Monitoring the Shelf Life of Refined Vegetable Oils under Market Storage Conditions—A Kinetic Chemofoodmetric Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Storage
2.3. Physico-Chemical Testing and Analytical Equipment
2.4. Kinetic Modelling and Shelf Life Estimation
3. Result and Discussion
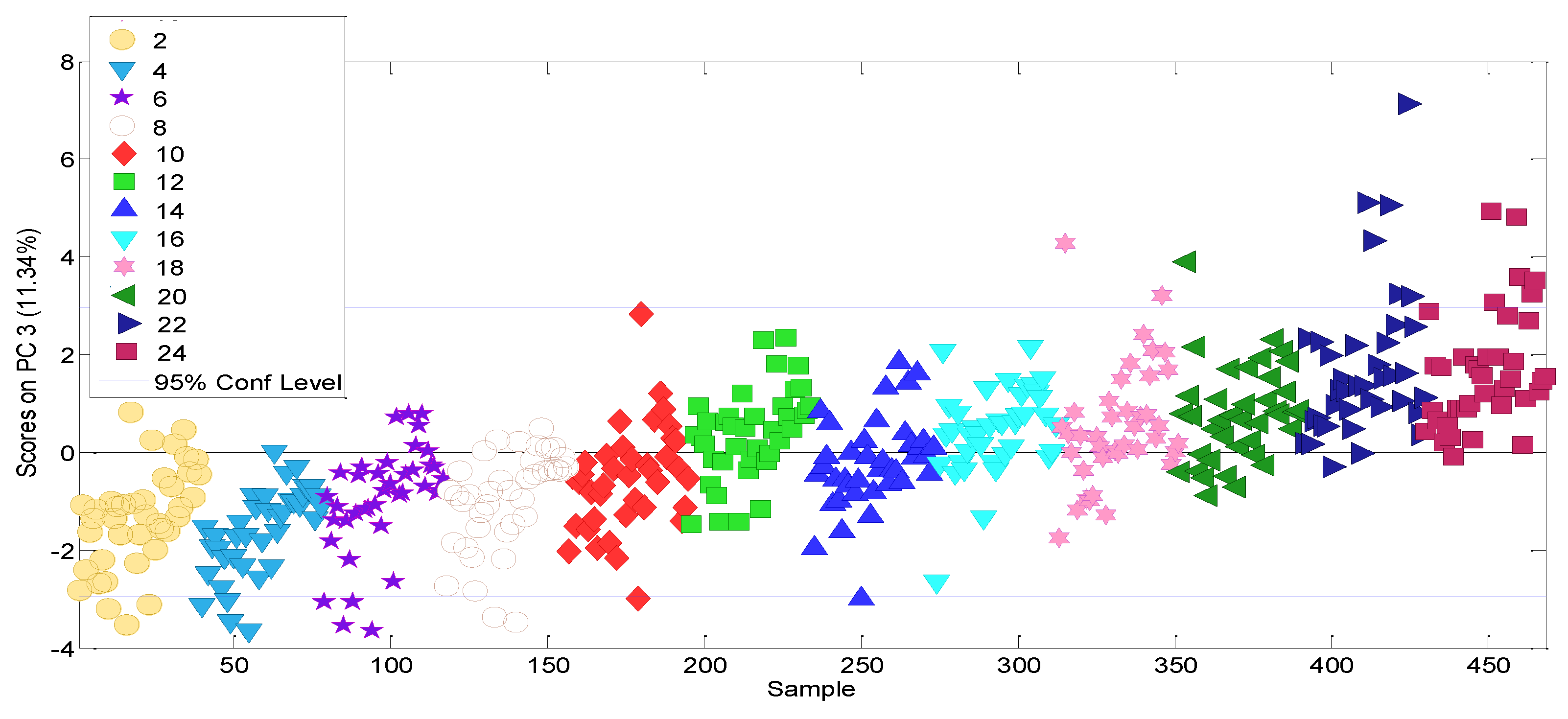
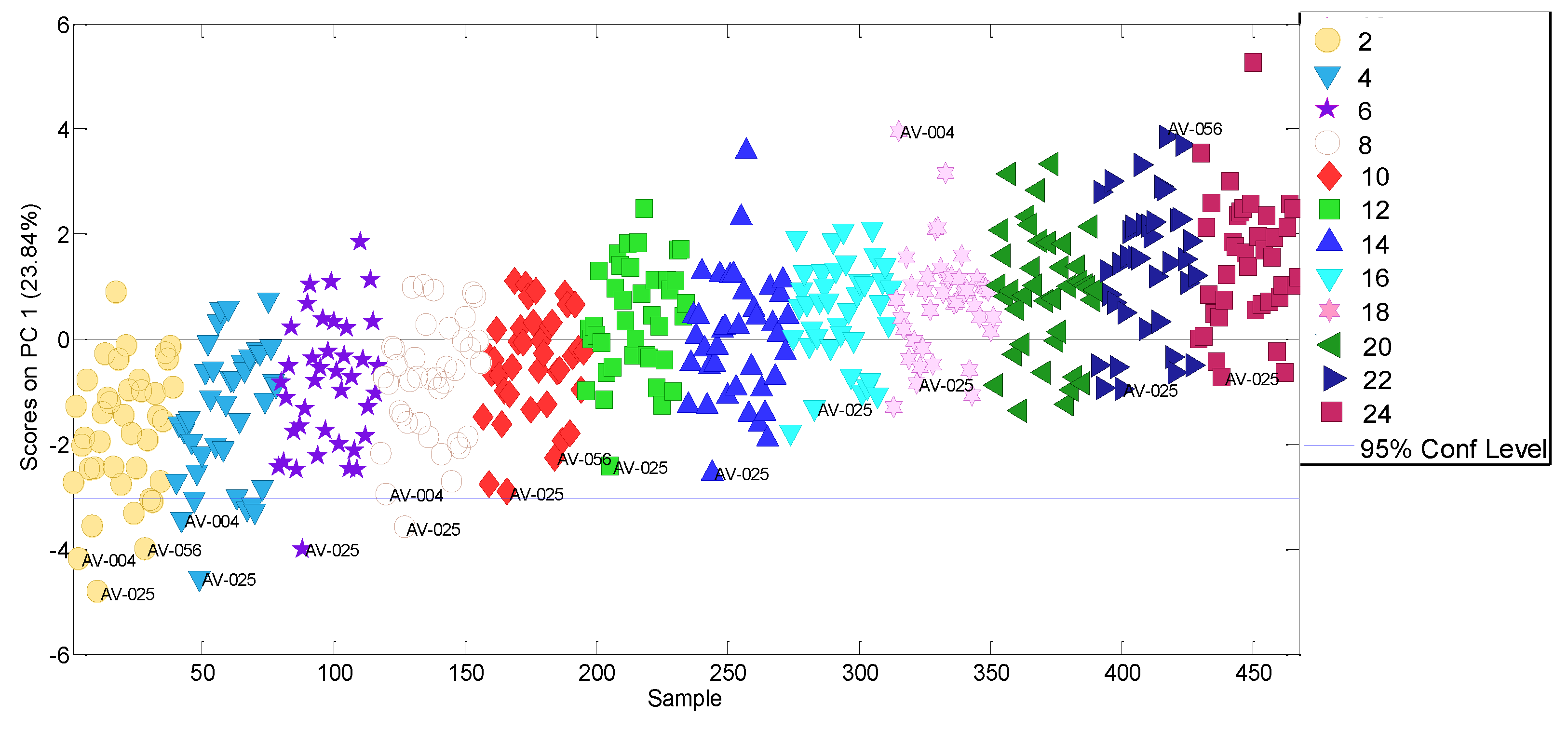
3.1. Selecting of Influential Volatile Variables
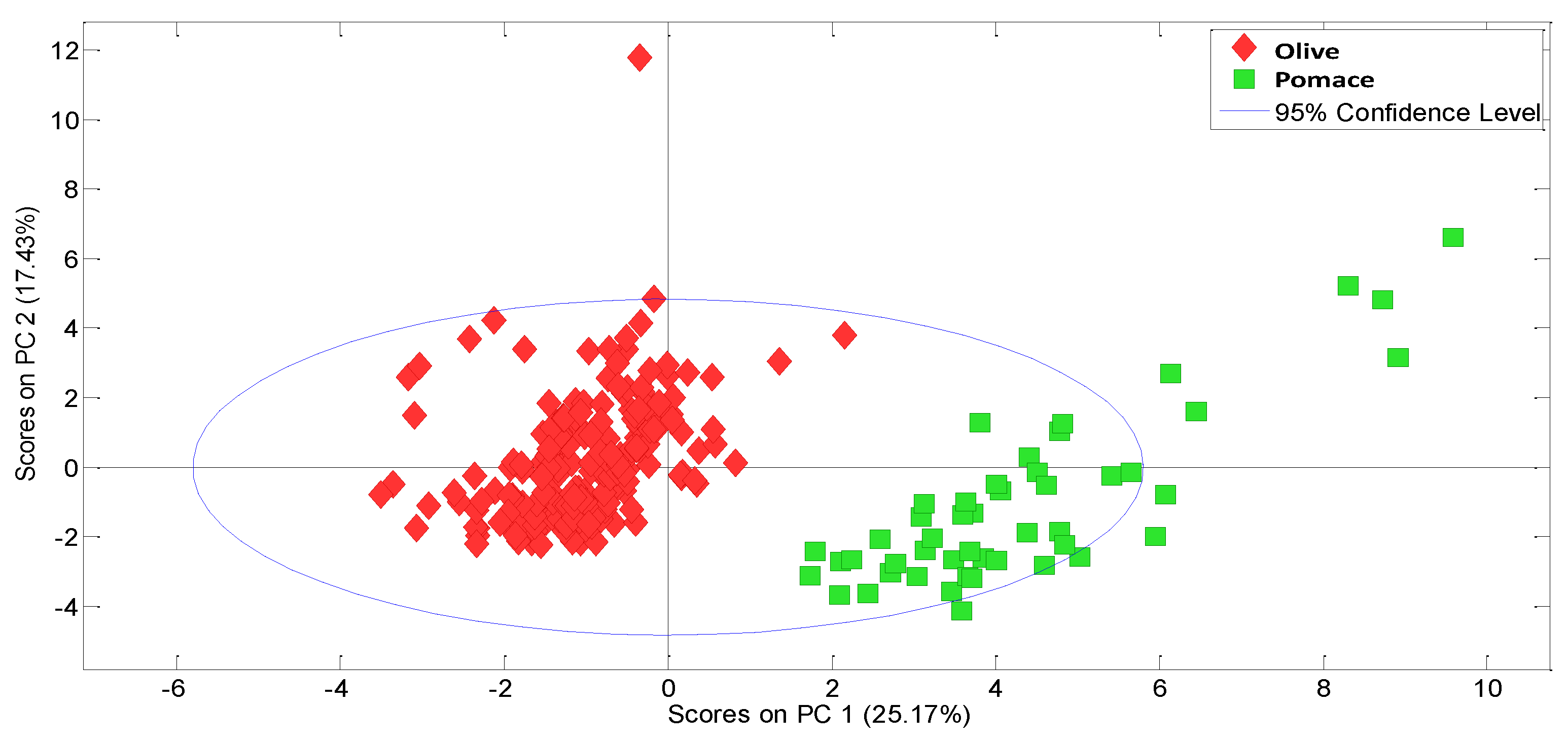
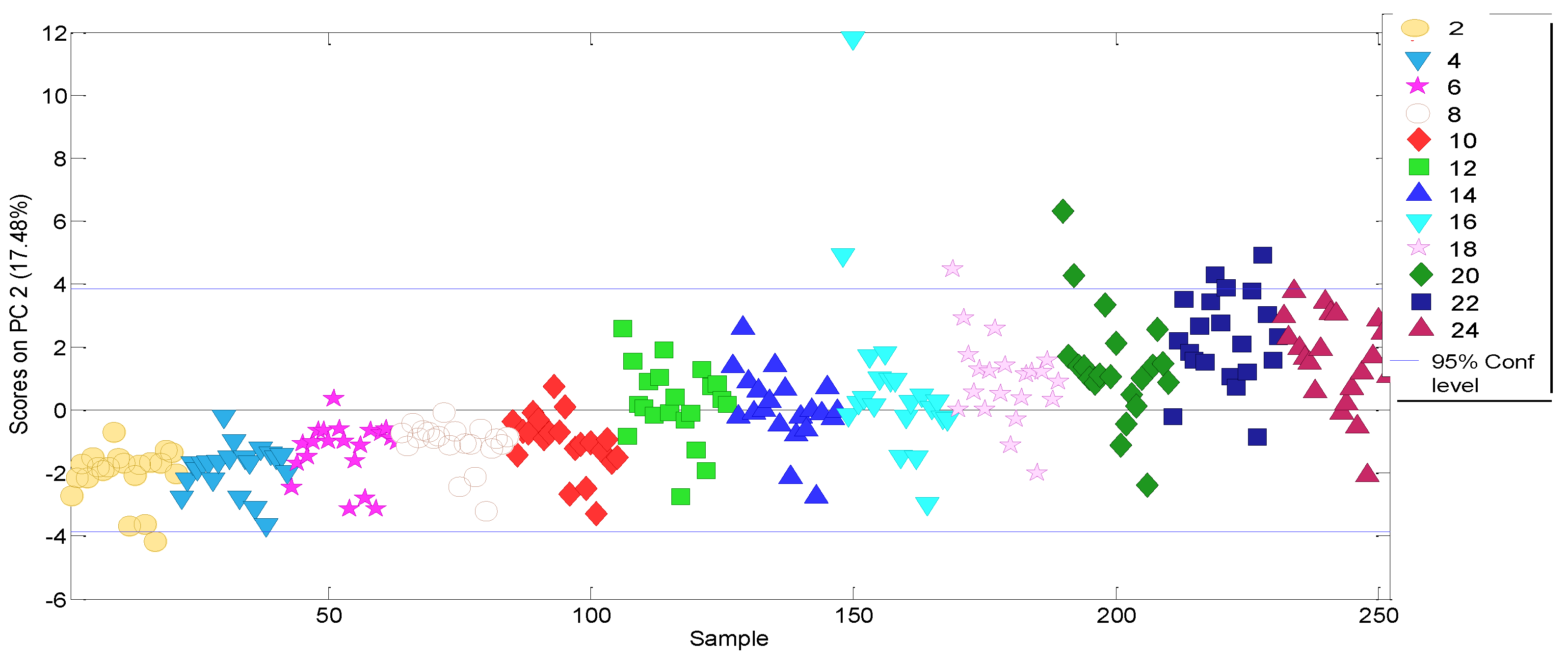
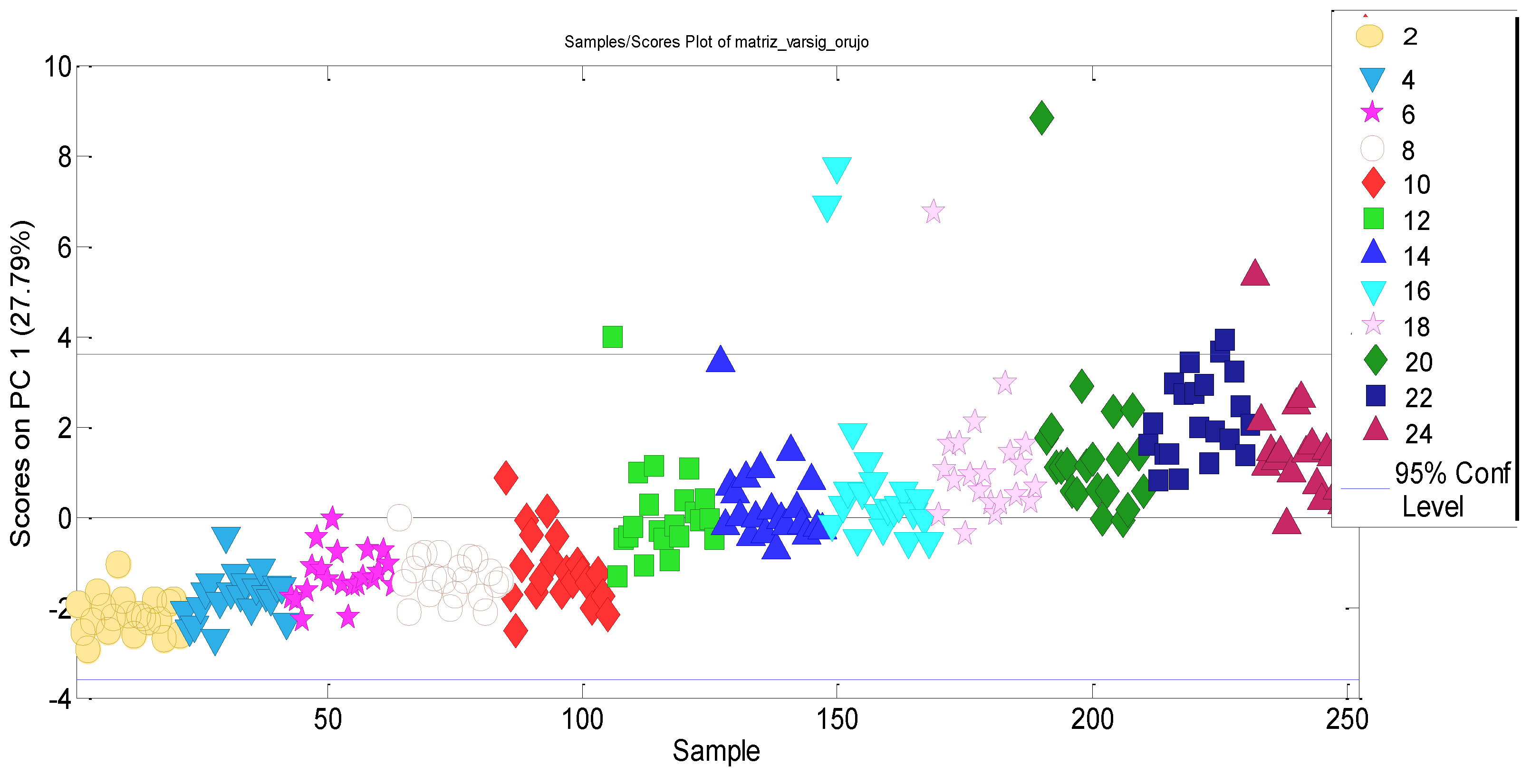
3.2. Experimental Data Matrix of Ageing
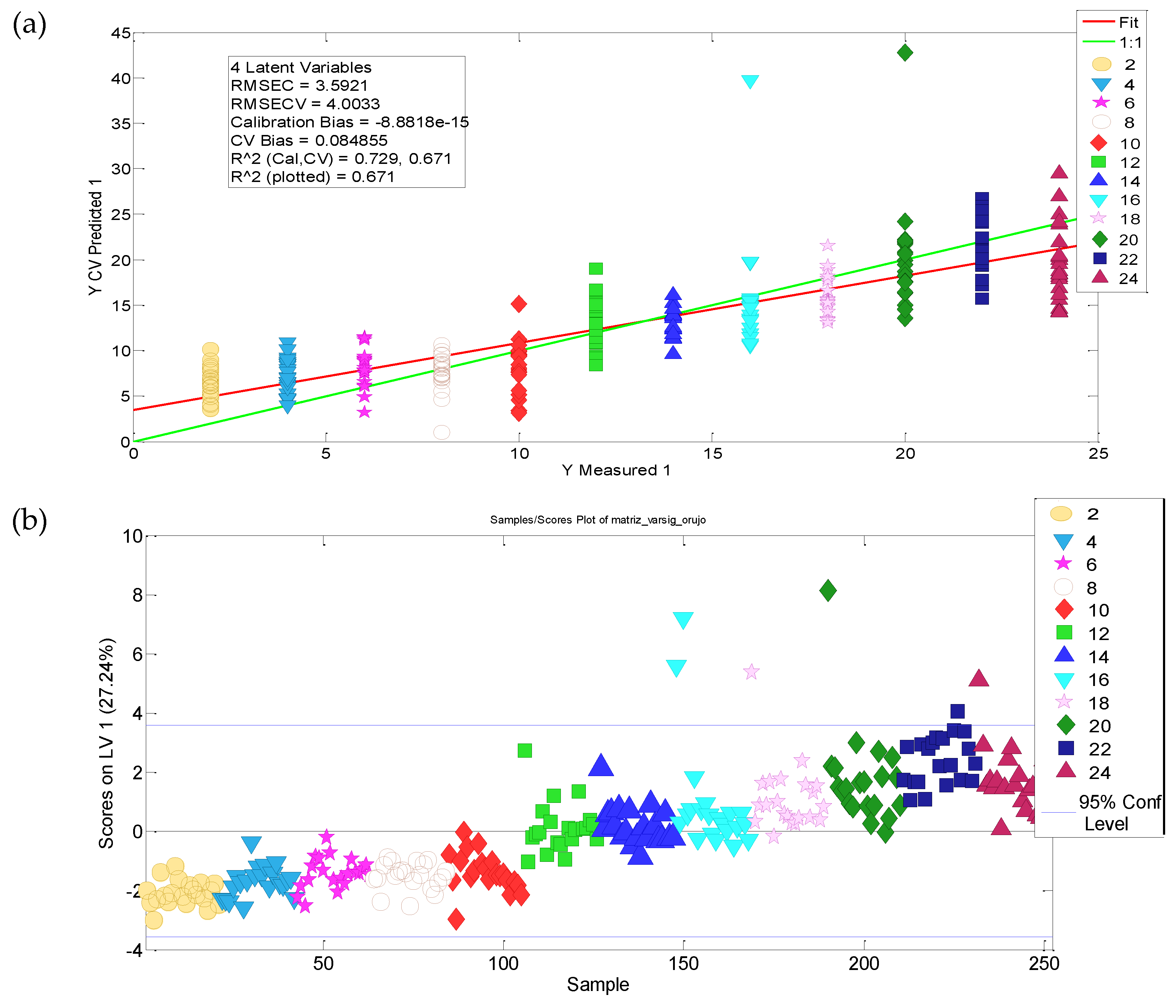
3.3. Modelling the Ageing of Refined Olive-Pomace Oils
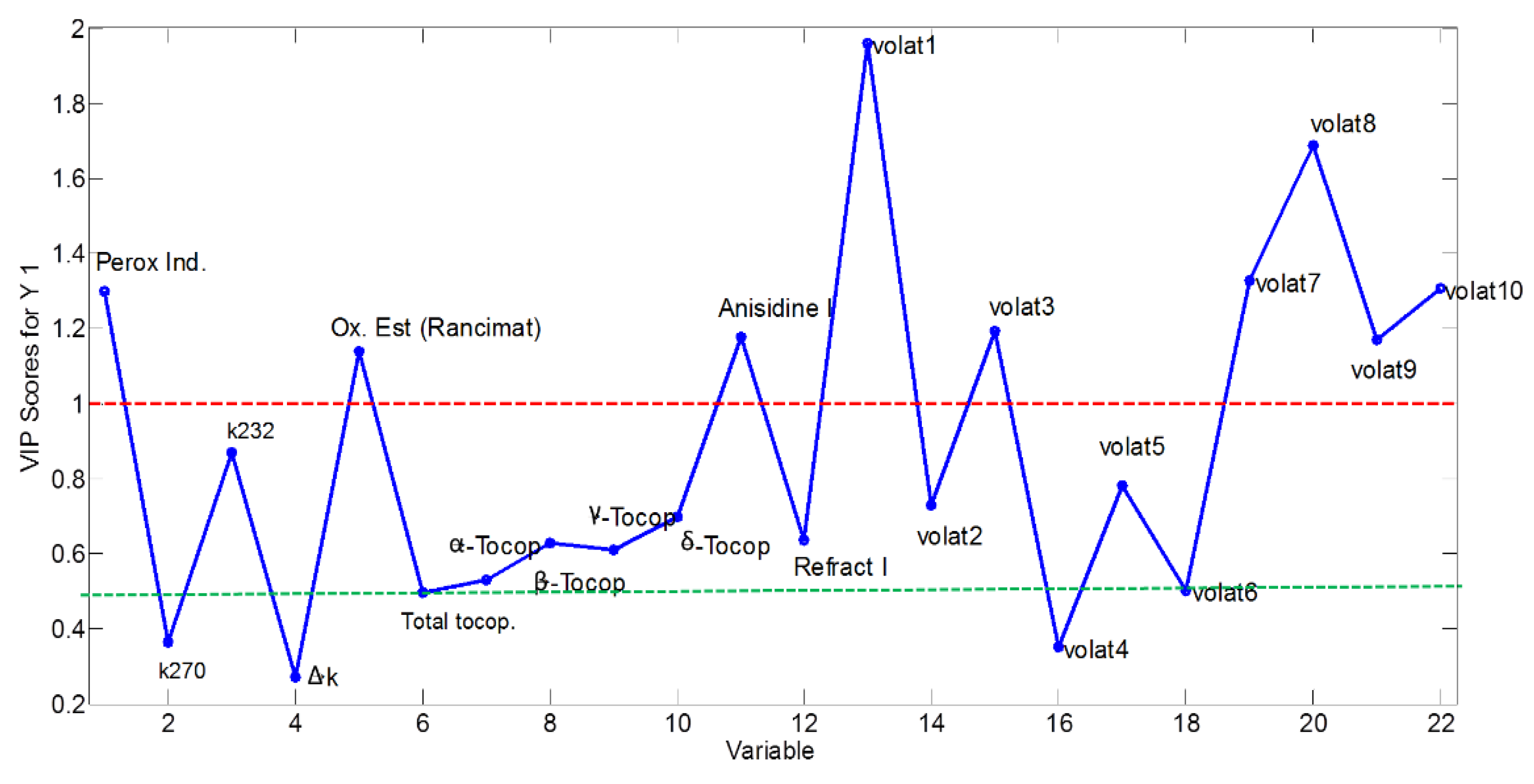
3.3.1. Selection of Significant Variables
3.3.2. Kinetic Parameters
3.3.3. Shelf Life Modelling
3.3.4. Peroxide Value-Based Modelling—Univariate Approach
3.4. Modelling the Ageing of Refined Seed Oils
3.4.1. Selection of Significant Variables
3.4.2. Kinetic Parameters
3.4.3. Shelf Life Modelling
3.5. Shelf Life Index and Ageing Rate
3.5.1. Comparison of Results (Multivariate and Univariate Approaches)
3.5.2. Verifying the Shelf Life Prediction Capability (Multivariate and Univariate Approaches)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McGuire, P.C. Refining vegetable oils: Chemical and physical refining. Sci. World J. 2022, 6627013, 1–10. [Google Scholar] [CrossRef]
- Statista. Consumo Doméstico de los Principales Aceites Vegetales en el Mundo en la Campaña de 2021/2022 Según Tipo. (In Spanish). 2022. Available online: https://es.statista.com/estadisticas/564768/consumo-domestico-de-los-principales-aceites-vegetales-segun-tipo/ (accessed on 4 August 2022).
- Roszkowska, B.; Tanska, M.; Czaplicki, S.; Konopka, I. Variation in the composition and oxidative stability of comercial rapeseed oils during their shelf life. Sci Eur. J. Lipid. Technol. 2015, 117, 673–683. [Google Scholar] [CrossRef]
- Logan, A.; Nienaber, U.; Pan, X.S. Lipid Oxidation: Challenges in Food Systems; AOCS Press: Urbana, IL, USA, 2013. [Google Scholar]
- Hu, M.; Jacobsen, C. Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Academic Press: London, UK, 2016. [Google Scholar]
- Tatum, V.; Chow, C.K. Effects of processing and storage on fatty acids in edible oils. In Fatty Acids in Foods and Their Health Implication, 3rd ed.; Chow, C.K., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 507–524. [Google Scholar]
- Serrano, A.; De la Rosa, R.; Sanchez-Ortiz, A.; Cano, J.; Pérez, A.G.; Sanz, C.; Arias-Calderón, R.; Velasco, L.; León, L. Chemical components influencing oxidative stability and sensory properties of extra virgin olive oil and effect of genotype and location on their expression. LWT Food Sci. Tech. 2021, 136, 110257. [Google Scholar] [CrossRef]
- CXS 19-1981; Standard for Edible Fats and Oils not Covered by Individual Standards. Codex Alimentarius, Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019.
- Farhoosh, R. Shelf-life prediction of edible fats and oils using Rancimat. Lipid Technol. 2007, 19, 232–234. [Google Scholar] [CrossRef]
- Grauwet, T.; Vervoort, L.; Colle, I.; Van Loey, A.; Hendrickx, M. From fingerprinting to kinetics in evaluating food quality changes. Trends Biotechnol. 2014, 32, 125–131. [Google Scholar] [CrossRef]
- Chaudhry, M.A.M.; Amodio, M.L.; Babellahi, F.; de Chiara, M.L.V.; Amigo Rubio, J.M.; Colelli, G. Hyperspectral imaging and multivariate accelerated shelf life testing (MASLT) approach for determining shelf life of rocket leaves. J. Food Eng. 2018, 238, 122–133. [Google Scholar] [CrossRef]
- Martín Torres, S.; Ruiz Castro, L.; Jimenez Carvelo, A.M.; Cuadros Rodríguez, L. Applications of multivariate data analysis in shelf life studies of edible vegetal oils—A review of the few past years. Food Packag. Shelf Life 2022, 31, 100790. [Google Scholar] [CrossRef]
- ISO 280; Essentials Oils—Determination of Refractive Index. International Organization for Standardization (ISO): Geneva, Switzerland, 1998.
- COI/T.20/Doc. No 19/ Rev.5; Spectrophotometric Investigation in the Ultraviolet. International Olive Council (IOC): Madrid, Spain, 2019.
- ISO 6886; Animal and Vegetable Fats and Oils—Determination of Oxidative Stability (Accelerated Oxidation Test). International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- COI/T.20/Doc. No 35/ Rev.1; Determination of Peroxide Value. International Olive Council (IOC): Madrid, Spain, 2017.
- ISO 6885; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ISO 9936; Animal and Vegetable Fats and Oils. Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- Dieffenbacher, A.M.; Poclington, W.D. Determination of antioxidants by high performance liquid chromatography. In Standard Methods for the Analysis of Oils, Fats and Derivatives; 1st Supplement to the 7th Edition; Blackwell Scientific Publication: Alden Press, Oxford, 1992. [Google Scholar]
- Ortega Gavilan, F.; Valverde Son, L.; Rodríguez García, F.P.; Cuadros Rodríguez, C.; Bagur González, M.G. Homogeneity assessment of reference materials for sensory analysis of liquid foodstuffs. The virgin olive oil as case study. Food Chem. 2020, 322, 126743. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M.A.J.S. Kinetic modelling of food quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Oliveri, P.; Malegori, C.; Casale, M. Chemometrics: Multivariate analysis of chemical data. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 33–76. [Google Scholar]
- European Commission. Regulation (EU) 2019/1604 of 27 September 2019 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; L250/14-48; Official Journal of the European Union; Official Journal of the European Union; 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1604 (accessed on 10 August 2022).
- CXS 210-1999; Standard for Named Vegetable Oils CXS 210-1999. Codex Alimentarius. Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019.
- Wójcicki, K.; Khmelinskii, I.; Sikorski, M.; Sikorska, E. Near and mid infrared spectroscopy and multivariate data analysis in studies of oxidation of edible oils. Food Chem. 2015, 187, 416–423. [Google Scholar] [CrossRef] [PubMed]

| (i) Olive-Pomace Oil Matrix | (ii) Seed Oil Matrix | ||||
|---|---|---|---|---|---|
| Name | Variable Number | Retention Time Interval (min) | Name | Variable Number | Retention Time Interval (min) |
| Volat1 | 77 | 1.60–1.68 | Volat1 | 76 | 1.58–1.69 |
| Volat2 | 167 | 1.77–1.79 | Volat2 | 196 | 1.82–1.84 |
| Volat3 | 195 | 1.81–1.84 | Volat3 | 521 | 2.34–2.48 |
| Volat4 | 337 | 2.04–2.12 | Volat4 | 846 | 2.89–2.93 |
| Volat5 | 491 | 2.31–2.32 | Volat5 | 871 | 2.93–3.01 |
| Volat6 | 518 | 2.33–2.47 | |||
| Volat7 | 845 | 2.90–2.92 | |||
| Volat8 | 869 | 2.93–2.97 | |||
| Volat9 | 2092 | 4.96–5.01 | |||
| Volat10 | 2124 | 5.03–5.06 | |||
| No | Parameter | Brief Description |
|---|---|---|
| 1. | Peroxide value | Peroxide content expressed in terms of milliequivalents of active oxygen per kilogram of vegetable oil. |
| 2. | K270 | The specific absorbances are calculated for a concentration of 1% (m/V) in a 10 mm cell (absorbance units × (g/100 mL)–1 × cm–1). |
| 3. | K232 | |
| 4. | ΔK | |
| 5. | Refractive index | At 20 °C as reference temperature (no units). |
| 6. | Oxidative stability | Rancimat induction period (in hours) at 120 °C. |
| 7. | Anisidine value | Rate of increase of absorbance, at 350 nm in a 10 mm cell, when reacted with p-anisidine under specific conditions (no units). |
| 8. | Total tocopherols | Absolute content of each type of tocopherol expressed in milligrams per kilogram of vegetable oil, determined by liquid chromatography. |
| 9. | α-tocopherol | |
| 10. | β-tocopherol | |
| 11. | γ-tocopherol | |
| 12. | δ-tocopherol | |
| 13. | Volat1 | Normalized chromatographic intensities (heights) at specific retention time values previously selected, extracted from the corresponding volatile chromatographic fingerprint (no units). |
| 14. | Volat2 | |
| 15. | Volat3 | |
| 16. | Volat4 | |
| 17. | Volat5 | |
| 18. | Volat6 | |
| 19. | Volat7 | |
| 20. | Volat8 | |
| 21. | Volat9 | |
| 22. | Volat10 |
| Selected Variables | Excluded Variables | ||
|---|---|---|---|
| Peroxide value | Volat1 | K270 | Volat4 |
| Oxidative stability | Volat2 | K232 * | Volat6 |
| Anisidine value | Volat3 | ΔK | Volat10 * |
| α-Tocopherol | Volat5 | Total tocopherols | |
| γ-Tocopherol | Volat7 | β-Tocopherol * | |
| Volat8 | δ-Tocopherol | ||
| Volat9 | Refractive index * | ||
| Variable | Acceptability Limit | Variable | Acceptability Limit |
|---|---|---|---|
| Peroxide value | 15.0 | Volat1 | 0.196 |
| Oxidative stability | 13.4 | Volat2 | 0.143 |
| Anisidine value | 5.4 | Volat3 | 0.130 |
| α-Tocopherol | 174.7 | Volat5 | 0.086 |
| γ-Tocopherol | 10.3 | Volat7 | 0.108 |
| Volat8 | 0.084 | ||
| Volat9 | 0.073 |
| Variable | Acceptability Limit | Variable | Acceptability Limit |
|---|---|---|---|
| Peroxide value | 10.00 | Anisidine value | 4.65 |
| K270 | 4.24 | Volat1 | 0.125 |
| Oxidative stability | 4.50 | Volat2 | 0.023 |
| Total tocopherols | 665.11 | Volat3 | 0.789 |
| β-Tocopherol | 28.66 | Volat5 | 0.044 |
| Multivariate Approach PLS (LV1 Scores) Model | Univariate ApproachEmpirical (Peroxide Value) Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Ageing Time | ti | ISL | %Age | PV | ti | ISL | %Age |
| AV002 | 2 | 3.7 | 10.3 | 26 | 2.5 | 0.8 | 15.2 | 5 |
| 4 | 2.5 | 11.5 | 18 | 3.8 | 1.7 | 14.3 | 11 | |
| 6 | 3.5 | 10.5 | 25 | 6.2 | 3.8 | 12.2 | 24 | |
| 8 | 10.0 | 4.0 | 72 | 15.8 | 17.7 | −1.7 * | 111 * | |
| 10 | 14.3 | −0.3 * | 102 * | 26.7 | 42.1 | −26.1 * | 263 * | |
| AV019 | 2 | 2.0 | 12.0 | 15 | 5.1 | 2.7 | 13.3 | 17 |
| 4 | 4.0 | 10.0 | 28 | 7.0 | 4.6 | 11.4 | 29 | |
| 6 | 5.5 | 8.5 | 39 | 8 | 5.7 | 10.3 | 36 | |
| 8 | 7.7 | 6.3 | 55 | 8.8 | 6.7 | 9.3 | 42 | |
| 10 | 8.3 | 5.7 | 59 | 9.1 | 7.1 | 8.9 | 44 | |
| 12 | 12.2 | 1.8 | 87 | 9.2 | 7.2 | 8.8 | 45 | |
| 14 | 16.6 | −2.6 * | 118 * | 12.8 | 12.5 | 3.5 | 78 | |
| AV026 | 2 | 3.3 | 10.7 | 24 | 2 | 0.6 | 15.4 | 4 |
| 4 | 5.8 | 8.2 | 41 | 12.0 | 11.2 | 4.8 | 70 | |
| 6 | 10.6 | 4.4 | 75 | 8 | 5.7 | 10.3 | 36 | |
| 8 | 9.2 | 4.8 | 66 | 10 | 8.3 | 7.7 | 52 | |
| 10 | 11.4 | 2.6 | 81 | 10.5 | 9 | 7.0 | 56 | |
| 12 | 18.2 | −4.3 * | 130 * | 10.5 | 9 | 7.0 | 56 | |
| 14 | 18.0 | −4.0 * | 129 * | 9.4 | 7.5 | 8.5 | 47 | |
| 16 | 23.0 | −9.0 * | 164 * | 12 | 11.2 | 4.8 | 70 | |
| AV030 | 14 | 13.7 | 0.3 | 98 | 10.5 | 9 | 7.0 | 56 |
| 16 | 16.9 | −2.9 * | 121 * | 20 | 26.1 | −10.1 * | 163 * | |
| 24 | 19.6 | −5.6 * | 140 * | 12.9 | 12.7 | 3.3 | 79 | |
| AV031 | 2 | 8.1 | 5.9 | 58 | 10 | 8.3 | 7.7 | 52 |
| 4 | 11.5 | 2.5 | 82 | 14 | 14.5 | 1.5 | 91 | |
| 6 | 13.1 | 0.9 | 94 | 15 | 16.2 | −0.2 * | 101 * | |
| 8 | 11.4 | 2.6 | 81 | 16.3 | 18.6 | −2.6 * | 116 * | |
| 10 | 13.0 | 1.0 | 93 | 19.4 | 24.8 | −8.8 * | 155 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Torres, S.; Tello-Jiménez, J.A.; López-Blanco, R.; González-Casado, A.; Cuadros-Rodríguez, L. Monitoring the Shelf Life of Refined Vegetable Oils under Market Storage Conditions—A Kinetic Chemofoodmetric Approach. Molecules 2022, 27, 6508. https://doi.org/10.3390/molecules27196508
Martín-Torres S, Tello-Jiménez JA, López-Blanco R, González-Casado A, Cuadros-Rodríguez L. Monitoring the Shelf Life of Refined Vegetable Oils under Market Storage Conditions—A Kinetic Chemofoodmetric Approach. Molecules. 2022; 27(19):6508. https://doi.org/10.3390/molecules27196508
Chicago/Turabian StyleMartín-Torres, Sandra, Juan Antonio Tello-Jiménez, Rafael López-Blanco, Antonio González-Casado, and Luis Cuadros-Rodríguez. 2022. "Monitoring the Shelf Life of Refined Vegetable Oils under Market Storage Conditions—A Kinetic Chemofoodmetric Approach" Molecules 27, no. 19: 6508. https://doi.org/10.3390/molecules27196508
APA StyleMartín-Torres, S., Tello-Jiménez, J. A., López-Blanco, R., González-Casado, A., & Cuadros-Rodríguez, L. (2022). Monitoring the Shelf Life of Refined Vegetable Oils under Market Storage Conditions—A Kinetic Chemofoodmetric Approach. Molecules, 27(19), 6508. https://doi.org/10.3390/molecules27196508





