Factors Influencing the Bioavailability of Organic Molecules to Bacterial Cells—A Mini-Review
Abstract
1. Introduction
2. Challenges in Defining Microbial Bioavailability
3. Significance of Bioavailability
3.1. Bioavailability in Biotechnological Processes
3.2. Bioavailability in Antimicrobial Therapies
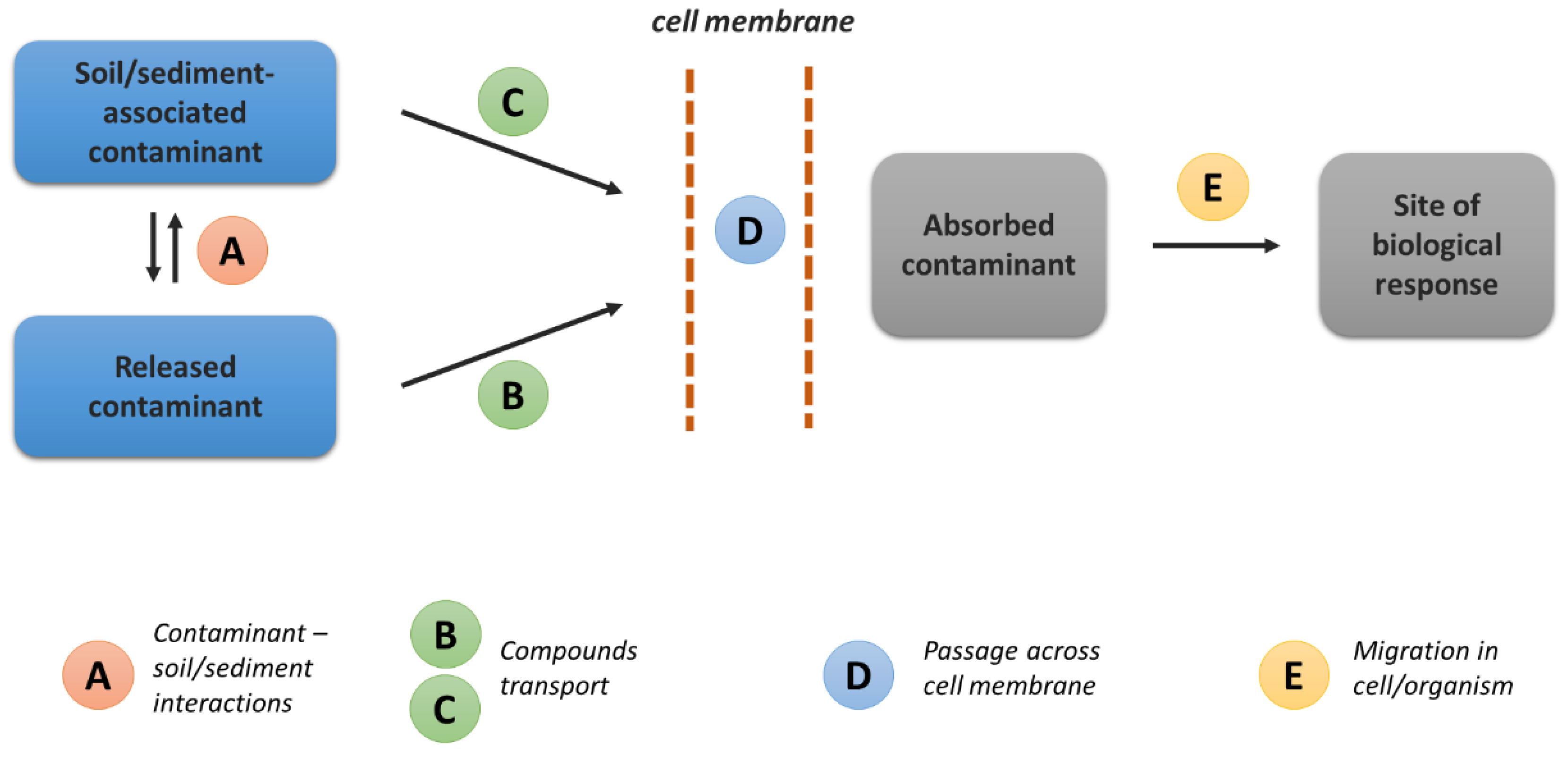
4. Factors Influencing Bioavailability
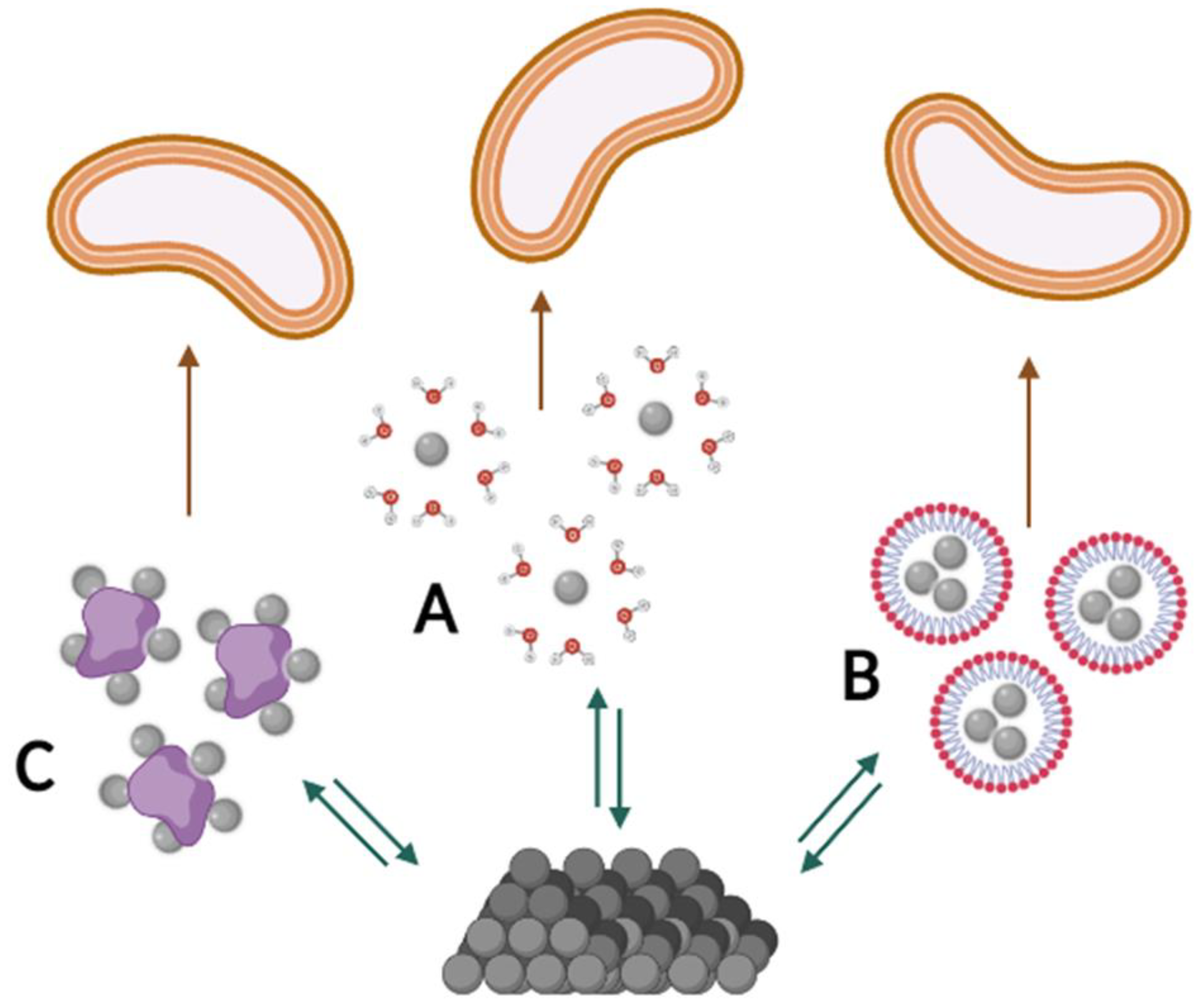
4.1. Bioavailability at the Physicochemical Level
- Chemical structure
- pH of the surrounding environment
- Ionic strength and co-dissolved compounds
- Crystallinity and amorphousness
- Stabilisers and carriers
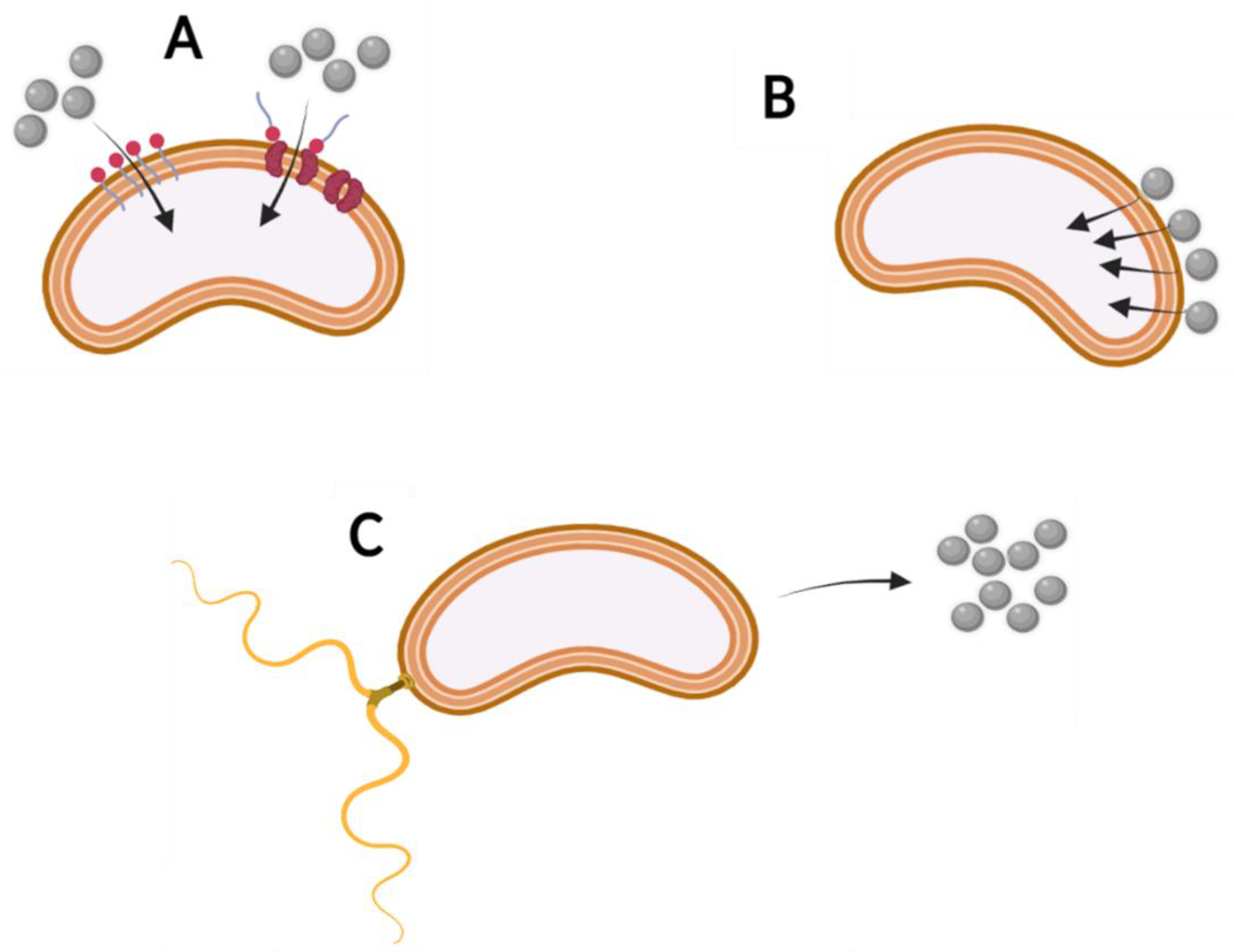
4.2. Bioavailability at the Microbiological Level
- Adsorption on cell surfaces
- Cell wall and membrane permeability
- Positive and negative chemotaxis
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hobot, J.A. Bacterial Ultrastructure. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 7–32. ISBN 978-0-12-397169-2. [Google Scholar]
- Cretel, E.; Pierres, A.; Benoliel, A.-M.; Bongrand, P. How Cells Feel Their Environment: A Focus on Early Dynamic Events. Cell. Mol. Bioeng. 2008, 1, 5–14. [Google Scholar] [CrossRef][Green Version]
- Prindle, A.; Liu, J.; Asally, M.; Ly, S.; Garcia-Ojalvo, J.; Süel, G.M. Ion channels enable electrical communication in bacterial communities. Nature 2015, 527, 59–63. [Google Scholar] [CrossRef]
- Vergalli, J.; Bodrenko, I.V.; Masi, M.; Moynié, L.; Acosta-Gutiérrez, S.; Naismith, J.H.; Davin-Regli, A.; Ceccarelli, M.; van den Berg, B.; Winterhalter, M.; et al. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 2020, 18, 164–176. [Google Scholar] [CrossRef]
- Troman, L.; Collinson, I. Pushing the Envelope: The Mysterious Journey Through the Bacterial Secretory Machinery, and Beyond. Front. Microbiol. 2021, 12, 782900. [Google Scholar] [CrossRef]
- Reintjes, G.; Arnosti, C.; Fuchs, B.M.; Amann, R. An alternative polysaccharide uptake mechanism of marine bacteria. ISME J. 2017, 11, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. In Site-Specific Protein Labeling; Gautier, A., Hinner, M.J., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1266, pp. 29–53. ISBN 978-1-4939-2271-0. [Google Scholar]
- Tardif, R.; Brodeur, J. Pharmacokinetics/Toxicokinetics. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 383–390. ISBN 978-0-12-369400-3. [Google Scholar]
- Wolverton, S.E. Basic Principles of Pharmacology. In Comprehensive Dermatologic Drug Therapy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–11. ISBN 978-0-323-61211-1. [Google Scholar]
- Willhite, C.; Karyakina, N.A.; Wiles, A.; Yenugadhati, N.; Momoli, F.; Wisniewski, T.; Krewski, D. Overview of Potential Aluminum Health Risks. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 817–830. ISBN 978-0-444-63952-3. [Google Scholar]
- Vallero, D.A. Environmental Biochemodynamic Processes. In Environmental Biotechnology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 89–150. ISBN 978-0-12-407776-8. [Google Scholar]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Peer reviewed: Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.-J.; Harmsen, J.; Parsons, J.R.; Semple, K.T.; Aitken, M.D.; Ajao, C.; Eadsforth, C.; Galay-Burgos, M.; Naidu, R.; Oliver, R.; et al. From Bioavailability Science to Regulation of Organic Chemicals. Environ. Sci. Technol. 2015, 49, 10255–10264. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, L.J.; Luthy, R.G. Peer Reviewed: Contaminant Bioavailability in Soil and Sediment. Environ. Sci. Technol. 2003, 37, 295A–302A. [Google Scholar] [CrossRef]
- Reichenberg, F.; Mayer, P. Two Complementary Sides of Bioavailability: Accessibility and Chemical Activity of Organic Contaminants in Sediments and Soils. Environ. Toxicol. Chem. 2006, 25, 1239. [Google Scholar] [CrossRef]
- Ropponen, H.-K.; Richter, R.; Hirsch, A.K.H.; Lehr, C.-M. Mastering the Gram-negative bacterial barrier—Chemical approaches to increase bacterial bioavailability of antibiotics. Adv. Drug Deliv. Rev. 2021, 172, 339–360. [Google Scholar] [CrossRef]
- Harmsen, J. Measuring Bioavailability: From a Scientific Approach to Standard Methods. J. Environ. Qual. 2007, 36, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Riding, M.J.; Doick, K.J.; Martin, F.L.; Jones, K.C.; Semple, K.T. Chemical measures of bioavailability/bioaccessibility of PAHs in soil: Fundamentals to application. J. Hazard. Mater. 2013, 261, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.F.; Hassellöv, I.-M.; Dahllöf, I. PAH effects on meio- and microbial benthic communities strongly depend on bioavailability. Aquat. Toxicol. 2014, 146, 230–238. [Google Scholar] [CrossRef]
- Gharasoo, M.; Centler, F.; Van Cappellen, P.; Wick, L.Y.; Thullner, M. Kinetics of Substrate Biodegradation under the Cumulative Effects of Bioavailability and Self-Inhibition. Environ. Sci. Technol. 2015, 49, 5529–5537. [Google Scholar] [CrossRef]
- Luo, J.; Wu, L.; Feng, Q.; Fang, F.; Cao, J.; Zhang, Q.; Su, Y. Synergistic effects of iron and persulfate on the efficient production of volatile fatty acids from waste activated sludge: Understanding the roles of bioavailable substrates, microbial community & activities, and environmental factors. Biochem. Eng. J. 2019, 141, 71–79. [Google Scholar] [CrossRef]
- Harms, H. Bioavailability and Bioaccessibility as Key Factors in Bioremediation. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 83–94. ISBN 978-0-08-088504-9. [Google Scholar]
- Liu, N.; Santala, S.; Stephanopoulos, G. Mixed carbon substrates: A necessary nuisance or a missed opportunity? Curr. Opin. Biotechnol. 2020, 62, 15–21. [Google Scholar] [CrossRef]
- Borrero-de Acuña, J.M.; Aravena-Carrasco, C.; Gutierrez-Urrutia, I.; Duchens, D.; Poblete-Castro, I. Enhanced synthesis of medium-chain-length poly(3-hydroxyalkanoates) by inactivating the tricarboxylate transport system of Pseudomonas putida KT2440 and process development using waste vegetable oil. Process Biochem. 2019, 77, 23–30. [Google Scholar] [CrossRef]
- Jaehme, M.; Slotboom, D.J. Diversity of membrane transport proteins for vitamins in bacteria and archaea. Biochim. Biophys. Acta (BBA) 2015, 1850, 565–576. [Google Scholar] [CrossRef]
- Idrees, M.; Mohammad, A.R.; Karodia, N.; Rahman, A. Multimodal Role of Amino Acids in Microbial Control and Drug Development. Antibiotics 2020, 9, 330. [Google Scholar] [CrossRef]
- Breton, J.; Tennoune, N.; Lucas, N.; Francois, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guérin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut Commensal E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. Cell Metab. 2016, 23, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Arigony, A.L.V.; de Oliveira, I.M.; Machado, M.; Bordin, D.L.; Bergter, L.; Prá, D.; Pêgas Henriques, J.A. The Influence of Micronutrients in Cell Culture: A Reflection on Viability and Genomic Stability. BioMed Res. Int. 2013, 2013, 597282. [Google Scholar] [CrossRef]
- Kim, M.H.; Fan, C.; Pan, S.-Y.; Lee, I.; Lin, Y.; Kim, H. Kinetics of competitive cometabolism under aerobic conditions. Water-Energy Nexus 2020, 3, 62–70. [Google Scholar] [CrossRef]
- Luo, J.; Wu, J.; Zhang, Q.; Feng, Q.; Wu, L.; Cao, J.; Li, C.; Fang, F. Efficient production of short-chain fatty acids from anaerobic fermentation of liquor wastewater and waste activated sludge by breaking the restrictions of low bioavailable substrates and microbial activity. Bioresour. Technol. 2018, 268, 549–557. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Yu, J.; Yi, H.; Ye, S.; Deng, R. Sorption, transport and biodegradation—An insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 2018, 610–611, 1154–1163. [Google Scholar] [CrossRef]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smułek, W. The Impact of Biosurfactants on Microbial Cell Properties Leading to Hydrocarbon Bioavailability Increase. Colloids Interfaces 2018, 2, 35. [Google Scholar] [CrossRef]
- Smułek, W.; Pacholak, A.; Kaczorek, E. Modification of the Bacterial Cell Wall—Is the Bioavailability Important in Creosote Biodegradation? Processes 2020, 8, 147. [Google Scholar] [CrossRef]
- Smułek, W.; Bielan, Z.; Pacholak, A.; Zdarta, A.; Zgoła-Grześkowiak, A.; Zielińska-Jurek, A.; Kaczorek, E. Nitrofurazone Removal from Water Enhanced by Coupling Photocatalysis and Biodegradation. Int. J. Mol. Sci. 2021, 22, 2186. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Narasimhulu, K.; PydiSetty, Y. Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J. Clean. Prod. 2018, 198, 1602–1631. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, L.; Li, F. Influences and mechanisms of surfactants on pyrene biodegradation based on interactions of surfactant with a Klebsiella oxytoca strain. Bioresour. Technol. 2013, 142, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Smułek, W.; Zdarta, A.; Sawczuk, A.; Zgoła-Grześkowiak, A. Influence of saponins on the biodegradation of halogenated phenols. Ecotoxicol. Environ. Saf. 2016, 131, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, M.; Smułek, W.; Kaczorek, E.; Prochaska, K. Langmuir Monolayer Techniques for the Investigation of Model Bacterial Membranes and Antibiotic Biodegradation Mechanisms. Membranes 2021, 11, 707. [Google Scholar] [CrossRef]
- Pacholak, A.; Burlaga, N.; Guzik, U.; Kaczorek, E. Investigation of the bacterial cell envelope nanomechanical properties after long-term exposure to nitrofurans. J. Hazard. Mater. 2021, 407, 124352. [Google Scholar] [CrossRef]
- Ibrar, M.; Khan, S.; Hasan, F.; Yang, X. Biosurfactants and chemotaxis interplay in microbial consortium-based hydrocarbons degradation. Environ. Sci. Pollut. Res. 2022, 29, 24391–24410. [Google Scholar] [CrossRef]
- Al-Tahhan, R.A.; Sandrin, T.R.; Bodour, A.A.; Maier, R.M. Rhamnolipid-Induced Removal of Lipopolysaccharide from Pseudomonas aeruginosa: Effect on Cell Surface Properties and Interaction with Hydrophobic Substrates. Appl. Environ. Microbiol. 2000, 66, 3262–3268. [Google Scholar] [CrossRef]
- Liu, S. How Cells Grow. In Bioprocess Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 629–697. ISBN 978-0-444-63783-3. [Google Scholar]
- Brautaset, T.; Ellingsen, T.E. Lysine. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 541–554. ISBN 978-0-08-088504-9. [Google Scholar]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300. [Google Scholar] [CrossRef]
- Li, Z.; Velkov, T. Polymyxins: Mode of Action. In Polymyxin Antibiotics: From Laboratory Bench to Bedside; Li, J., Nation, R.L., Kaye, K.S., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1145, pp. 37–54. ISBN 978-3-030-16371-6. [Google Scholar]
- Zhang, Y.; Boyd, S.A.; Teppen, B.J.; Tiedje, J.M.; Li, H. Role of Tetracycline Speciation in the Bioavailability to Escherichia coli for Uptake and Expression of Antibiotic Resistance. Environ. Sci. Technol. 2014, 48, 4893–4900. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Antimicrobial agents targeting bacterial cell walls and cell membranes. Rev. Sci. Tech. OIE 2012, 31, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Rogacheva, E.; Kremleva, A.; Morozkina, S.; Uspenskaya, M.; Kraeva, L. In-Vitro Antibacterial Activity of Curcumin-Loaded Nanofibers Based on Hyaluronic Acid against Multidrug-Resistant ESKAPE Pathogens. Pharmaceutics 2022, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- de Melo, A.L.F.; Rossato, L.; Barbosa, M.D.S.; Palozi, R.A.C.; Alfredo, T.M.; Antunes, K.A.; Eduvirgem, J.; Ribeiro, S.M.; Simionatto, S. From the environment to the hospital: How plants can help to fight bacteria biofilm. Microbiol. Res. 2022, 261, 127074. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-L.; Zhao, J.; Xu, R. Recent Advances in Oral Nano-Antibiotics for Bacterial Infection Therapy. Int. J. Nanomed. 2020, 15, 9587–9610. [Google Scholar] [CrossRef]
- Le, H.; Karakasyan, C.; Jouenne, T.; Le Cerf, D.; Dé, E. Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance. Appl. Sci. 2021, 11, 10695. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Gao, Y.; Boyd, S.A.; Zhu, D.; Li, H. Influence of Dissolved Organic Matter on Tetracycline Bioavailability to an Antibiotic-Resistant Bacterium. Environ. Sci. Technol. 2015, 49, 10903–10910. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, J.; Zhang, S.; Zhao, Y.; Cui, H.; Wei, Z.; Luo, S.; Cao, J. Bioavailability Evaluation of Dissolved Organic Matter Derived from Compost-Amended Soils. J. Agric. Food Chem. 2019, 67, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.P.; Parry, J.D.; Hamilton-Taylor, J. Further evidence of elemental composition as an indicator of the bioavailability of humic substances to bacteria. Limnol. Oceanogr. 2000, 45, 237–241. [Google Scholar] [CrossRef]
- Okpokwasili, G.C.; Nweke, C.O. Microbial growth and substrate utilization kinetics. Afr. J. Biotechnol. 2005, 5, 305–317. [Google Scholar]
- Li, J.-L.; Chen, B.-H. Surfactant-mediated Biodegradation of Polycyclic Aromatic Hydrocarbons. Materials 2009, 2, 76–94. [Google Scholar] [CrossRef]
- Krell, T.; Lacal, J.; Reyes-Darias, J.A.; Jimenez-Sanchez, C.; Sungthong, R.; Ortega-Calvo, J.J. Bioavailability of pollutants and chemotaxis. Curr. Opin. Biotechnol. 2013, 24, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Di Gioacchino, M.; Bruni, F.; Imberti, S.; Ricci, M.A. Hydration of Carboxyl Groups: A Route toward Molecular Recognition? J. Phys. Chem. B 2020, 124, 4358–4364. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. Predicting aqueous solubility from structure. Drug Discov. Today 2005, 10, 289–295. [Google Scholar] [CrossRef]
- Sieger, P.; Cui, Y.; Scheuerer, S. pH-dependent solubility and permeability profiles: A useful tool for prediction of oral bioavailability. Eur. J. Pharm. Sci. 2017, 105, 82–90. [Google Scholar] [CrossRef]
- Hamed, R.; Awadallah, A.; Sunoqrot, S.; Tarawneh, O.; Nazzal, S.; AlBaraghthi, T.; Al Sayyad, J.; Abbas, A. pH-Dependent Solubility and Dissolution Behavior of Carvedilol—Case Example of a Weakly Basic BCS Class II Drug. AAPS PharmSciTech 2016, 17, 418–426. [Google Scholar] [CrossRef]
- Shoghi, E.; Fuguet, E.; Bosch, E.; Ràfols, C. Solubility–pH profiles of some acidic, basic and amphoteric drugs. Eur. J. Pharm. Sci. 2013, 48, 291–300. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Luthman, K.; Artursson, P. Accuracy of calculated pH-dependent aqueous drug solubility. Eur. J. Pharm. Sci. 2004, 22, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Litou, C.; Psachoulias, D.; Vertzoni, M.; Dressman, J.; Reppas, C. Measuring pH and Buffer Capacity in Fluids Aspirated from the Fasted Upper Gastrointestinal Tract of Healthy Adults. Pharm. Res. 2020, 37, 42. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shin, W.S.; Kim, H.T. Effects of pH, dissolved organic matter, and salinity on ibuprofen sorption on sediment. Environ. Sci. Pollut. Res. 2016, 23, 22882–22889. [Google Scholar] [CrossRef]
- Zdarta, A.; Smułek, W.; Bielan, Z.; Zdarta, J.; Nguyen, L.N.; Zgoła-Grześkowiak, A.; Nghiem, L.D.; Jesionowski, T.; Kaczorek, E. Significance of the presence of antibiotics on the microbial consortium in wastewater—The case of nitrofurantoin and furazolidone. Bioresour. Technol. 2021, 339, 125577. [Google Scholar] [CrossRef] [PubMed]
- Hutacharoen, P.; Dufal, S.; Papaioannou, V.; Shanker, R.M.; Adjiman, C.S.; Jackson, G.; Galindo, A. Predicting the Solvation of Organic Compounds in Aqueous Environments: From Alkanes and Alcohols to Pharmaceuticals. Ind. Eng. Chem. Res. 2017, 56, 10856–10876. [Google Scholar] [CrossRef]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a Molecular Understanding of Protein Solubility: Increased Negative Surface Charge Correlates with Increased Solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Boyd, S.A.; Teppen, B.J.; Tiedje, J.M.; Li, H. Organic acids enhance bioavailability of tetracycline in water to Escherichia coli for uptake and expression of antibiotic resistance. Water Res. 2014, 65, 98–106. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Nason, S.L. Importance of Soil Properties and Processes on Bioavailability of Organic Compounds. In Bioavailability of Organic Chemicals in Soil and Sediment; Ortega-Calvo, J.J., Parsons, J.R., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2020; Volume 100, pp. 7–41. ISBN 978-3-030-57918-0. [Google Scholar]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Qian, K.K.; Bogner, R.H. Spontaneous Crystalline-to-Amorphous Phase Transformation of Organic or Medicinal Compounds in the Presence of Porous Media, Part 1: Thermodynamics of Spontaneous Amorphization. J. Pharm. Sci. 2011, 100, 2801–2815. [Google Scholar] [CrossRef] [PubMed]
- Choucair, A.; Eisenberg, A. Interfacial Solubilization of Model Amphiphilic Molecules in Block Copolymer Micelles. J. Am. Chem. Soc. 2003, 125, 11993–12000. [Google Scholar] [CrossRef]
- Li, X.-Y.; Li, X.-S.; Wang, Y.; Li, G.; Zhang, Y.; Hu, H.-Q.; Wan, K.; Zeng, H.-P. Influence of Particle Size on the Heat and Mass Transfer Characteristics of Methane Hydrate Formation and Decomposition in Porous Media. Energy Fuels 2021, 35, 2153–2164. [Google Scholar] [CrossRef]
- Din, F.U.; Saleem, S.; Aleem, F.; Ahmed, R.; Huda, N.U.; Ahmed, S.; Khaleeq, N.; Shah, K.U.; Ullah, I.; Zeb, A.; et al. Advanced colloidal technologies for the enhanced bioavailability of drugs. Cogent Med. 2018, 5, 1480572. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021, 81, 101081. [Google Scholar] [CrossRef] [PubMed]
- Tamang, N.; Shrestha, P.; Khadka, B.; Mondal, M.H.; Saha, B.; Bhattarai, A. A Review of Biopolymers’ Utility as Emulsion Stabilizers. Polymers 2021, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Shao, X.; Chen, Y.; Cheng, Z. Increasing entropy for colloidal stabilization. Sci. Rep. 2016, 6, 36836. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Pisano, R. Surfactants as stabilizers for biopharmaceuticals: An insight into the molecular mechanisms for inhibition of protein aggregation. Eur. J. Pharm. Biopharm. 2018, 128, 98–106. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: A review. Trends Food Sci. Technol. 2020, 104, 49–59. [Google Scholar] [CrossRef]
- Jiang, L.; Li, S.; Yu, W.; Wang, J.; Sun, Q.; Li, Z. Interfacial study on the interaction between hydrophobic nanoparticles and ionic surfactants. Colloids Surf. Physicochem. Eng. Asp. 2016, 488, 20–27. [Google Scholar] [CrossRef]
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant–soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef]
- Rao, V.; Nerurkar, M.; Pinnamaneni, S.; Rinaldi, F.; Raghavan, K. Co-solubilization of poorly soluble drugs by micellization and complexation. Int. J. Pharm. 2006, 319, 98–106. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.-H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.G.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P.; et al. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef]
- Zhang, Y.; Boyd, S.A.; Teppen, B.J.; Tiedje, J.M.; Zhang, W.; Zhu, D.; Li, H. Bioavailability of tetracycline to antibiotic resistant Escherichia coli in water-clay systems. Environ. Pollut. 2018, 243, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.-X.; Silveira, A.P.; Morais, J.A.V.; Sampaio, M.C.; Muehlmann, L.A.; Zhang, J.; Jiang, C.-S.; Liu, S.-K. Recent Advances in Antimicrobial Nano-Drug Delivery Systems. Nanomaterials 2022, 12, 1855. [Google Scholar] [CrossRef]
- Su, F.-Y.; Chen, J.; Son, H.-N.; Kelly, A.M.; Convertine, A.J.; West, T.E.; Skerrett, S.J.; Ratner, D.M.; Stayton, P.S. Polymer-augmented liposomes enhancing antibiotic delivery against intracellular infections. Biomater. Sci. 2018, 6, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Rong, F.; Tang, Y.; Li, M.; Feng, T.; Zhou, Q.; Li, P.; Huang, W. Targeted polymer-based antibiotic delivery system: A promising option for treating bacterial infections via macromolecular approaches. Prog. Polym. Sci. 2021, 116, 101389. [Google Scholar] [CrossRef]
- Mohanty, S.; Jasmine, J.; Mukherji, S. Practical Considerations and Challenges Involved in Surfactant Enhanced Bioremediation of Oil. BioMed Res. Int. 2013, 2013, 328608. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Łuczak, M.; Krawczyk, P.; Jesionowski, T.; Kaczorek, E. Sapindus saponins’ impact on hydrocarbon biodegradation by bacteria strains after short- and long-term contact with pollutant. Colloids Surf. B Biointerfaces 2016, 142, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Müllertz, A.; Ogbonna, A.; Ren, S.; Rades, T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, S.; Huang, Q.; Cai, P. Bacterial cell surface properties: Role of loosely bound extracellular polymeric substances (LB-EPS). Colloids Surf. B Biointerfaces 2015, 128, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yue, T.; Yuan, Y.; Wang, Z.; Ye, M.; Cai, R. A new insight into the adsorption mechanism of patulin by the heat-inactive lactic acid bacteria cells. Food Control 2015, 50, 104–110. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Kennes, C.; Chen, J.; Chen, D.; Ye, J.; Zhang, S.; Dionysiou, D.D. Differences of cell surface characteristics between the bacterium Pseudomonas veronii and fungus Ophiostoma stenoceras and their different adsorption properties to hydrophobic organic compounds. Sci. Total Environ. 2019, 650, 2095–2106. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Nuri, R.; Shprung, T.; Shai, Y. Defensive remodeling: How bacterial surface properties and biofilm formation promote resistance to antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, A.; Smułek, W.; Kaczorek, E. Multilevel changes in bacterial properties on long-term exposure to hydrocarbons and impact of these cells on fresh-water communities. Sci. Total Environ. 2020, 729, 138956. [Google Scholar] [CrossRef]
- Smułek, W.; Burlaga, N.; Hricovíni, M.; Medveďová, A.; Kaczorek, E.; Hricovíniová, Z. Evaluation of surface active and antimicrobial properties of alkyl D-lyxosides and alkyl L-rhamnosides as green surfactants. Chemosphere 2021, 271, 129818. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Hua, X.; Guo, Z. Inhibitory effects of extracellular polymeric substances on ofloxacin sorption by natural biofilms. Sci. Total Environ. 2018, 625, 178–184. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Pacholak, A.; Runka, T.; Kaczorek, E. Increased biological removal of 1-chloronaphthalene as a result of exposure: A study of bacterial adaptation strategies. Ecotoxicol. Environ. Saf. 2019, 185, 109707. [Google Scholar] [CrossRef] [PubMed]
- Torresi, E.; Polesel, F.; Bester, K.; Christensson, M.; Smets, B.F.; Trapp, S.; Andersen, H.R.; Plósz, B.G. Diffusion and sorption of organic micropollutants in biofilms with varying thicknesses. Water Res. 2017, 123, 388–400. [Google Scholar] [CrossRef]
- Cao, D.-Q.; Yang, W.-Y.; Wang, Z.; Hao, X.-D. Role of extracellular polymeric substance in adsorption of quinolone antibiotics by microbial cells in excess sludge. Chem. Eng. J. 2019, 370, 684–694. [Google Scholar] [CrossRef]
- Cinquin, B.; Maigre, L.; Pinet, E.; Chevalier, J.; Stavenger, R.A.; Mills, S.; Réfrégiers, M.; Pagès, J.-M. Microspectrometric insights on the uptake of antibiotics at the single bacterial cell level. Sci. Rep. 2015, 5, 17968. [Google Scholar] [CrossRef]
- Shi, K.; Xue, J.; Xiao, X.; Qiao, Y.; Wu, Y.; Gao, Y. Mechanism of Degrading Petroleum Hydrocarbons by Compound Marine Petroleum-Degrading Bacteria: Surface Adsorption, Cell Uptake, and Biodegradation. Energy Fuels 2019, 33, 11373–11379. [Google Scholar] [CrossRef]
- Wang, X.; Lin, L.; Zhou, J. Links among extracellular enzymes, lignin degradation and cell growth establish the models to identify marine lignin-utilizing bacteria. Environ. Microbiol. 2021, 23, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Herrán, A.R.; Pérez-Andrés, J.; Caminero, A.; Nistal, E.; Vivas, S.; de Morales, J.M.R.; Casqueiro, J. Gluten-degrading bacteria are present in the human small intestine of healthy volunteers and celiac patients. Res. Microbiol. 2017, 168, 673–684. [Google Scholar] [CrossRef]
- Mutanda, I.; Sun, J.; Jiang, J.; Zhu, D. Bacterial membrane transporter systems for aromatic compounds: Regulation, engineering, and biotechnological applications. Biotechnol. Adv. 2022, 59, 107952. [Google Scholar] [CrossRef]
- Hua, F.; Wang, H.Q. Uptake and trans-membrane transport of petroleum hydrocarbons by microorganisms. Biotechnol. Biotechnol. Equip. 2014, 28, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 update. Nucleic Acids Res. 2021, 49, D461–D467. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.N.; Koech, P.K.; Plymale, A.E.; Landorf, E.V.; Konopka, A.; Collart, F.R.; Lipton, M.S.; Romine, M.F.; Wright, A.T. Live Cell Discovery of Microbial Vitamin Transport and Enzyme-Cofactor Interactions. ACS Chem. Biol. 2016, 11, 345–354. [Google Scholar] [CrossRef]
- Robinson, J.A. Folded Synthetic Peptides and Other Molecules Targeting Outer Membrane Protein Complexes in Gram-Negative Bacteria. Front. Chem. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef]
- Nowak, A.; Greń, I.; Mrozik, A. Changes in fatty acid composition of Stenotrophomonas maltophilia KB2 during co-metabolic degradation of monochlorophenols. World J. Microbiol. Biotechnol. 2016, 32, 198. [Google Scholar] [CrossRef]
- Zdarta, A.; Tracz, J.; Luczak, M.; Guzik, U.; Kaczorek, E. Hydrocarbon-induced changes in proteins and fatty acids profiles of Raoultella ornithinolytica M03. J. Proteom. 2017, 164, 43–51. [Google Scholar] [CrossRef]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Gould, K. Antibiotics: From prehistory to the present day. J. Antimicrob. Chemother. 2016, 71, 572–575. [Google Scholar] [CrossRef]
- Gülez, G.; Dechesne, A.; Smets, B.F. The Pressurized Porous Surface Model: An improved tool to study bacterial behavior under a wide range of environmentally relevant matric potentials. J. Microbiol. Methods 2010, 82, 324–326. [Google Scholar] [CrossRef]
- Aujla, I.S.; Paulitz, T.C. An Improved Method for Establishing Accurate Water Potential Levels at Different Temperatures in Growth Media. Front. Microbiol. 2017, 8, 1497. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, L.; Zhang, W.; Peng, A.; Sallach, J.B.; Luo, Y.; Li, H. NaCl salinity enhances tetracycline bioavailability to Escherichia coli on agar surfaces. Chemosphere 2022, 302, 134921. [Google Scholar] [CrossRef]
- Gao, B.; Taghizadeh, E.; Wood, B.D.; Ford, R.M. Transport of chemotactic bacteria in granular media with randomly distributed chemoattractant-containing NAPL ganglia: Modeling and simulation. Adv. Water Resour. 2022, 159, 104065. [Google Scholar] [CrossRef]
- Colin, R.; Sourjik, V. Emergent properties of bacterial chemotaxis pathway. Curr. Opin. Microbiol. 2017, 39, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, K.; Mao, R.; Liu, B.; Cheng, L.; Shi, X. Unveiling the chemotactic response and mechanism of Shewanella oneidensis MR-1 to nitrobenzene. J. Hazard. Mater. 2022, 431, 128629. [Google Scholar] [CrossRef] [PubMed]
- Frank, V.; Piñas, G.E.; Cohen, H.; Parkinson, J.S.; Vaknin, A. Networked Chemoreceptors Benefit Bacterial Chemotaxis Performance. mBio 2016, 7, e01824-16. [Google Scholar] [CrossRef]
- Stock, J.B.; Baker, M.D. Chemotaxis. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 71–78. ISBN 978-0-12-373944-5. [Google Scholar]
- Hoffmann, S.; Schmidt, C.; Walter, S.; Bender, J.K.; Gerlach, R.G. Scarless deletion of up to seven methyl-accepting chemotaxis genes with an optimized method highlights key function of CheM in Salmonella Typhimurium. PLoS ONE 2017, 12, e0172630. [Google Scholar] [CrossRef]
- Bi, S.; Sourjik, V. Stimulus sensing and signal processing in bacterial chemotaxis. Curr. Opin. Microbiol. 2018, 45, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Zhu, D.; Sun, J. Bacterial chemotaxis: A way forward to aromatic compounds biodegradation. Environ. Sci. Eur. 2020, 32, 52. [Google Scholar] [CrossRef]
- Bodhankar, G.A.; Tohidifar, P.; Foust, Z.L.; Ordal, G.W.; Rao, C.V. Characterization of Opposing Responses to Phenol by Bacillus subtilis Chemoreceptors. J. Bacteriol. 2022, 204, e00441-21. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smułek, W.; Kaczorek, E. Factors Influencing the Bioavailability of Organic Molecules to Bacterial Cells—A Mini-Review. Molecules 2022, 27, 6579. https://doi.org/10.3390/molecules27196579
Smułek W, Kaczorek E. Factors Influencing the Bioavailability of Organic Molecules to Bacterial Cells—A Mini-Review. Molecules. 2022; 27(19):6579. https://doi.org/10.3390/molecules27196579
Chicago/Turabian StyleSmułek, Wojciech, and Ewa Kaczorek. 2022. "Factors Influencing the Bioavailability of Organic Molecules to Bacterial Cells—A Mini-Review" Molecules 27, no. 19: 6579. https://doi.org/10.3390/molecules27196579
APA StyleSmułek, W., & Kaczorek, E. (2022). Factors Influencing the Bioavailability of Organic Molecules to Bacterial Cells—A Mini-Review. Molecules, 27(19), 6579. https://doi.org/10.3390/molecules27196579








