and cerebral changes in rats: Molecular docking and in vivo studies. BioMed Res. Int. 2022, 2022, 8962149. [Google Scholar] [CrossRef]
- Kreft, S.; Štrukelj, B.; Gaberščik, A.; Kreft, I. Rutin in buckwheat herbs grown at diferent UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002, 53, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Honda, Y.; Mukasa, Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in Tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005, 168, 1303–1307. [Google Scholar] [CrossRef]
- Gfeller, A.; Glauser, G.; Etter, C.; Signarbieux, C.; Wirth, J. Fagopyrum esculentum alters its root exudation after Amaranthus retroflexus recognition and suppresses weed growth. Front. Plant Sci. 2018, 9, 50. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Honda, Y.; Mukasa, Y.; Kim, S. Effects of lipase, lipoxygenase, peroxidase and rutin on quality deteriorations in buckwheat flour. J. Agric. Food Chem. 2005, 53, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Nakagawa, H. Purification and characterization of the rutin-degrading enzymes in Tartary buckwheat seeds. Phytochemistry 1994, 37, 133–136. [Google Scholar] [CrossRef]
- Fujita, K.; Yoshihashi, T. Heat-treatment of Tartary buckwheat (Fagopyrum tataricum Gaertn.) provides dehulled and gelatinized product with denatured rutinosidase. Food Sci. Technol. Res. 2019, 25, 613–618. [Google Scholar] [CrossRef]
- Fabjan, N. Zel in Zrnje Tatarske Ajde (Fagopyrum tataricum Gaertn.) Kot Vir Flavonoidov. Ph.D. Thesis, Biotechnical Faculty, Ljubljana, Slovenia, 2007; p. 104. [Google Scholar]
- Kitabayashi, H.; Ujihara, A.; Hirose, T.; Minami, M. On the genotypic differences for rutin content in tartary buckwheat Fagopyrum tataricum Gaertn. Breed. Sci. 1995, 45, 189–194. [Google Scholar] [CrossRef][Green Version]
- Briggs, C.J.; Campbell, C.; Pierce, G.; Jiang, P. Bioflavonoid Analysis and Antioxidant Properties of Tartary Buckwheat Accessions. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2022; Faberová, I., Dvořáček, V., Čepková, P., Hon, I., Holubec, V., Stehno, Z., Eds.; Research Institute of Crop Production: Prague, Czech Republic, 2004; pp. 593–597. [Google Scholar]
- Park, B.J.; Park, J.I.; Chang, K.J.; Park, C.H. Comparison in Rutin Content in Seed and Plant of Tartary Buckwheat (Fagopyrum tataricum). In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2022; Faberová, I., Dvořáček, V., Čepková, P., Hon, I., Holubec, V., Stehno, Z., Eds.; Research Institute of Crop Production: Prague, Czech Republic, 2004; pp. 626–629. [Google Scholar]
- Yan, C.; Baili, F.; Yingang, H.; Jinfeng, G.; Xiaoli, G. Analysis on the Variation of Rutin Vontent in Different Buckwheat Genotypes. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2022; Faberová, I., Dvořáček, V., Čepková, P., Hon, I., Holubec, V., Stehno, Z., Eds.; Research Institute of Crop Production: Prague, Czech Republic, 2004; pp. 688–691. [Google Scholar]
- Buratti, S.; Giovanelli, G.; Benedetti, S.; Marti, A. Impact of gelatinization on common (Fagopyrum esculentum) and Tartary (Fagopyrum tataricum) buckwheat: Effect on taste and flavor assessed by e-senses in relation to phenolic compounds. Eur. Food Res. Technol. 2022, 248, 2521–2530. [Google Scholar] [CrossRef]
- Suzuki, T.; Kim, S.J.; Yamauchi, H.; Takigawa, S.; Honda, Y.; Mukasa, Y. Characterization of flavonoid 3-O-glucosyltransferase and its activity during cotyledon growth in buckwheat (Fagopyrum esculentum). Plant Sci. 2005, 169, 943–948. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X. Determination of rutin content on chinese buckwheat cultivars. In Proceedings of the 10th International Symposium on Buckwheat, Yangling, China, 14–18 August 2007; Chai, Y., Zhang, Z., Eds.; Northwest A & F University Press: Yangling, China, 2007; pp. 465–468. [Google Scholar]
- Morishita, T.; Yamaguchi, H.Y.; Degi, K. The contribution of polyphenols to antioxidative activity in common buckwheat and tartary buckwheat grain. Plant Prod. Sci. 2007, 10, 99–104. [Google Scholar] [CrossRef]
- Kim, S.J.; Zaidul, I.S.M.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and Tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Sulewska, H.; Kreft, I.; Gujska, E.; Walkowiak, J. The content of dietary fibre and polyphenols in morphological parts of buckwheat (Fagopyrum tataricum). Plant Foods Hum. Nutr. 2018, 73, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Vombergar, B.; Škrabanja, V.; Luthar, Z.; Germ, M. Izhodišča za raziskave učinkov flavonoidov, taninov in skupnih beljakovin v frakcijah zrn navadne ajde (Fagopyrum esculentum Moench) in tatarske ajde (Fagopyrum tataricum Gaertn.). Folia Biol. Geol. 2017, 58, 101–145. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Evaluation of the Mutagenicity Potential of Trace-Rutinosidase Variety of Tartary Buckwheat (Fagopyrum tataricum Gaertn.) Using the Ames Test. J. Agric. Chem. Environ. 2016, 5, 100–105. [Google Scholar] [CrossRef][Green Version]
- Vogrinčič, M. Primerjava Biotskih Učinkov Rutina in Kvercetina Iz Navadne (Fagopyrum esculentum Moench) in Tatarske (Fagopyrum tataricum Gaertn.) Ajde. Ph.D. Thesis, Biotechnical Faculty, Ljubljana, Slovenia, 2013; p. 70. [Google Scholar]
- Bhinder, S.; Singh, N.; Kaur, A. Impact of germination on nutraceutical, functional and gluten free muffin making properties of Tartary buckwheat (Fagopyrum tataricum). Food Hydrocoll. 2022, 124, 107268. [Google Scholar] [CrossRef]
- Ren, R.; Zeng, H.; Mei, Q.; Xu, Z.; Mazhar, M.; Qin, L. Effects of Monascus purpureus-fermented Tartary buckwheat extract on the blood lipid profile, glucose tolerance and antioxidant enzyme activities in KM mice. J. Cereal Sci. 2022, 105, 103465. [Google Scholar] [CrossRef]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive compounds in different buckwheat species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Kreft, I.; Golob, A. Hydrothermal treatments affecting the concentration of neochlorogenic acid in dough of Tartary buckwheat. Agriculture 2020, 10, 601. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef]
- Thurow, T. Effect of Chlorogenic Acid and Neochlorogenic Acid on Human Colon Cancer Cells. Ph.D. Thesis, University of Arkansas, Fayetteville, NC, USA, 2012; p. 32. [Google Scholar]
- Valvasor, J.V. Die Ehre dess Hertzogthums Crain, das Ist, Wahre, Gründliche und Recht Eigendliche Belegen- und Beschaffenheit Dieses; Römisch-Keyserlichen Herrlichen Erblandes: Ljubljana, Slovenia, 1689; Volume 1, p. 187. [Google Scholar]
- Costantini, L.; Lukšic, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-freebread using Tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Vombergar, B.; Kreft, I.; Horvat, M.; Vorih, S. Ajda—Buckwheat; Kmečki Glas: Ljubljana, Slovenia, 2018; p. 143. [Google Scholar]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and Tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Gambelli, L.; Fabjan, N.; Kreft, I. Trace elements in flour and bran from common and tartary buckwheat. Food Chem. 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Gao, J.; Kreft, I.; Chao, G.; Wang, Y.; Liu, W.; Wang, L.; Wang, P.; Gao, X.; Feng, B. Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chem. 2016, 190, 552–558. [Google Scholar] [CrossRef]
- Atalay, M.H.; Bılgıçlı, N.; Elgün, A.; Demır, M.K. Effects of buckwheat (Fagopyrum esculentum Moench) milling products, transglutaminase and sodium stearoyl-2-lactylate on bread properties. J. Food Process. Preserv. 2013, 37, 1–9. [Google Scholar] [CrossRef]
- Koval, D.; Plockova, M.; Kyselka, J.; Skřivan, P.; Sluková, M.; Horáčková, S. Buckwheat secondary metabolites: Potential antifungal agents. J. Agric. Food Chem. 2020, 68, 11631–11643. [Google Scholar] [CrossRef]
- Zou, S.; Wang, L.; Wang, A.; Zhang, Q.; Li, Z.; Qiu, J. Effect of moisture distribution changes induced by different cooking temperature on cooking quality and texture properties of noodles made from whole Tartary buckwheat. Foods 2021, 10, 2543. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Development of rutin-rich noodles using trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’. Food Sci. Technol. Res. 2019, 25, 915–920. [Google Scholar] [CrossRef]
- Janeš, D.; Prosen, H.; Kreft, I.; Kreft, S. Aroma compounds in buckwheat (Fagopyrum esculentum Moench) groats, flour, bran and husk. Cereal Chem. 2010, 87, 41–143. [Google Scholar] [CrossRef]
- Skrabanja, V.; Kreft, I. Resistant Starch Formation Following Autoclaving of Buckwheat (Fagopyrum esculentum Moench) Groats. An In Vitro Study. J. Agric. Food Chem. 1998, 46, 2020–2023. [Google Scholar] [CrossRef]
- Škrabanja, V.; Laerke, H.; Kreft, I. Effects of hydrothermal processing of buckwheat (Fagopyrum esculentum Moench) groats on starch enzymatic availability in vitro and in vivo in rats. J. Cereal Sci. 1998, 28, 209–214. [Google Scholar] [CrossRef]
- Škrabanja, V.; Liljeberg Elmståhl, H.G.M.; Kreft, I.; Björck, I.M.E. Nutritional properties of starch in buckwheat products: Studies in vitro and in vivo. J. Agric. Food Chem. 2001, 49, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Ishiguro, K.; Suzuki, T.; Morishita, T. Physicochemical properties and in vitro digestibility of starch from a trace-rutinosidase variety of Tartary buckwheat ‘Manten-Kirari’. Molecules 2022, 27, 6172. [Google Scholar] [CrossRef]
- Ikeda, K.; Ishida, Y.; Ikeda, S.; Asami, Y.; Lin, R. Tartary, but not common, buckwheat inhibits α-glucosidase activity: Its nutritional implications. Fagopyrum 2017, 34, 13–18. [Google Scholar] [CrossRef]
- Kreft, I. Grenko Seme Tatarske Ajde; SAZU: Ljubljana, Slovenia, 2020; p. 79. [Google Scholar]
- Eguchi, K.; Anase, T.; Osuga, H. Development of a high-performance liquid chromatography method to determine the fagopyrin content of Tartary buckwheat (Fagopyrum tartaricum Gaertn.) and common buckwheat (F. esculentum Moench). Plant Prod. Sci. 2009, 12, 475–480. [Google Scholar] [CrossRef]
- Benkovič, E.T.; Žigon, D.; Friedrich, M.; Plavec, J.; Kreft, S. Isolation, analysis and structures of phototoxic fagopyrins from buckwheat. Food Chem. 2014, 143, 432–439. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, K.T. Fagopyrins in different parts of common buckwheat (Fagopyrum esculentum) and Tartary buckwheat (F. tataricum) during growth. J. Food Compos. Anal. 2020, 86, 103354. [Google Scholar] [CrossRef]
- Szymański, S.; Majerz, I. Theoretical studies on the structure and intramolecular interactions of fagopyrins—Natural photosensitizers of Fagopyrum. Molecules 2022, 27, 3689. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Sytar, O.; Svediene, J.; Loziene, K.; Paskevicius, A.; Kosyan, A.; Taran, N. Antifungal properties of hypericin, hypericin tetrasulphonic acid and fagopyrin on pathogenic fungi and Spoilage yeasts. Pharm. Biol. 2016, 54, 3121–3125. [Google Scholar] [CrossRef]
- Yin, Q.; Han, X.; Han, Z.; Chen, Q.; Shi, Y.; Gao, H.; Zhang, T.; Dong, G.; Xiong, C.; Song, C.; et al. Genome-wide analyses reveals a glucosyltransferase involved in rutin and emodin glucoside biosynthesis in tartary buckwheat. Food Chem. 2020, 318, 126478. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, J.B.; Hu, L.X.; Zhao, J.L.; Xiang, D.B.; Zou, L.; Gang Zhao, G. Rapid and simple method for the determination of emodin in Tartary buckwheat (Fagopyrum tataricum) by high-performance liquid chromatography coupled to a diode array detector. J. Agric. Food Chem. 2013, 61, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Vombergar, B.; Škrabanja, V.; Germ, M. Flavonoid concentration in milling fractions of Tartary and common buckwheat. Fagopyrum 2020, 37, 11–21. [Google Scholar] [CrossRef]
- Ghosh, N.; Saha, I.; Sharma, N.; Sarkar, J.P. Human miRNAs to identify potential regions of SARS-CoV-2. ACS Omega 2022, 7, 21086–21101. [Google Scholar] [CrossRef] [PubMed]
- Park, M.O.; Kim, H.J.; Choi, I.Y.; Park, C.H. Development and utilization of buckwheat sprouts in Korea. Fagopyrum 2022, 39, 19–26. [Google Scholar] [CrossRef]
- Germ, M.; Vollmannova, A.; Timoracka, M.; Melichacova, S.; Stibilj, V.; Vogrinčič, M.; Kreft, I. Antioxidative substances of Tartary buckwheat sprouts and impact of Se and Zn on the sprout development. In Proceedings of the International Symposium of Buckwheat Sprouts—Developement and Utilization of Buckwheat as Medicinal Natural Products, Bongpyoung, South Korea, 7–9 September 2009; pp. 46–53. [Google Scholar]
- Lee, H.S.; Park, C.H.; Park, B.J.; Kwon, S.M.; Chang, K.J.; Kim, S.L. Rutin, catechin derivatives, and chemical components of Tartary buckwheat (Fagopyrum tartaricum Gaertn.) sprouts. Korean J. Crop Sci. 2006, 51, 277–282. [Google Scholar]
- Kim, S.J.; Zaidul, I.S.M.; Maeda, T.; Suzuki, T.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H.A. Time-course study of flavonoids in the sprouts of Tartary (Fagopyrum tataricum Gaertn.) buckwheats. Sci. Hortic. 2007, 115, 13–18. [Google Scholar] [CrossRef]
- Kuwabara, T.; Han, K.H.; Hashimoto, N.; Yamauchi, H.; Shimada, K.I.; Sekikawa, M.; Fukushima, M. Tartary buckwheat sprout powder lowers plasma cholesterol level in rats. J. Nutr. Sci. Vitaminol. 2007, 53, 501–507. [Google Scholar] [CrossRef]
- Nam, T.G.; Lee, S.M.; Park, J.H.; Kim, D.O.; Back, N.I.; Eom, S.H. Flavonoid analysis of buckwheat sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef]
- Kim, H.Y.; Woo, S.Y.; Seo, W.D.; Lee, M.J. Changes of antioxidant activity as affected by cultivation period in buckwheat (Fagopyrum species) sprouts. J. Korean Soc. Food Cult. 2020, 35, 590–596. [Google Scholar]
- Pongrac, P.; Potisek, M.; Fras, A.; Likar, M.; Budič, B.; Myszka, K.; Boros, D.; Nečemer, M.; Kelemen, M.; Vavpetič, P.; et al. Composition of mineral elements and bioactive compounds in Tartary buckwheat and wheat sprouts as affected by natural mineral-rich water. J. Cereal Sci. 2016, 69, 9–16. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Potisek, M.; Kovačec, E.; Budič, B.; Kump, P.; Regvar, M.; Kreft, I. Mineral and trace element composition and importance for nutritional value of buckwheat grain, groats, and sprouts. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: London, UK, 2016; pp. 261–271. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kang, M.J.; Park, J.I.; Yang, J.Y.; Kim, G.D. Effect of LED light strength for enhancing rutin content in Tartary buckwheat sprouts and antioxidant activity. J. Life Sci. 2018, 28, 977–984. [Google Scholar]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effect of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sim, U.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Effect of calcium chloride and sucrose on the composition of bioactive compounds and antioxidant activities in buckwheat sprouts. Food Chem. 2020, 312, 126075. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qian, G.; Chen, Y.; Liu, T.; Wan, H.; Wang, S.; Meng, X.; Chen, W.; Atia-tul-Wahab; Su, Y.; et al. Profiling of polyphenols for in-depth understanding of Tartary buckwheat sprouts: Correlation between cultivars and active components, dynamic changes and the effects of ultraviolet B stress. Food Chem. 2022, 14, 100295. [Google Scholar] [CrossRef] [PubMed]


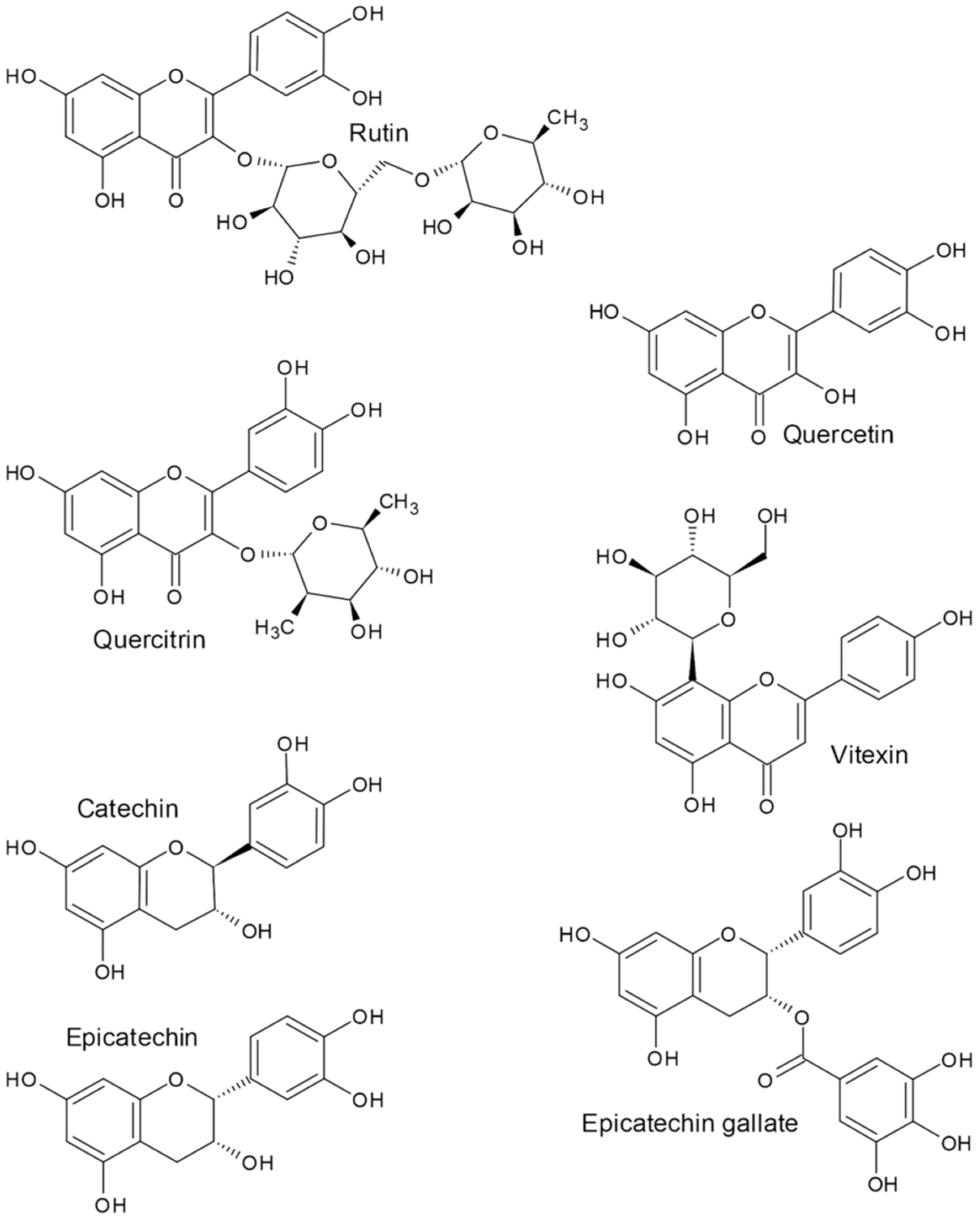
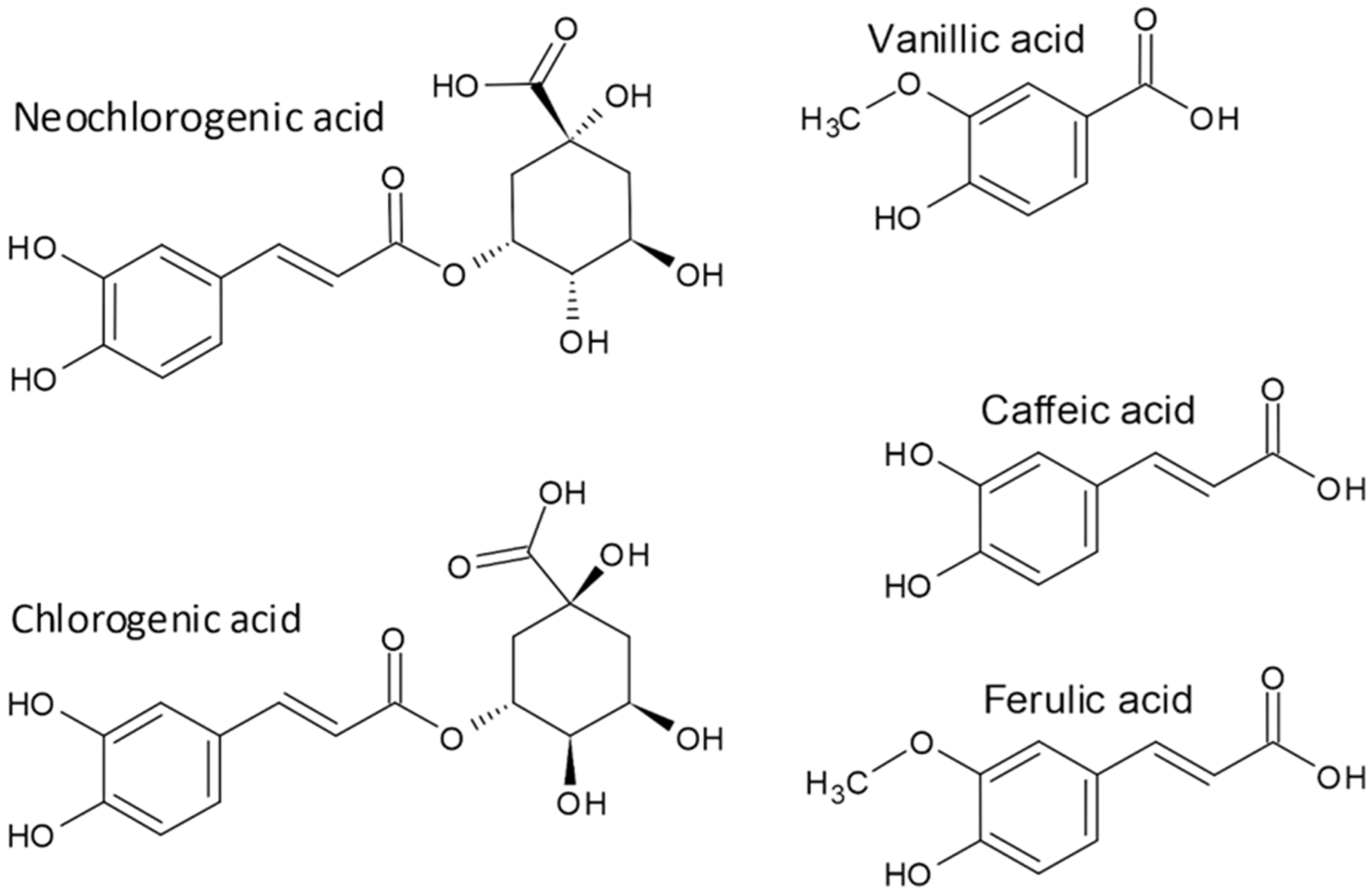


| Compounds Name | Content in Dry Weight | References | ||||
|---|---|---|---|---|---|---|
| Roots | Stem | Leaves | Flowers | Seeds | ||
| Total polyphenols (mg/g) | 19.76 | / | 32.51 | / | / | [49] |
| Total polyphenols (µg/mg) | / | 96.5–109.8 | / | / | 208 | [50] |
| Total flavonoids (mg/g) | / | 17 | 100 | 160 | / | [51] |
| Total flavonoids (%) | / | / | / | / | 2.04 | [52] |
| Total flavonoids (µg/mg) | / | 38.1 | / | / | 142.2 | [50] |
| Total flavonoids (mg/g) | / | / | 76.40 | 145.4 | 20.24 | [53] |
| Total flavonoids YTB (mg/g) | / | / | / | 49.07 | / | [54] |
| Total flavonoids BTB (mg/g) | / | / | / | 52.81 | / | [54] |
| Total flavonoids (mg/g) | / | / | 213.66 | / | / | [55] |
| Compounds Name | Content in Dry Weight | References | ||||
|---|---|---|---|---|---|---|
| Roots | Stem | Leaves | Flowers | Seeds | ||
| Rutin (mg/g) | / | / | / | / | 16.7 | [73] |
| Rutin (%) | / | / | / | / | 0.8–1.7 | [5] |
| Rutin (g/100 g) | / | / | / | / | 1.83–1.97 | [74] |
| Rutin (g/100 g) | 22.3 | 482.6 | 2876.0 | 3518.6 | 1469.8 | [75] |
| Rutin (mg/g) | / | / | / | / | 8.68–13.34 | [76] |
| Rutin (mg/g) | / | / | / | / | 7.56–8.9 | [77] |
| Rutin (mg/g) | / | / | / | / | 11.99–21.4 | [66,78] |
| Rutin (%) different varieties | / | / | / | / | 1.19–2.91 | [79] |
| Rutin (mg/g) | / | / | / | / | 16.69 | [52] |
| Rutin (mg/g) different varieties | / | / | / | / | 6.5–16.64 | [72] |
| Rutin (mg/g) | / | / | / | / | 18.08–18.53 | [80] |
| Rutin (mg/g) | / | / | / | / | 14.1 | [81] |
| Rutin (mg/g) | / | / | / | / | 11.97 | [50] |
| Rutin (%) | / | / | 6.06 % | 7.77 % | 1.35 % | [53] |
| Rutin (mg/g) | / | / | / | / | 14.1 | [82] |
| Rutin (mg/g) | 0.8 | 3.0 | 28 | 38 | 18 | [42] |
| Rutin (mg/mg) YTB | / | / | / | 35.93 | / | [54] |
| Rutin (mg/mg) BTB | / | / | / | 38.80 | / | [54] |
| Rutin (μg/g) | 1963.4 | 2949.3 | 2253.8 | / | [83] | |
| Rutin (mg/g) | 3–8 | 6–14 | / | 47–63 | / | [46] |
| Quercetin (mg/g) different varieties | / | / | / | / | 0.47–0.9 | [72] |
| Quercetin (μg/g) | 7.2 | 2.1 | 172.1 | 844.7 | / | [83] |
| Quercetin(mg/mg) YTB | / | / | / | 3.06 | / | [54] |
| Quercetin(mg/mg) BTB | / | / | / | 6.49 | / | [54] |
| Kaempferol(mg/mg) YTB | / | / | / | 0.09 | / | [54] |
| Kaempferol(mg/mg) BTB | / | / | / | 0.06 | / | [54] |
| Myricetin (mg/mg) YTB | / | / | / | 0.40 | / | [54] |
| Myricetin (mg/mg) BTB | / | / | / | 0.43 | [54] | |
| Catechin (mg/mg) | / | / | / | / | 0.12 | [84] |
| Catechin (μg/g) | 5.3 | 1.1 | 9.9 | 11.9 | / | [83] |
| Epicatechin (mg/g) | / | / | / | / | 0.04 | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Vollmannová, A.; Kreft, S.; Luthar, Z. Phytochemistry, Bioactivities of Metabolites, and Traditional Uses of Fagopyrum tataricum. Molecules 2022, 27, 7101. https://doi.org/10.3390/molecules27207101
Kreft I, Germ M, Golob A, Vombergar B, Vollmannová A, Kreft S, Luthar Z. Phytochemistry, Bioactivities of Metabolites, and Traditional Uses of Fagopyrum tataricum. Molecules. 2022; 27(20):7101. https://doi.org/10.3390/molecules27207101
Chicago/Turabian StyleKreft, Ivan, Mateja Germ, Aleksandra Golob, Blanka Vombergar, Alena Vollmannová, Samo Kreft, and Zlata Luthar. 2022. "Phytochemistry, Bioactivities of Metabolites, and Traditional Uses of Fagopyrum tataricum" Molecules 27, no. 20: 7101. https://doi.org/10.3390/molecules27207101
APA StyleKreft, I., Germ, M., Golob, A., Vombergar, B., Vollmannová, A., Kreft, S., & Luthar, Z. (2022). Phytochemistry, Bioactivities of Metabolites, and Traditional Uses of Fagopyrum tataricum. Molecules, 27(20), 7101. https://doi.org/10.3390/molecules27207101










