“Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses
Abstract
1. Introduction
2. Results
2.1. Ponderal Yields
2.2. 1H-NMR and LC-DAD-MSn Analysis
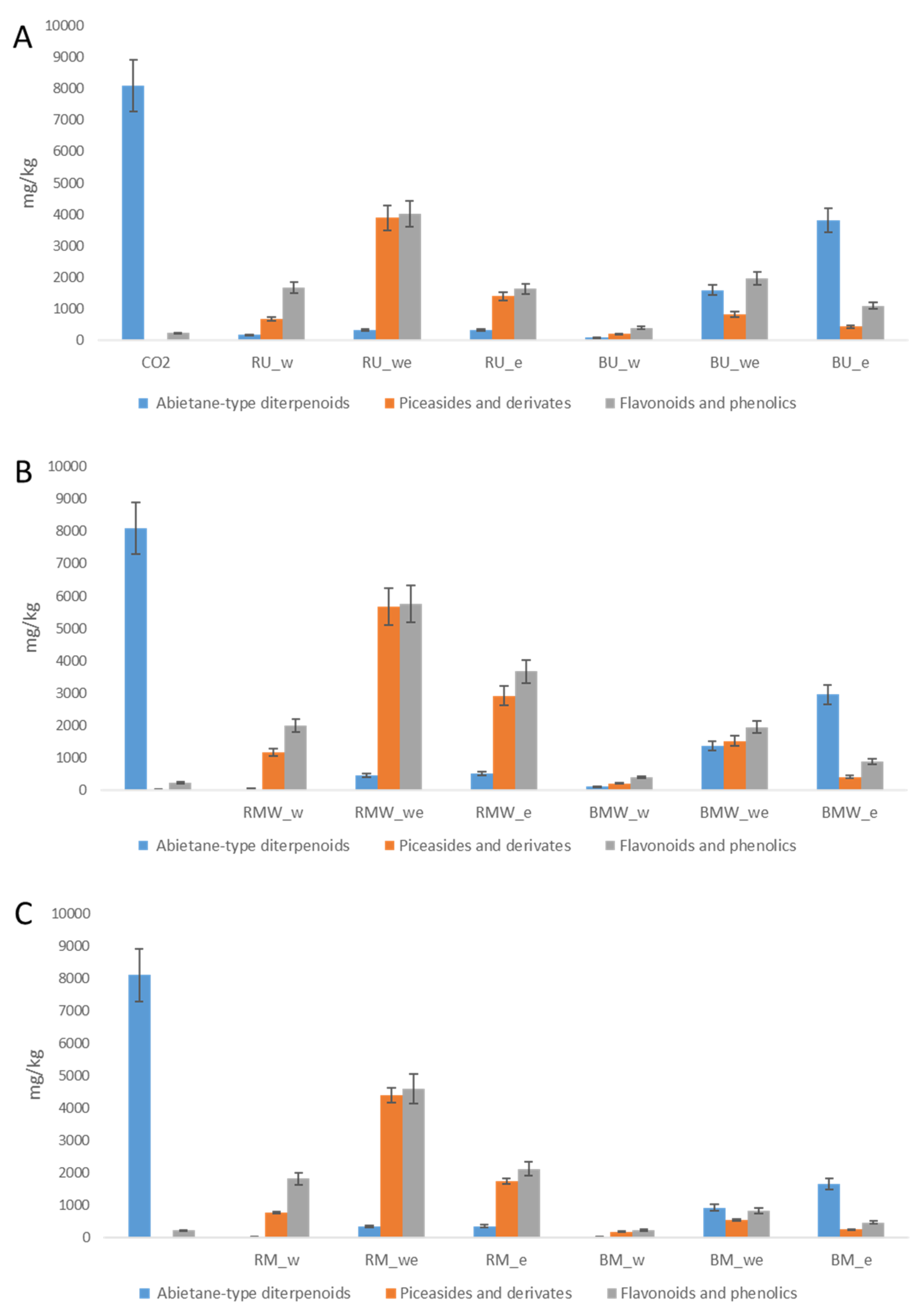
2.3. Quantitative Analysis of Secondary Metabolites in P. abies Extracts
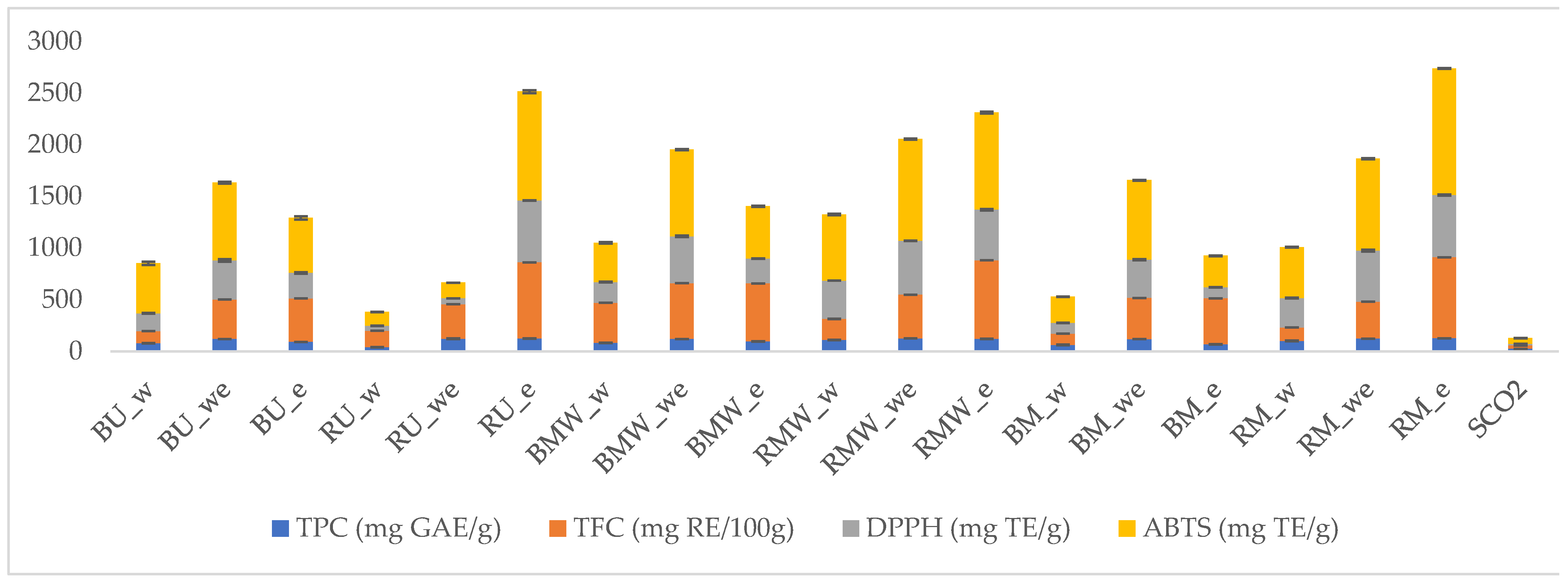
2.4. Total Phenolic and Flavonoid Content in P. abies Extracts
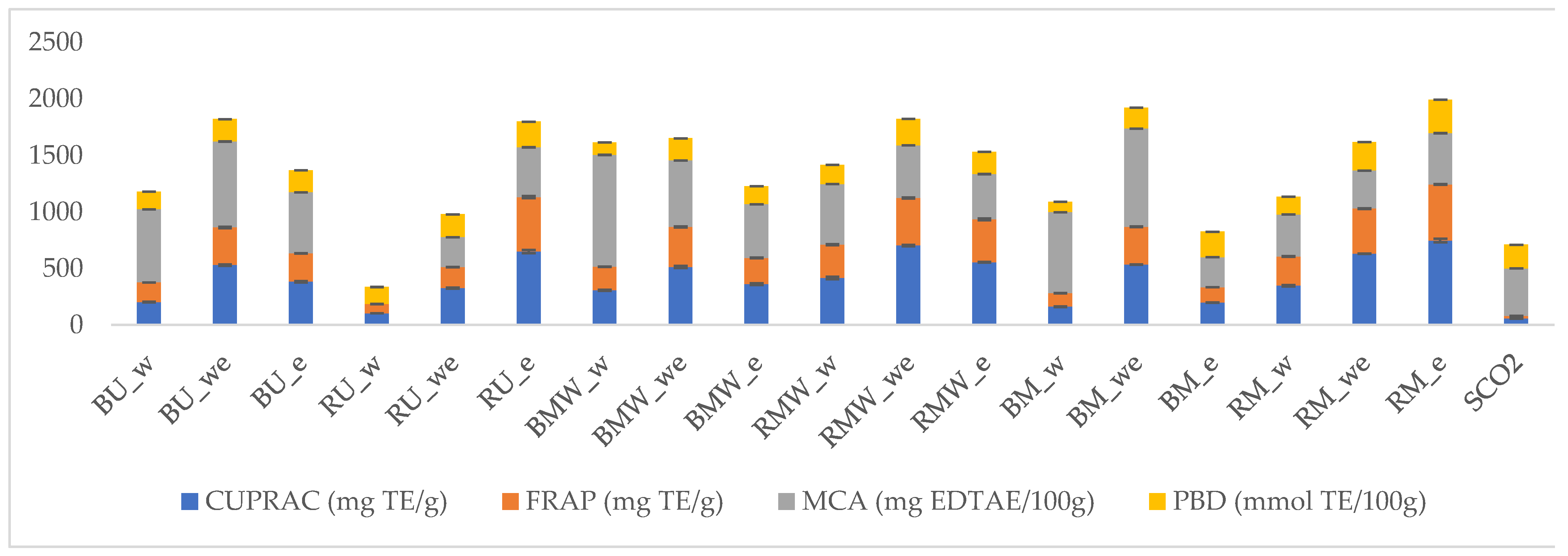
2.5. Antioxidant Properties of Analyzed Extracts
2.6. Enzyme Inhibitory Properties
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and General Materials
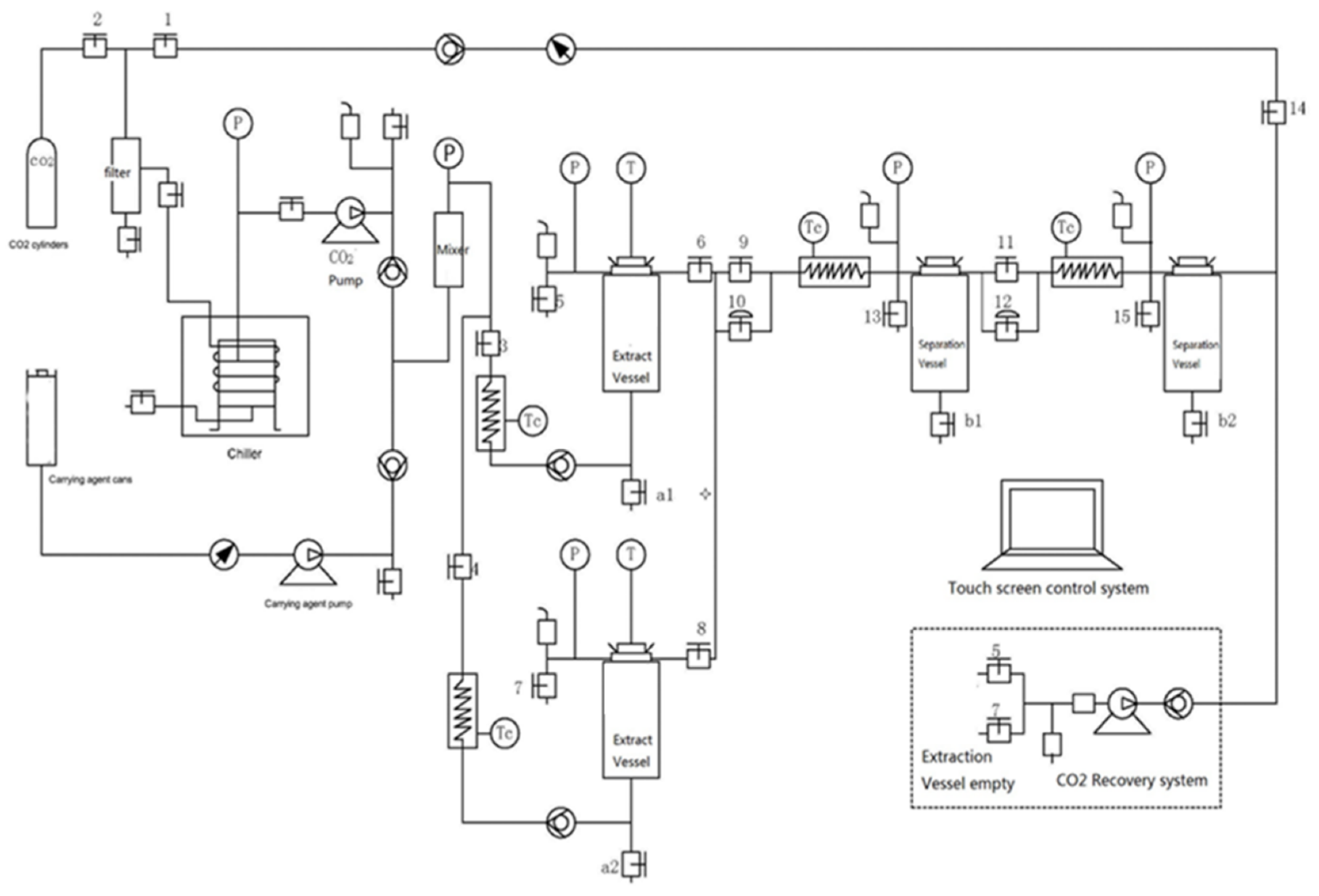
4.3. SCO2 Extraction
4.4. Solvent Based Extraction
4.4.1. Maceration (M)
4.4.2. Microwave-Assisted Extraction (MAE)
4.4.3. Ultrasound-Assisted Extraction (UAE)
4.5. Calculation of Extraction Yield
4.6. NMR Analysis
4.7. Liquid Chromatography–Diode Array Detector–Mass Spectrometry (LC-DAD-MSn)
4.8. Total Phenolic and Flavonoid Content
4.9. Antioxidant Assays
4.10. Enzyme Inhibitory Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brosowski, A.; Thrän, D.; Mantau, U.; Mahro, B.; Erdmann, G.; Adler, P.; Stinner, W.; Reinhold, G.; Hering, T.; Blanke, C. A review of biomass potential and current utilisation—Status quo for 93 biogenic wastes and residues in Germany. Biomass Bioenergy 2016, 95, 257–272. [Google Scholar]
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [PubMed]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar]
- Vieito, C.; Fernandes, É.; Velho, M.V.; Pires, P. The effect of different solvents on extraction yield, total phenolic content and antioxidant activity of extracts from pine bark (Pinus pinaster subsp. atlantica). Chem. Eng. Trans. 2018, 64, 127–132. [Google Scholar]
- Koutsaviti, A.; Ioannou, E.; Couladis, M.; Tzakou, O.; Roussis, V. 1H and 13C NMR spectral assignments of abietane diterpenes from Pinus heldreichii and Pinus nigra subsp. nigra. Magn. Reson. Chem. 2017, 55, 772–778. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Ferrarese, I.; Maccari, E.; Teobaldo, E.; De Zordi, N.; Bertoni, P.; Peron, G.; Dall’acqua, S. The bark of Picea abies L., a waste from sawmill, as a source of valuable compounds: Phytochemical investigations and isolation of a novel pimarane and a stilbene derivative. Plants 2021, 10, 2106. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Ž.; Popa, V.I. Isolation of bioactive compounds from spruce bark waste using sub- and supercritical fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [PubMed]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Phytochemical Profile and Biological Effects of Spruce (Picea abies) Bark Subjected to Ultrasound Assisted and Microwave-Assisted Extractions. Plants 2021, 10, 870. [Google Scholar] [PubMed]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar]
- Strižincová, P.; Ház, A.; Burčová, Z.; Feranc, J.; Kreps, F.; Šurina, I.; Jablonský, M. Spruce bark—A source of polyphenolic compounds: Optimizing the operating conditions of supercritical carbon dioxide extraction. Molecules 2019, 24, 4049. [Google Scholar] [CrossRef]
- Senhaji, S.; Lamchouri, F.; Boulfia, M.; Lachkar, N.; Bouabid, K.; Toufik, H. Mineral composition, content of phenolic compounds and in vitro antioxidant and antibacterial activities of aqueous and organic extracts of the seeds of Peganum harmala L. S. Afr. J. Bot. 2022, 147, 697–712. [Google Scholar]
- Ben Jalloul, A.; Chaar, H.; Tounsi, M.S.; Abderrabba, M. Variations in phenolic composition and antioxidant activities of Scabiosa maritima (Scabiosa atropurpurea sub. maritima L.) crude extracts and fractions according to growth stage and plant part. S. Afr. J. Bot. 2022, 146, 703–714. [Google Scholar] [CrossRef]
- Bou Dargham, M.; Matar Boumosleh, J.; Farhat, A.; Abdelkhalek, S.; Bou-Maroun, E.; El Hosry, L. Antioxidant and anti-diabetic activities in commercial and homemade pomegranate molasses in Lebanon. Food Biosci. 2022, 46, 101540. [Google Scholar] [CrossRef]
- Ben Mahmoud, K.; Wasli, H.; Ben Mansour, R.; Jemai, N.; Selmi, S.; Jemmali, A.; Ksouri, R. Antidiabetic, antioxidant and chemical functionalities of Ziziphus jujuba (Mill.) and Moringa oleifera (Lam.) plants using multivariate data treatment. S. Afr. J. Bot. 2022, 144, 219–228. [Google Scholar]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; Renault, J.-H. Bio-guided isolation of methanol-soluble metabolites of common spruce (Picea abies) bark by-products and investigation of their dermo-cosmetic properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Jehan, N. Natural Products as a Potential Enzyme Inhibitors from Medicinal Plants. In Enzyme Inhibitors and Activators; IntechOpen: London, UK, 2017. [Google Scholar]
- Naz, S.; Holloway, P.; Ata, A.; Sener, B. Exploring medicinal plants for the development of natural enzyme inhibitors. In Evidence-Based Validation of Herbal Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 671–690. ISBN 9780323855426. [Google Scholar]
- Senol, F.S.; Orhan, I.E.; Ustun, O. In vitro cholinesterase inhibitory and antioxidant effect of selected coniferous tree species. Asian Pac. J. Trop. Med. 2015, 8, 269–275. [Google Scholar] [CrossRef]
- Chai, Y.H.; Yusup, S.; Kadir, W.N.A.; Wong, C.Y.; Rosli, S.S.; Ruslan, M.S.H.; Chin, B.L.F.; Yiin, C.L. Valorization of tropical biomass waste by supercritical fluid extraction technology. Sustainability 2021, 13, 233. [Google Scholar] [CrossRef]
- Barbini, S.; Jaxel, J.; Karlström, K.; Rosenau, T.; Potthast, A. Multistage fractionation of pine bark by liquid and supercritical carbon dioxide. Bioresour. Technol. 2021, 341, 125862. [Google Scholar] [CrossRef]
- Bento, A.; Escórcio, R.; Tomé, A.S.; Robertson, M.; Gaugler, E.C.; Malthus, S.J.; Raymond, L.G.; Hill, S.J.; Silva Pereira, C. Pinus radiata bark sequentially processed using scCO2 and an ionic liquid catalyst yields plentiful resin acids and alkanoic acids enriched suberin. Ind. Crops Prod. 2022, 185, 115172. [Google Scholar] [CrossRef]
- Ku, C.S.; Jang, J.P.; Mun, S.P. Exploitation of polyphenol-rich pine barks for potent antioxidant activity. J. Wood Sci. 2007, 53, 524–528. [Google Scholar] [CrossRef]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochemistry 2016, 32, 191–197. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochemistry 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Chmelová, D.; Škulcová, D.; Legerská, B.; Horník, M.; Ondrejovič, M. Ultrasonic-assisted extraction of polyphenols and antioxidants from Picea abies bark. J. Biotechnol. 2020, 314–315, 25–33. [Google Scholar] [CrossRef]
- Sládková, A.; Benedeková, M.; Stopka, J.; Šurina, I.; Ház, A.; Strižincová, P.; Čižová, K.; Škulcová, A.; Burčová, Z.; Kreps, F.; et al. Yield of Polyphenolic Substances Extracted From Spruce (Picea abies) Bark by Microwave-Assisted Extraction. BioResources 2016, 11, 9912–9921. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative study regarding the chemical composition and biological activity of pine (Pinus nigra and P. sylvestris) bark extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour. Technol. 2019, 292, 121893. [Google Scholar] [CrossRef] [PubMed]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjöberg, P.J.R.; Turner, C. Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents. Phytochem. Anal. 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 481–488. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and Enzyme Inhibitory Potential of Two Potentilla species (P. speciosa L. and P. reptans Willd.) and Their Chemical Composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
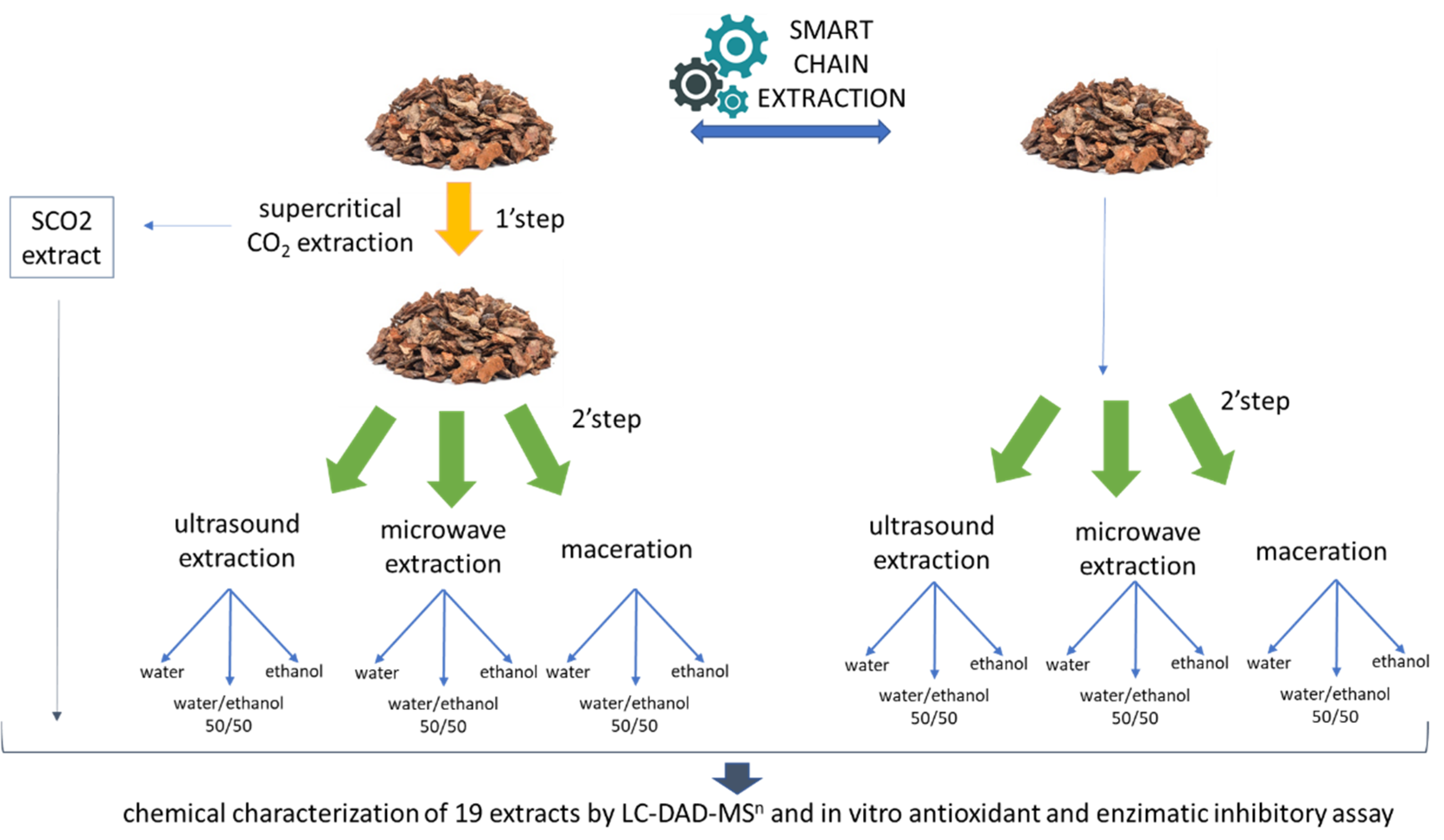

| Extraction Technique | Starting Bark Material | Solvent | Sample | % Yield |
|---|---|---|---|---|
| UAE | bark | water | BU_w | 1.07% |
| ethanol/water 50% | BU_we | 2.33% | ||
| ethanol | BU_e | 2.87% | ||
| bark after SCO2 | water | RU_w | 2.78% | |
| ethanol/water 50% | RU_we | 3.98% | ||
| ethanol | RU_e | 1.9% | ||
| MAE | bark | water | BMW_w | 2.04% |
| ethanol/water 50% | BMW_we | 3.30% | ||
| ethanol | BMW_e | 2.59% | ||
| bark after SCO2 | water | RMW_w | 1.99% | |
| ethanol/water 50% | RMW_we | 4.79% | ||
| ethanol | RMW_e | 4.39% | ||
| M | bark | water | BM_w | 1.48% |
| ethanol/water 50% | BM_we | 2.37% | ||
| ethanol | BM_e | 1.74% | ||
| bark after SCO2 | water | RM_w | 2.63% | |
| ethanol/water 50% | RM_we | 4.57% | ||
| ethanol | RM_e | 2.19% | ||
| SCO2 | bark | SCO2 | SCO2 | 5.00% |
| Signal Number in Spectrum | δ H | Tentative Identification |
|---|---|---|
| 1 | 7.60–6.45 | Trans olefine signals of hydroxycinnamic acid derivatives |
| 2 | 6.80–7.20 | Aromatic signals ascribable to hydroxycinnamic derivatves, phenolics, flavonoids, stilbenoids |
| 3 | 6.00–6.8 | Aromatic signals ascribable to abietic acid derivatives or similar diterpene |
| 4 | 5.60–5.80 | Olefine of abietic acid derivatives ascribable to positions 7-8-13-14 |
| 5 | 5.20–5.40 | Olefine signals of unsaturated fatty acids |
| 6 | 5.20–4.80 | Exocyclic sp2 olefine signals in diterpene derivatives |
| 7 | 4.10–4.30 | Oxigenated CH |
| 8 | 3.80 | Methoxy signal |
| Compound | [M − H]− m/z | ESI-MSn m/z |
|---|---|---|
| Hydroxy-piceaside derivative | 665 | 485-443-305-243 |
| Benzoic acid derivative | 313 | 151-282 |
| Caffeoyl-hexoside | 341 | 203-179-131 |
| Quinic acid * | 191 | 127-111 |
| Caffeic acid derivative | 377 | 341-179 |
| Procyanidin trimer B | 865 | 695-577-407 |
| Protocatechuic acid-hexoside | 315 | 153-109 |
| Ferulic acid * | 193 | 173-145 |
| (epi)-Catechin * | 289 | 245-203 |
| Hydroxy-piceaside derivative | 665 | 485-443 |
| Isorhamnetin * | 315 | 299 |
| Taxifolin-7-O-glucoside * | 465 | 447-303-285 |
| Luteolin-7-O-rhamnoside * | 431 | 285-241 |
| Hydroxy-piceaside derivative | 665 | 485-443-305 |
| Trans-astringin * | 405 | 243 |
| Hydroxy-piceaside derivative | 665 | 503-445-297 |
| Ellagic acid hexoside | 463 | 301 |
| Piceaside A/B | 809 | 647-485-375 |
| Hydroxy-piceaside derivative | 665 | 503-445-297 |
| Piceaside A/B | 809 | 647-485-375-229 |
| Isorhapontigenin | 257 | 241-213 |
| Piceatannol | 243 | 225-201 |
| Hydroxy-piceaside derivative | 665 | 503-445-243 |
| Piceaside A/B | 809 | 647-485-375 |
| Piceaside A/B | 809 | 647-485-375-318 |
| Piceaside G/H | 809 | 646-405 |
| Piceaside C/D | 823 | 661-499 |
| Piceaside C/D | 823 | 661-499 |
| Piceaside C/D | 823 | 661-499 |
| Piceaside G/H | 809 | 646-405-243 |
| Piceaside C/D | 823 | 661-499-257 |
| Taxifolin * | 303 | 285-241-213 |
| Isorhamnetin-pentoside | 447 | 315-300 |
| Piceaside E/F | 823 | 661-499-241 |
| 7-hydroxy-matairesinol * | 373 | 355-311-296 |
| Piceaside G/H | 809 | 646-405 |
| Piceaside E/F | 823 | 661-499-241 |
| Piceaside G/H | 809 | 646-405-243 |
| Piceatannol derivative | 647 | 485-243 |
| Methoxy-piceatannol hexoside | 661 | 499-241 |
| Piceaside E/F | 823 | 661-499-241 |
| Methoxy-piceatannol | 499 | 467-389-241 |
| Quercetin * | 301 | 179-151 |
| Methyl abietate | 315 | 301-257 |
| Dehydroabietic acid | 299 | 255 |
| Abietic acid | 301 | 257 |
| 12β hydroxy abieta 7-13 18 oic acid | 333 | 289 |
| 7-Oxodehydroabietic acid | 313 | 269 |
| Abienol | 289 | 191-163 |
| 13-Epi-manool | 289 | 215 |
| Sample | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|
| BU_w | 0.78 ± 0.05 | 1.70 ± 0.01 | 1.53 ± 0.09 | 0.05 ± 0.01 | 2.53 ± 0.01 |
| BU_we | 3.57 ± 0.03 | 3.37 ± 0.16 | 57.80 ± 0.67 | 0.40 ± 0.01 | Na |
| BU_e | 4.01 ± 0.07 | 4.30 ± 0.21 | 66.15 ± 1.10 | 0.37 ± 0.02 | 2.49 ± 0.01 |
| RU_w | Na | 4.60 ± 0.06 | 41.47 ± 0.58 | 0.19 ± 0.01 | 2.54 ± 0.01 |
| RU_we | 3.70 ± 0.04 | 3.71 ± 0.15 | 57.08 ± 0.45 | 0.41 ± 0.01 | Na |
| RU_e | 3.98 ± 0.03 | 4.63 ± 0.08 | 67.67 ± 0.37 | 0.33 ± 0.01 | Na |
| BMW_w | 0.52 ± 0.02 | 1.32 ± 0.07 | 2.80 ± 0.86 | 0.09 ± 0.01 | Na |
| BMW_we | 3.52 ± 0.06 | 3.04 ± 0.10 | 60.92 ± 0.75 | 0.39 ± 0.01 | Na |
| BMW_e | 3.75 ± 0.07 | 4.34 ± 0.23 | 63.11 ± 1.01 | 0.35 ± 0.01 | 2.35 ± 0.02 |
| RMW_w | 0.96 ± 0.03 | 2.25 ± 0.30 | 12.28 ± 0.76 | 0.05 ± 0.01 | Na |
| RMW_we | 3.69 ± 0.02 | 2.82 ± 0.09 | 60.36 ± 0.46 | 0.41 ± 0.03 | Na |
| RMW_e | 4.04 ± 0.03 | 4.77 ± 0.04 | 65.22 ± 0.66 | 0.32 ± 0.01 | Na |
| BM_w | 0.59 ± 0.11 | 0.76 ± 0.05 | Na | 0.05 ± 0.01 | 2.46 ± 0.01 |
| BM_we | 3.45 ± 0.07 | 2.64 ± 0.12 | 53.13 ± 0.91 | 0.33 ± 0.01 | Na |
| BM_e | 3.81 ± 0.08 | 4.54 ± 0.12 | 48.34 ± 1.00 | 0.35 ± 0.01 | 2.41 ± 0.01 |
| RM_w | 1.14 ± 0.06 | 2.52 ± 0.14 | 13.76 ± 0.73 | 0.05 ± 0.01 | Na |
| RM_we | 3.81 ± 0.01 | 3.68 ± 0.05 | 57.77 ± 0.20 | 0.32 ± 0.02 | Na |
| RM_e | 4.07 ± 0.04 | 4.76 ± 0.16 | 66.71 ± 0.35 | 0.29 ± 0.02 | Na |
| SCO2 | 3.49 ± 0.10 | 4.50 ± 0.25 | 36.19 ± 0.76 | 0.45 ± 0.01 | Na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sut, S.; Maccari, E.; Zengin, G.; Ferrarese, I.; Loschi, F.; Faggian, M.; Paolo, B.; De Zordi, N.; Dall’Acqua, S. “Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses. Molecules 2022, 27, 6719. https://doi.org/10.3390/molecules27196719
Sut S, Maccari E, Zengin G, Ferrarese I, Loschi F, Faggian M, Paolo B, De Zordi N, Dall’Acqua S. “Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses. Molecules. 2022; 27(19):6719. https://doi.org/10.3390/molecules27196719
Chicago/Turabian StyleSut, Stefania, Erica Maccari, Gokhan Zengin, Irene Ferrarese, Francesca Loschi, Marta Faggian, Bertoni Paolo, Nicola De Zordi, and Stefano Dall’Acqua. 2022. "“Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses" Molecules 27, no. 19: 6719. https://doi.org/10.3390/molecules27196719
APA StyleSut, S., Maccari, E., Zengin, G., Ferrarese, I., Loschi, F., Faggian, M., Paolo, B., De Zordi, N., & Dall’Acqua, S. (2022). “Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses. Molecules, 27(19), 6719. https://doi.org/10.3390/molecules27196719








