Abstract
By using the chemical bath deposition approach, binary bismuth sulphides (Bi2S3) and chromium-doped ternary bismuth sulphides (Bi2−xCrxS3) thin films were effectively produced, and their potential for photovoltaic applications was examined. Structural elucidation revealed that Bi2S3 deposited by this simple and cost-effective method retained its orthorhombic crystal lattice by doping up to 3 at.%. The morphological analysis confirmed the crack-free deposition, hence making them suitable for solar cell applications. Optical analysis showed that deposited thin films have a bandgap in the range of 1.30 to 1.17 eV, values of refractive index (n) from 2.9 to 1.3, and an extinction coefficient (k) from 1.03 to 0.3. From the Hall measurements, it followed that the dominant carriers in all doped and undoped samples are electrons, and the carrier density in doped samples is almost two orders of magnitude larger than in Bi2S3. Hence, this suggests that doping is an effective tool to improve the optoelectronic behavior of Bi2S3 thin films by engineering the compositional, structural, and morphological properties.
1. Introduction
To satisfy the need for renewable energy, new efforts are required to efficiently gather incident photons [1,2,3]. First-generation photovoltaic devices, such as single-crystal silicon-based devices, although having an efficiency of up to 15%, are expensive to manufacture and install. While second-generation devices, i.e., polycrystalline semiconductor thin film-based solar cells, are cost-effective, their poor efficiency limits their applicability [4,5,6]. In the quest to design efficient, inexpensive solar cell absorbers, dye-sensitized solar cells [7] and lead halide perovskite, an emerging material with an efficiency ∼30%, have paved the way for the design of newer materials [8,9]. However, there are two main concerns for the mass production of conventional perovskite. First is the issue of the intrinsic chemical instability of lead halide perovskites and second is their toxic constituent, i.e., Pb [10]. To address these issues, lead-free and non-halide perovskites with earth-abundant constituency can be an option, although, as yet, no such candidate has demonstrated comparable performance [3]. The capture and transmission of charge carriers inside semiconductor networks have always been fundamental problems that have to be addressed in order to ensure efficient charge separation. To increase the overall effectiveness of light energy conversion, several strategies to enhance photoinduced charge separation and electron transfer processes have been put forward [11,12,13]. Additionally, it is a popular issue right now to concentrate future research efforts on the creation and use of unique nanostructures for the advancement of next-generation solar cells [14,15,16].
Metal chalcogenides are a kind of solar energy material with good optical and electrical characteristics that may be used in photovoltaic applications [5,17]. A number of binary and ternary chalcogenide semiconductor materials, such as CdSe, CdS1−xSex, CdS, ZnSe, ZnS, Zn1−xCdxS, Cd1−xZnXS, Sb2S3, Sb2Se3, Bi2Se3, and Bi2S3, have been employed as semiconductor electrodes in solar cells [14,18,19,20]. Bi2S3 (bismuth sulphide) is a semiconductor material that belongs to the V-VI family. It is known as ‘bismuth glance’ or ‘bismuthinite’ when it occurs naturally in a grey crystalline form. Bi2S3 is a potential semiconducting material for optoelectronic appliances, with a band gap energy of 1.2–1.7 eV, making it suitable in thermoelectric and optoelectronic devices with a decent incoming photon to electron conversion efficiency (5%) [21]. Bi2S3 may be made using a variety of techniques, including electrodeposition, vapor deposition, sputtering, a solution gas interface, spray, and a chemical bath [22,23,24] in both non-aqueous and aqueous media. For the effective, straightforward, and easy deposition of large surface area thin films, the chemical bath deposition approach has been used [25]. In the chemical bath deposition method, both metal ions and chalcogen ions are released in a single bath and are then precipitated onto a film to form metal chalcogenides [26,27,28].
D block metals have high stability when used as dopants, decreasing semiconductor materials’ photo-corrosion restrictions. By causing reticular distortions in the semiconductor lattice, transition metals increase the percentage of faults, resulting in improved electron hole charge separation efficiency [29,30,31,32]. The dopant concentration and distribution, as well as the electron configuration and metal ion-electron donor density, all play roles in deciding the fate of the designed semiconductors [33,34,35]. Chromium is a lustrous, steely grey, hard, and brittle transition metal with the atomic number 24, which belongs to group 6. The strong corrosion resistance and hardness of chromium make it a valuable metal. In the current study, the inclusion of earth-abundant compatible Cr3+ ions into the Bi site of the orthorhombic crystal lattice is attempted in order to alter and enhance the characteristic behavior of Bi2S3.
The goal of this study is to evaluate the efficacy of the chemical bath deposition method to deposit chromium-doped Bi2S3 thin films for solar harvesting. Since impurity-induced chemical modification in semiconductor networks creates a favorable environment for the optoelectronic response, in the current study, we attempt to enhance optoelectronic properties of Bi2S3 thin films via structural and morphological manipulation with the help of a trivalent cation, i.e., Cr3+.
2. Results and Discussion
Figure 1 illustrates how an ellipsometeric method was used to gauge the films’ thickness. Film formation often slows as time goes on as a result of reactant consumption in reactions that typically start off quickly [32]. It is possible that the precipitation process was altered based on the thickness of the films with various Cr concentrations for the same deposition duration. The selectivity of EDTA for one metal ion over another and the ensuing difference in the strength of one metal-EDTA complex over another, i.e., Bi and Cr, are attributed to variations in the precipitation process and, finally, the film thickness [36]. Figure 1 reveals that Cr addition slowed down the precipitation reaction by strong chelation, developed between the Cr-EDTA [37,38,39], which resulted in a slow precipitation process by slowly releasing the Cr ions for the doped samples for the same period of deposition time, i.e., six hours.
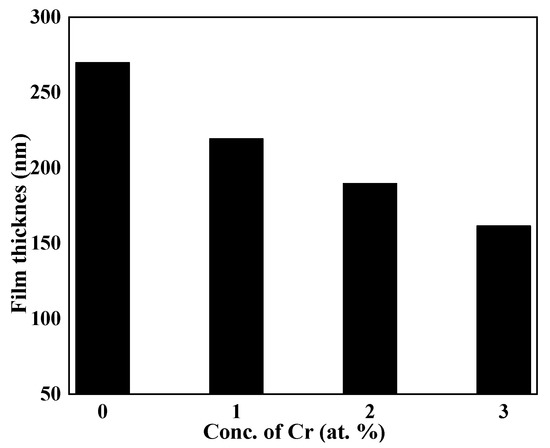
Figure 1.
Variation in thickness versus concentration of dopant of undoped and Cr-doped Bi2S3thin films.
The XRD patterns are shown in Figure 2. The polycrystalline structure of the deposited thin films is evident by sharp and well-defined peaks. XRD analysis shows that both undoped and doped materials (Bi2S3 and Bi2−xCrxS3) fit the bismuthinite phase of bismuth sulphide, with an orthorhombic structure (ICSD No: 01-075-1306), as shown by black vertical lines in Figure 2. The lack of additional peaks matching Cr or Cr-related, as well as Bi-related, peaks, suggests the formation of a single phase of Bi2S3 with high Cr homogeneity. For doped samples, a preferred orientation along the 021 plane is observed. Upon doping, the thin film growth process is influenced, resulting in the shifting of preferred planes [40].
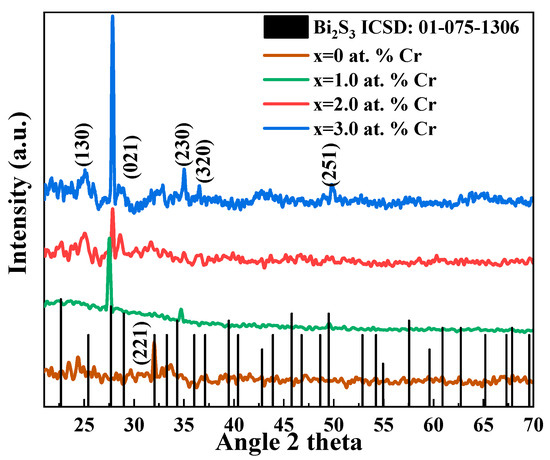
Figure 2.
Bi2S3 thin films that have been Cr-doped and left undoped show various XRD patterns.
Defects that are introduced as a result of dopant inclusion cause lattice deformation and shifts in the XRD peaks. The XRD peak locations move to either a higher or lower angle as a function of the external entity, i.e., dopant [41]. Change in the preferred orientation of thin films while transforming into the doped ones, as in the current case instead of the (221) plane to (021), is a common phenomenon [40]. Slide shifting of diffracted peaks at 2θ~35.0° and 49.0° towards larger angles give a clear indication of the incorporation of Cr ions in the Bi2S3 lattice [42]. Grain sizes were found to be decreased with the addition of Cr ions, as with the addition of Cr, more nucleation centers and sites are created for crystal growth. As both cations, i.e., Bi and Cr, act as seed nuclei, with the incorporation and increasing concentration of Cr ions, nucleation cites increased, resulting in a greater number of grains with a consequent reduction in size [43].
Lattice factors “a, b, and c”, unit cell volume “Vcell”, Scherrer crystallite size “D” [44], X-ray density “ρX-ray” dislocation density and microstrain were calculated using Equations (1)–(6).
where β is the full width at half maximum intensity, λ is the X-ray wavelength and is equal to 0.15406 nm, θ is Bragg’s angle, k is the constant equal to 0.94, Z is the number of molecules per formula unit, and M is the molar mass. Vcell and NA have their usual meanings. Crystallographic parameters calculated for both Bi2S3 and Bi2−xCrxS3 thin films calculated from XRD data are tabulated in Table 1, which seem to be influenced by Cr addition. Transitions from binary to ternary, elemental to compound, and complex compounds often result in compositional and positional chaos [45].

Table 1.
Crystallographic parameters calculated from XRD data for thin films.
The surface morphologies of undoped Bi2S3 and Cr-doped Bi2S3 thin films are shown in Figure 3. A noticeable difference was observed between the morphological properties of films with the addition of Cr from 0–3 at.%. Figure 3a depicts the surface morphology of an undoped sample, which has compact, homogenous, and interconnected particles; Figure 3b depicts the surface morphology of a sample with 1% Cr, which has incredibly small particles that are comparable to those of pure Bi2S3, while the texture of the particles was preserved after Cr insertion. Upon further increase in the dopant, Figure 3c,d indicates irregular-shaped particles with a broad range of sizes. The particle size is in the range of 150 to 80 nm for all the deposited samples, which is in agreement with the XRD findings. Upon increasing the dopant concentration, the particle size decreased. As both cations, i.e., Bi and Cr, serve as seed nuclei for the growth of nanoparticles by Ostwald’s ripening, particles were discovered to grow at the cost of previously deposited particles, resulting in agglomeration owing to the overgrowth of microscopic grains on previously deposited particles with uneven boundaries. Higher dopant concentrations resulted in loosely organized, smaller-particle-sized films on the substrate as evident by both SEM and AFM studies, hence validating the findings of XRD data. Atomic force microscopic studies (inset figures) showed that with an increasing doping concentration, the thickness increased from 51 to 57 nm by offering more surface area for photon interactions. The differences in the compositions of the deposited samples, which are determined by the Cr-to-Bi ratio, are connected to variations in their morphologies at various dopant concentrations.
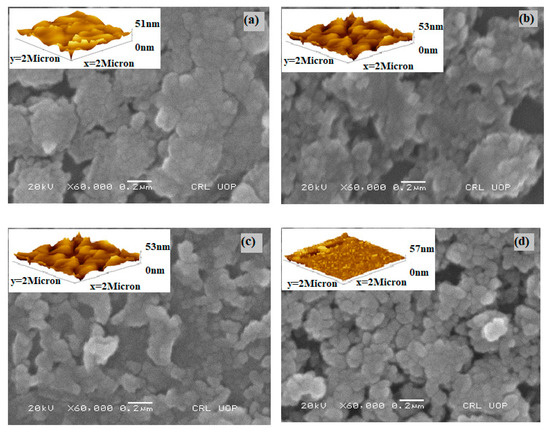
Figure 3.
Morphological analysis of (a) 1 at.% Cr, (b) 1 at.% Cr, (c) 2 at.% Cr, and (d) 3 at.% Cr doped Bi2S3 with the help of AFM and SEM micrographs.
The absorbance-versus-wavelength plot of chromium-doped bismuth sulphide thin film systems is shown in Figure 4. The strong absorbance region in this figure is between 400 and 800 nm, while in the infrared region, there is noteworthy absorbance. Furthermore, the absorption in the near-infrared region harvests more photons to invert into photocurrent [46]. The position of the absorption edge shifts red as the Cr content increases from 0 to 3 at. Percent. By modifying the ratio of Bi and S atoms, the addition of Cr to the system changes the average atomization energy, leading to this shift. The red shift in the absorption spectra will be helpful to enhance the ability of the synthesized materials to absorb a wider spectrum of light (more in the visible region). Additionally, Table 2 shows the compositions’ optical absorption coefficients, which ranged from 105 to 106 cm−1, and confirms their potential as effective absorber materials for solar applications.
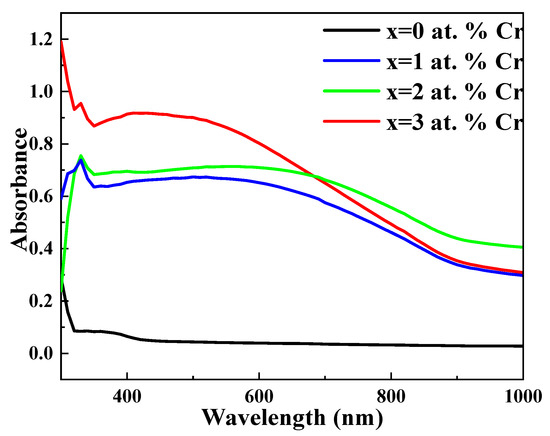
Figure 4.
Absorbance spectrum of undoped and Cr-doped Bi2S3 thin films.

Table 2.
Optical parameters of selected samples calculated by UV-vis spectroscopy at 535 nm.
Figure 5 illustrates the band gaps of the thin films, which were calculated using UV-Vis spectroscopy and the Tauc equation. These band gaps are in good agreement with known values and are appropriate for applications as visible light absorber materials [47]; the value of exponent n is 2, indicating a direct and allowed transition.
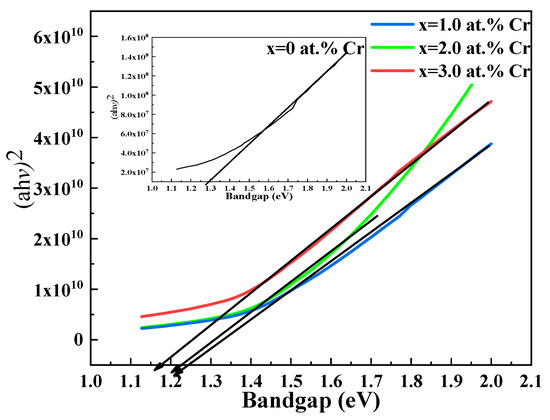
Figure 5.
Tauc plot for undoped and Cr-doped Bi2S3 thin films.
To calculate the band gap (Eg) of deposited films, the Tauc equation is used:
“h” stands for Planck’s constant (6.62 × 10−34 Js), “υ” stands for light frequency, A is the constant, and “α” stands for the absorption coefficient calculated from this relationship.
Regarding the dependence of the composition of thin films on the band gap, a decrease in the band gap as shown in Figure 6 is credited to manifestation in the band structure by introducing discrete impurity levels [48]. Crystallinity, an important factor, might also speculate its role in the decrement in the band gap [49].
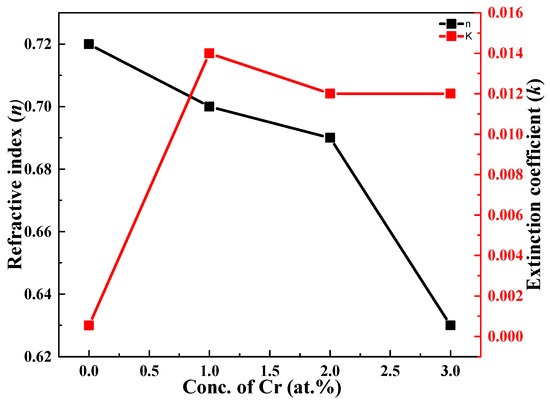
Figure 6.
Dependence of refractive index and extinction for undoped and Cr-doped Bi2S3 thin films.
Transmittance (T) and absorbance (A) are inter-related through the following equation:
A = −log (T)
The absorption coefficient (α) is calculated by the following equation:
The value of the extinction coefficient is calculated using the absorption coefficient (α) and optical wavelength (λo).
Equation (11) demonstrates how to use the reflectance (R) and extinction coefficient (k) data to obtain the refractive index (n).
The refractive index (n) and extinction coefficient are related to the dielectric constant (k).
The dielectric constant is connected to certain substances, such as those used in capacitors, printed circuit board substrates, and cable insulation. It is a complex number, with the imaginary component corresponding to dielectric losses and the real part (εr) indicating the degree of the polarizability of a material. They were calculated by the relations:
Electrical conductivity (σe) is estimated from the values of the wavelength (λ), refractive index (n), and speed of light (c = 2.8 × 108 m/s). Equation (15) may be used to determine it mathematically.
Thermal conductivity (σt) is assessed by Equation (16).
L is the Lorentz number, 2.45 × 10−8 W Ω K−2 and T is the temperature.
Table 2 shows absorption coefficient values that are suitable for use as an absorber layer in photovoltaic applications [50]. The real portion (εr) of the complex dielectric constant describes how much light is retarded in the material, while the imaginary part (εi) describes how much energy is absorbed from an electric field owing to the dipole signal. The real component of the dielectric constant is bigger than the imaginary part in this case, suggesting that the material’s reaction to light is visible and distinct [51]. Hence, the dielectric properties (ε) of materials contribute to mainly dipolar or orientation polarization, which arises from molecules that change their dipolar orientation when an electric field is applied. Values of both real and imaginary dielectric constants lie in the visible region, and this behavior leads to increased electronic transfers through the material from the valence band to the conduction band [50]. The Urbach energy, another critical optical characteristic of the material, is related to the width of the band tail of the localized states in the bandgap (Euo). The Urbach energy value is determined by the degree of defect in the chalcogenides [52]. The Urbach slope is calculated by plotting the logarithm of alpha versus photon energy and fitting a line after determining the linear zone. The width of tail states into the forbidden gap is quantified by Euo, which is the inverse of that slope. Table 2 shows the Eu values determined for undoped and doped thin films. The value of Euo is zero in a perfect semiconductor. The Urbach energy was found to increase from 0.26 to 0.35 eV when the bandgap region below the bandgap became wider and included more tail-absorbing states. Euo’s behavior is shown to be influenced by Cr content, since Cr incorporation resulted in an increase in the number of abnormalities and diseases.
Figure 6 shows the considerable compositional dependence of doping and doping concentration on the refractive index and extinction coefficient. Values of the refractive index (n) decreased from 0.72 to 0.62, while an enhancement of the values of the extinction coefficient (k) was observed, i.e., from 0.001 to 0.012.
The incorporation of the Cr dopant strongly influenced the resistivity and conductivity of thin films, as shown in Figure 7a,b. The conductivity of binary thin films was enhanced up to 1.54 × 10−2 ohm−1 cm−1, and a consequent decrease in the resistivity of films from 299.9 to 59.0 Ω cm upon transforming the binary compound to a ternary compound is observed (Table 3). The value of sheet carrier mobility (μs) is calculated and found to be 47.7 cm2/V s, which is considered to be higher than those published before in the literature (i.e., 28 cm2/V s) [53]. The value of carrier concentrations (Ns) decreases while μs increases with an increasing concentration of Cr, as shown in Figure 7. Recombination of the stimulated carriers by the traps, which may be a shadow or deep, led to a decrease in mobility [54]. The behavior of thin films in the present study is n-type.
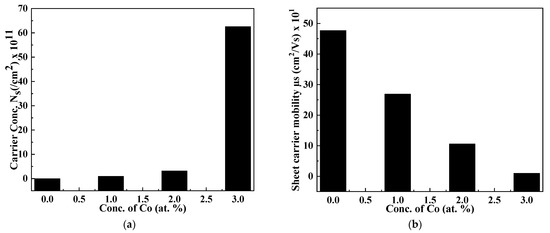
Figure 7.
Dependence of (a) carrier concentration and (b) sheet carrier mobility on dopant concentration.

Table 3.
Hall studies of undoped and Cr-doped Bi2S3 films.
Figure 8 depicts the IV behavior of undoped and selected Cr-doped thin films. It is clear that with an increasing Cr content, the diode behavior of the film is enhanced, hence making the ternary material more suitable for photovoltaic applications. An improvement in the photocurrent signal of treated Bi2S3 compared to that of pure Bi2S3 under visible-light irradiation has also been reported previously [55].
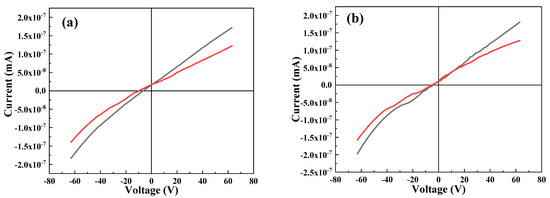
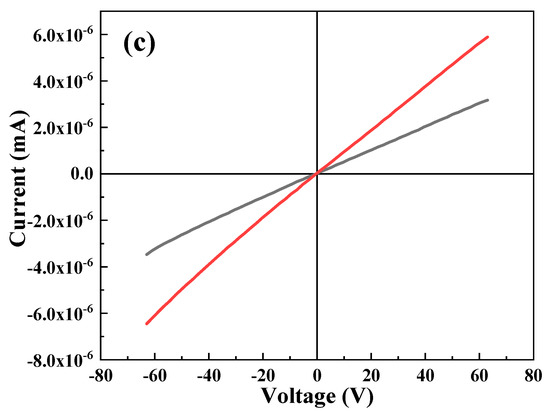
Figure 8.
IV study of (a) 0 at.% Cr, (b) 1. 0 at.% Cr, and (c) 3.0 at.% Cr.
3. Materials and Methods
Well-cleaned commercially available soda-lime microscopic glass slides were used as substrates. The chemicals used were Bi(NO3)3·5H2O, chromium nitrate pentahydrate (Cr(NO3)3·9H2O, Sigma, Schnelldorf, Germany, 98%), thioacetamide (CH3CSNH2, Aldrich, 99%), nitric acid (HNO3, Sigma), and ethylene-diamine-tetra-acetic-acid, (EDTA, Sigma, 99%). To deposit pure and chromium-doped samples in the range of 1–3 at%, four baths with varying concentrations of Cr(NO3)3·9H2O and Bi(NO3)3·5H2O were prepared to deposit undoped and doped films, labeled as 0 at.% Cr, 1.0 at.% Cr, 2.0 at.% Cr, and 3.0 at.% Cr. Equal-volume and equimolar (10 mL of 0.10 M) bismuth nitrate and EDTA solutions for the pure sample were mixed in a bath at pH 2. To synthesize the doped derivative samples, different concentrations of the chromium solution were added to the same bath. Thioacetamide (10 mL of 0.1 M) was added to the resultant mixture as the sulphur source. Pre-cleaned-glass films were placed vertically in the resultant mixture beaker for six hours at room temperature.
In order to assess how the planned material will behave, synthetic samples were exposed to various characterizations. Using a PANalytical Xpert’ Pro (Holland) X-ray Diffractometer, the phase composition of the deposited thin films was investigated using an X-ray diffraction study in the 20–700 range with Cu K irradiation (k = 0.15406 nm). Optical analysis was performed using the Perkin Elmer Lambda 25 spectrophotometer. Investigation of the morphology and content of samples was carried out using the JSM-6360A SEM and the ‘Contact mode AFM’ nasoscope digital equipment with a silicon nitride cantilever. Using a nano-chip dependability grade Hall effect device, the Hall experiments were examined. The optical properties of thin films were verified using the Systronics-117 spectrophotometer’s ellipsometry method (sensor). Additionally, the Keithley-2635A source meter was used to assess IV behavior while in ohmic contact with an Ag electrode.
4. Conclusions
Low bandgap energy, preferably in the visible range, high surface area, and conductivity are prerequisite properties for an efficient photocatalytic material. In the current study, chromium-doped bismuth sulphide thin films with good lateral homogeneity and an energy bandgap between 1.3 and 1.15 eV were successfully deposited in an acidic medium via the chemical bath deposition technique. The optical characteristics of the films were modified by dopant incorporation by modifying the lattice parameters and thickness of the films, according to a correlation between the optical band gap and lattice parameters of the films. According to the films’ optical properties, almost all of them were found to be efficient absorbers in the targeted UV-Vis range. Top-view scans and AFM observations indicate that the surfaces of the films were affected by the Cr contributions. We determined that the Cr concentration in the ternary chromium-doped bismuth sulphide chalcogenide had an effect on all of the distinctive characteristics of the deposited films without disrupting the crystal lattice. It is necessary to relate the influence of the dopant concentration on the distinctive characteristics at the same thickness by altering the deposition duration, since all optoelectronic properties rely on the thickness of the film.
Author Contributions
Conceptualization, writing-original draft Preparation, T.F. and S.I.; methodology, M.S.; software, B.I.; validation, project administration, N.S., H.A. and M.M.A.-A.; writing review and editing, E.B.E.; investigation, H.H.S.; resources, R.A.P.; data curation, E.A.; writing-original draft preparation, A.-E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR41), Email: rapasha@uqu.edu.sa. This work was supported by King Khalid University through a grant (KKU/RCAMS/22) under the Research Center for Advanced Materials Science (RCAMS) at King Khalid University, Saudi Arabia. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R7), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data will be available on request.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4320141DSR41), Email: rapasha@uqu.edu.sa. This work was supported by King Khalid University through a grant (KKU/RCAMS/22) under the Research Center for Advanced Materials Science (RCAMS) at King Khalid University, Saudi Arabia. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R7), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohammed, M.K.; Shalan, A.E.; Dehghanipour, M.; Mohseni, H. Improved mixed-dimensional 3D/2D perovskite layer with formamidinium bromide salt for highly efficient and stable perovskite solar cells. Chem. Eng. J. 2022, 428, 131185. [Google Scholar] [CrossRef]
- Savill, K.J.; Ulatowski, A.M.; Herz, L.M. Optoelectronic Properties of Tin–Lead Halide Perovskites. ACS Energy Lett. 2021, 6, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Schileo, G.; Grancini, G. Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells. J. Mater. Chem. C 2021, 9, 67–76. [Google Scholar] [CrossRef]
- Gao, J.; Ma, X.; Xu, C.; Wang, X.; Son, J.H.; Jeong, S.Y.; Zhang, Y.; Zhang, C.; Wang, K.; Niu, L. Over 17.7% efficiency ternary-blend organic solar cells with low energy-loss and good thickness-tolerance. Chem. Eng. J. 2022, 428, 129276. [Google Scholar] [CrossRef]
- Salmanogli, A.; Gokcen, D. Entanglement Sustainability Improvement Using Optoelectronic Converter in Quantum Radar (Interferometric Object-Sensing). IEEE Sens. J. 2021, 21, 9054–9062. [Google Scholar] [CrossRef]
- Jafari, A.; Khademi, S.; Farahmandjou, M. Nano-crystalline Ce-doped TiO2 powders: Sol-gel synthesis and optoelectronic properties. Mater. Res. Express 2018, 5, 095008. [Google Scholar] [CrossRef]
- Thomas, K.J.; Singh, P.; Baheti, A.; Hsu, Y.-C.; Ho, K.-C.; Lin, J.T.s. Electro-optical properties of new anthracene based organic dyes for dye-sensitized solar cells. Dye. Pigment. 2011, 91, 33–43. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Chen, X.; Zhang, L. High-performance flexible lead-free perovskite solar cells based on tin-halide perovskite films doped by reductant metal halide. Mater. Lett. 2022, 321, 132460. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Y.; Tang, R.; Zhang, J.; Chen, K.; Liu, H.; Wu, F.; Zhong, C.; Liu, X.; Zhu, L. Periphery group engineering in hole transport materials for efficient perovskite solar cells. Dye. Pigment. 2022, 206, 110671. [Google Scholar] [CrossRef]
- Dou, J.; Bai, Y.; Chen, Q. Challenges of lead leakage in perovskite solar cells. Mater. Chem. Front. 2022, 6, 2779–2789. [Google Scholar] [CrossRef]
- Bhandari, S.; Mondal, D.; Nataraj, S.K.; Balakrishna, R.G. Biomolecule-derived quantum dots for sustainable optoelectronics. Nanoscale Adv. 2019, 1, 913–936. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Azimi, M.; Santato, C.; Cicoira, F. Combining Aqueous Solution Processing and Printing for Fabrication of Flexible and Sustainable Tin Dioxide Ion-Gated Transistors. Adv. Mater. Techol. 2022, 7, 2100843. [Google Scholar] [CrossRef]
- Giraud, L.; Grelier, S.; Grau, E.; Hadziioannou, G.; Brochon, C.; Cramail, H.; Cloutet, E. Upgrading the chemistry of π-conjugated polymers toward more sustainable materials. J. Mater. Chem. C 2020, 8, 9792–9810. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Jiang, C.; Li, C.; Xiao, P.; Tang, R.; Gong, J.; Chen, G.; Chen, T.; Li, J. Regulating Energy Band Alignment via Alkaline Metal Fluoride Assisted Solution Post-Treatment Enabling Sb2(S,Se)3 Solar Cells with 10.7% Efficiency. Adv. Energy Mater. 2022, 12, 2103015. [Google Scholar] [CrossRef]
- Mustafa, G.M.; Zelai, T.; Bouzgarrou, S.; Alhossainy, M.; Mahmood, Q.; Mera, A.; Hegazy, H.; Alharthi, S.; Amin, M.A. First principle study of magnesium-based chalcogenides MgLa2(S/Se)4 for solar cells and renewable energy applications. Appl. Phys. A 2022, 128, 38. [Google Scholar] [CrossRef]
- Tao, K.; Tang, Y.; Rencus-Lazar, S.; Yao, Y.; Xue, B.; Gilead, S.; Wei, G.; Gazit, E. Bioinspired Supramolecular Packing Enables High Thermo-Sustainability. Angew. Chem. 2020, 59, 19037–19041. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.D.; Tang, L.; Giesbers, G.; Van Court, R.C.; Zhu, L.; Robinson, S.C.; Ostroverkhova, O.; Fang, C. Ultrafast Triplet State Formation in a Methylated Fungi-Derived Pigment: Toward Rational Molecular Design for Sustainable Optoelectronics. J. Phys. Chem. C 2021, 125, 17565–17572. [Google Scholar] [CrossRef]
- Rasal, A.S.; Chang, T.-W.; Korupalli, C.; Chang, J.-Y. Composition engineered ternary copper chalcogenide alloyed counter electrodes for high-performance and stable quantum dot-sensitized solar cells. Compos. Part B Eng. 2022, 232, 109610. [Google Scholar] [CrossRef]
- Guo, H.; Meng, R.; Wang, G.; Wang, S.; Wu, L.; Li, J.; Wang, Z.; Dong, J.; Hao, X.; Zhang, Y. Band-gap-graded Cu2ZnSn(S,Se)4 drives highly efficient solar cells. Energy Environ. Sci. 2022, 15, 693–704. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyum, M.A. The controlled synthesis of g-C3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025–2039. [Google Scholar] [CrossRef]
- Maria, C.C.S.; Patil, R.A.; Hasibuan, D.P.; Saragih, C.S.; Lai, C.-C.; Liou, Y.; Ma, Y.-R. White-light Photodetection Enhancement and Thin Film Impediment in Bi2S3 Nanorods/Thin-Films Homojunction Photodetectors. Appl. Surf. Sci. 2022, 584, 152608. [Google Scholar] [CrossRef]
- Pejova, B.; Grozdanov, I. Structural and optical properties of chemically deposited thin films of quantum-sized bismuth (III) sulfide. Mater. Chem. Phys. 2006, 99, 39–49. [Google Scholar] [CrossRef]
- Sakthivel, R.; Lin, L.-Y.; Lee, T.-H.; Liu, X.; He, J.-H.; Chung, R.-J. Disposable and cost-effective label-free electrochemical immunosensor for prolactin based on bismuth sulfide nanorods with polypyrrole. Bioelectrochemistry 2022, 143, 107948. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Missous, M.; Pinter, G.; Zhong, X.; Spencer, B.; Thomas, A.G.; Lewis, D.J. Sustainable ITO films with reduced indium content deposited by AACVD. J. Mater. Chem. C 2022, 10, 579–589. [Google Scholar] [CrossRef]
- Anwar, S.; Anwar, S.; Mishra, B.K. Synthesis and Characterization of Bismuth Selenide Thin Films by Chemical Bath Deposition Technique. Adv. Sci. Lett. 2014, 20, 854–856. [Google Scholar] [CrossRef]
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: Living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). Polym. Chem. 2014, 5, 3222–3230. [Google Scholar] [CrossRef]
- Lagonegro, P.; Giovanella, U.; Pasini, M. Carbon Dots as a Sustainable New Platform for Organic Light Emitting Diode. Coatings 2021, 11, 5. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Kumar, P.; Srivastava, V.K.; Sofer, Z. Surface Functionalization of 2D Transition Metal Oxides and Dichalcogenides via Covalent and Non-covalent Bonding for Sustainable Energy and Biomedical Applications. ACS Appl. Nano Mater. 2020, 3, 3116–3143. [Google Scholar] [CrossRef]
- Jenisha, M.A.; Kavirajan, S.; Harish, S.; Archana, J.; Kamalabharathi, K.; Kumar, E.S.; Navaneethan, M. Interfacial engineering effect and bipolar conduction of Ni-doped MoS2 nanostructures for thermoelectric application. J. Alloys Compd. 2022, 895, 162493. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Bahadur, A.; Ismail, B.; Abd-Rabboh, H.S.M.; Hameed, R.; Mahmood, Q.; Ibrar, A.; Nasar, M.S.; et al. Deposition of bismuth sulfide and aluminum doped bismuth sulfide thin films for photovoltaic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 42–53. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Anwer, S.; Ali, S.; Irfan, R.M.; Li, H.; Shoaib, M.; Raheel, M.; Anjum, T.A.; Zulqarnain, M.J.C.; et al. Effect of temperature and reaction time on the morphology of L-cysteine surface capped chalcocite (Cu2S) snowflakes dendrites nanoleaves and photodegradation study of methyl orange dye under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124984. [Google Scholar] [CrossRef]
- Tian, y.; Zhang, S.; Tan, W. Oxygen-doping to Bi2S3 thin film and its substrate-dependent resistive switching. Mater. Res. Express 2019, 6, 116324. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, L.; Wu, T.; Wang, J.; Wu, F.; Chen, L.; Li, Q.; Liu, W.; Lien, S.-Y.; Gao, P. Compositional engineering of metal-xanthate precursors toward (Bi1−xSbx)2S3 (0 ≤ x ≤ 0.05) films with enhanced room temperature thermoelectric performance. J. Mater. Chem. C 2022. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Mahmood, Q.; Ismail, B.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A.; Elkaeed, E.B. Optoelectronic, structural and morphological analysis of Cu3BiS3 sulfosalt thin films. Results Phys. 2022, 36, 105453. [Google Scholar] [CrossRef]
- Fazal, T.; Iqbal, S.; Shah, M.; Mahmood, Q.; Ismail, B.; Alzhrani, R.M.; Awwad, N.S.; Ibrahium, H.A.; Alam, S.; Yasir, M.; et al. Optoelectronic Analysis of Bismuth Sulfide and Copper-Doped Bismuth Sulfide Thin Films. JOM 2022, 74, 2809–2816. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Resende, J.E.; Gonçalves, M.A.; Oliveira, L.C.A.; da Cunha, E.F.F.; Ramalho, T.C. Use of Ethylenediaminetetraacetic Acid as a Scavenger for Chromium from “Wet Blue” Leather Waste: Thermodynamic and Kinetics Parameters. J. Chem. 2014, 2014, 754526. [Google Scholar] [CrossRef]
- Song, Y.; Ammami, M.-T.; Benamar, A.; Mezazigh, S.; Wang, H. Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environ. Sci. Pollut. Res. 2016, 23, 10577–10586. [Google Scholar] [CrossRef]
- Smith, S.W. The role of chelation in the treatment of other metal poisonings. J. Med. Toxicol 2013, 9, 355–369. [Google Scholar] [CrossRef]
- Azizah, N.m.; Muhammady, S.; Purbayanto, M.A.K.; Nurfani, E.; Winata, T.; Sustini, E.; Widita, R.; Darma, Y. Influence of Al doping on the crystal structure, optical properties, and photodetecting performance of ZnO film. Prog. Nat. Sci. Mater. Int. 2020, 30, 28–34. [Google Scholar] [CrossRef]
- Ginting, M.; Taslima, S.; Sebayang, K.; Aryanto, D.; Sudiro, T.; Sebayang, P. Preparation and characterization of zinc oxide doped with ferrite and chromium. AIP Conf. Proc. 2017, 1826, 030062. [Google Scholar]
- Taziwa, R.; Meyer, E.; Katwire, D.; Ntozakhe, L. Influence of carbon modification on the morphological, structural, and optical properties of zinc oxide nanoparticles synthesized by pneumatic spray pyrolysis technique. J. Nanomater. 2017, 2017, 9095301. [Google Scholar] [CrossRef]
- Yilmaz, M.; Aydoğan, Ş. The effect of Pb doping on the characteristic properties of spin coated ZnO thin films: Wrinkle structures. Mater. Sci. Semicond. Process. 2015, 40, 162–170. [Google Scholar] [CrossRef]
- Sabarish, R.; Suriyanarayanan, N.; Kalita, J.M.; Sarma, M.P.; Wary, G. Investigation on growth, structural, optical, electrical and X-ray sensing properties of chemically deposited zinc bismuth sulfide (ZnxBi2−xS3) thin films. Mater. Res. Express 2018, 5, 056402. [Google Scholar] [CrossRef]
- Ahire, R.; Deshpande, N.; Gudage, Y.; Sagade, A.; Chavhan, S.; Phase, D.; Sharma, R. A comparative study of the physical properties of CdS, Bi2S3 and composite CdS–Bi2S3 thin films for photosensor application. Sens. Actuators A Phys. 2007, 140, 207–214. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, X.; Wang, H.; Yan, D. Organic photovoltaic cells with near infrared absorption spectrum. Appl. Phys. Lett. 2007, 91, 253503. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Desai, J.; Lokhande, C. Chemical deposition of Bi2S3 thin films from thioacetamide bath. Mater. Chem. Phys. 1995, 41, 98–103. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Behfard, Z.; Amiri, O. Synthesis of bismuth sulfide nanostructures by using bismuth (III) monosalicylate precursor and fabrication of bismuth sulfide based p–n junction solar cells. Asia-Pac. J. Chem. Eng. 2014, 9, 16–23. [Google Scholar] [CrossRef]
- Mageshwari, K.; Sathyamoorthy, R. Nanocrystalline Bi2S3 thin films grown by thio-glycolic acid mediated successive ionic layer adsorption and reaction (SILAR) technique. Mater. Sci. Semicond. Process. 2013, 16, 43–50. [Google Scholar] [CrossRef]
- Moreno-García, H.; Messina, S.; Calixto-Rodriguez, M.; Martínez, H. Physical properties of chemically deposited Bi2S3 thin films using two post-deposition treatments. Appl. Surf. Sci. 2014, 311, 729–733. [Google Scholar] [CrossRef]
- Huang, L.; Nair, P.; Nair, M.; Zingaro, R.A.; Meyers, E.A. Chemical deposition of Bi2S3 thin films on glass substrates pretreated with organosilanes. Thin Solid Film. 1995, 268, 49–56. [Google Scholar] [CrossRef]
- Cabrita, J.; Ferreira, V.; Monteiro, O. Titanate nanofibers sensitized with nanocrystalline Bi2S3 as new electrocatalytic materials for ascorbic acid sensor applications. Electrochim. Acta 2014, 135, 121–127. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, S.; Tan, W. Improved optical and electrical switching in Bi2S3 nested nano-networks with broad trap distribution. Appl. Nanosci. 2022, 12, 2023–2030. [Google Scholar] [CrossRef]
- Xi, J.; Wang, H.; Zhang, B.; Hu, X.; Zhao, F.; Zeng, B. Type I Bi2S3@ZnS Core-shell Structured Photocatalyst for the Selective Photoelectrochemical Sensing of Cu2+. Anal. Methods 2019, 11, 2605–2610. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).