Effects of Heat Treatment Duration on the Electrical Properties, Texture and Color of Polymerized Whey Protein
Abstract
1. Introduction
2. Results and Discussion
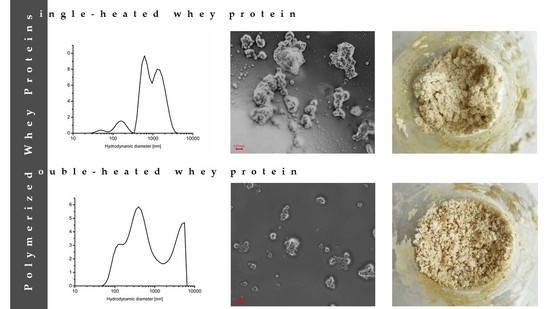
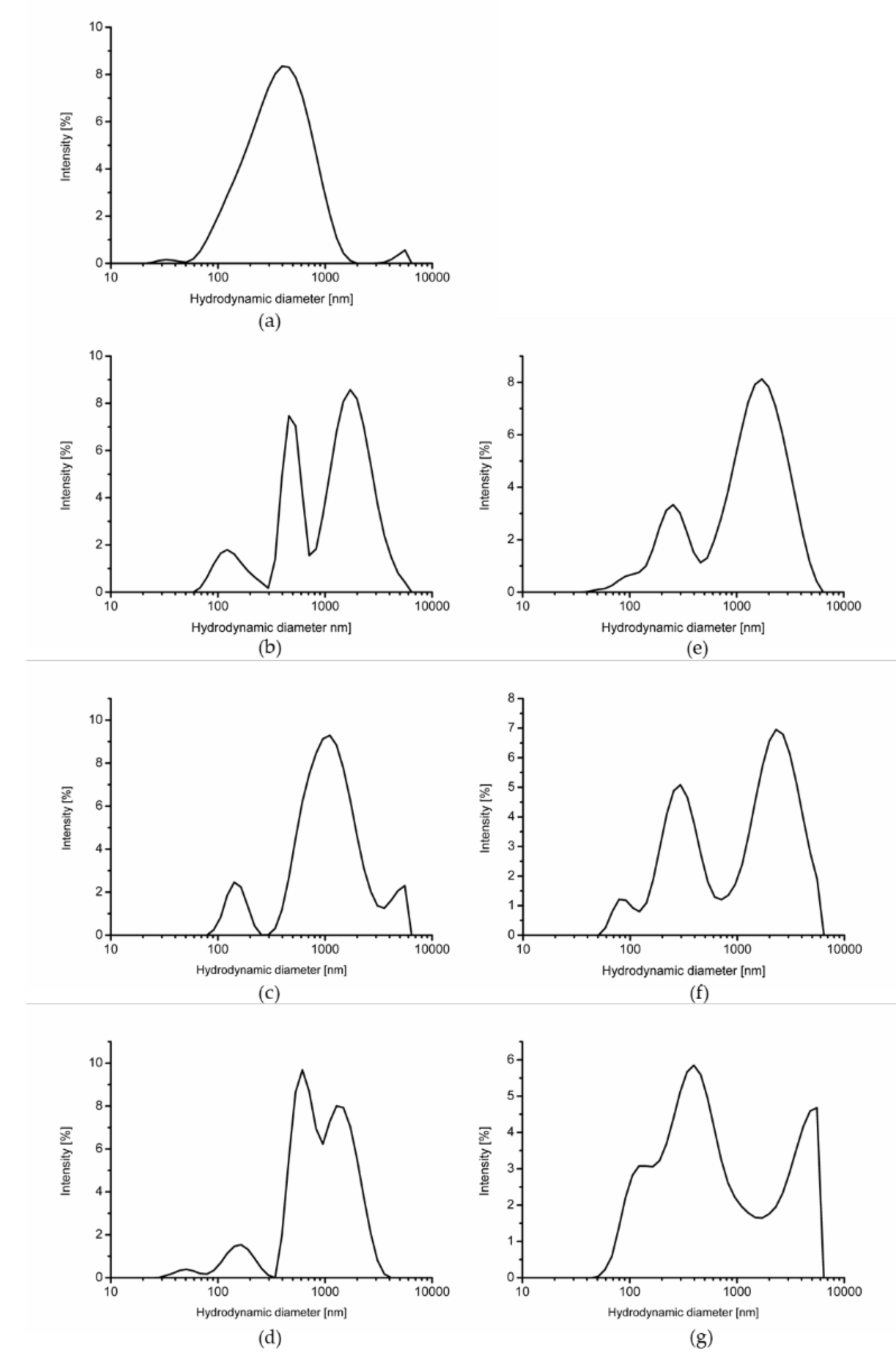
2.1. Electrical Properties and Particle Size
2.2. Color
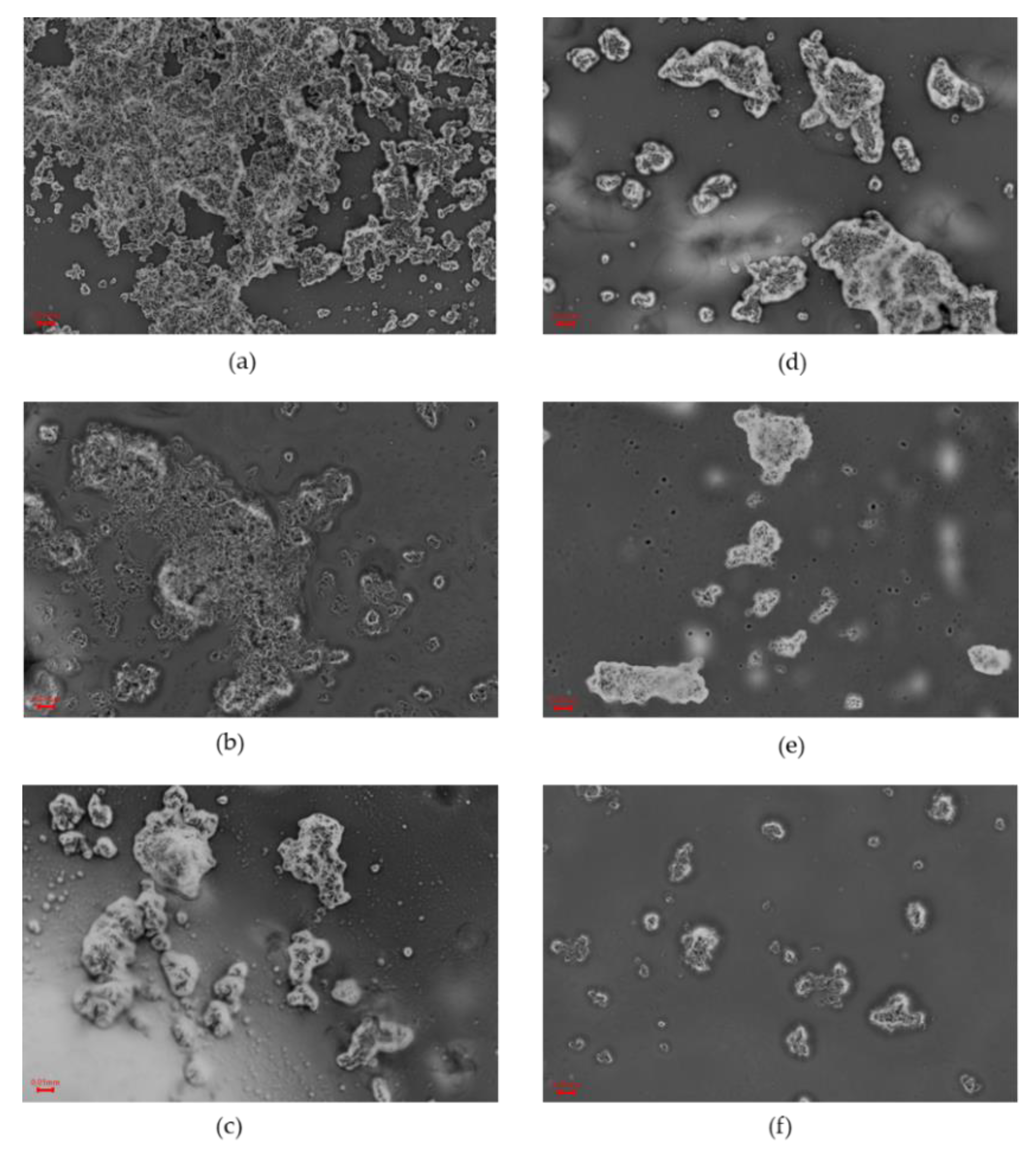
2.3. Texture and Microstructure
3. Materials and Methods
3.1. Polymerized Whey Protein
3.2. Particle Size and Zeta Potential
3.3. Electrical Conductivity
3.4. Color
where x = (a* + 1.750 · L*) · (5.645 · L* + a* − 3.012 · b*)−1
3.5. Texture
3.6. Microstructure
3.7. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khan, A.; Wang, C.; Sun, X.; Killpartrick, A.; Guo, M. Physicochemical and microstructural properties of polymerized whey protein encapsulated 3,3′-diindolylmethane nanoparticles. Molecules 2019, 24, 702. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Gao, F.; Wang, J.; Wang, C. Systematical characterization of physiochemical and rheological properties of thermal-induced polymerized whey protein. J. Sci. Food Agric. 2019, 99, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, M.; Riaublanc, A.; Lopez, C.; Hamon, P.; Rousseau, F.; Croguennec, T. Aggregated whey proteins and trace of caseins synergistically improve the heat stability of whey protein-rich emulsions. Food Hydrocoll. 2016, 61, 487–495. [Google Scholar] [CrossRef]
- Joyce, A.M.; Kelly, A.L.; O’Mahony, J.A. Controlling denaturation and aggregation of whey proteins during thermal processing by modifying temperature and calcium concentration. Int. J. Dairy Technol. 2018, 71, 446–453. [Google Scholar] [CrossRef]
- Li, R.; Lund, P.; Nielsen, S.B.; Lund, M.N. Formation of whey protein aggregates by partial hydrolysis and reduced thermal treatment. Food Hydrocoll. 2022, 124, 107206. [Google Scholar] [CrossRef]
- Jiang, S.; Hussain, M.A.; Cheng, J.; Jiang, Z.; Geng, H.; Sun, Y.; Sun, C.; Hou, J. Effect of heat treatment on physicochemical and emulsifying properties of polymerized whey protein concentrate and polymerized whey protein isolate. LWT 2018, 98, 134–140. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Foegeding, E.A.; McGuffey, M.K.; Daubert, C.R.; Swaisgood, H.E. Gelation properties of dispersions containing polymerized and native whey protein isolate. Food Hydrocoll. 2001, 15, 165–175. [Google Scholar] [CrossRef]
- Mahomud, M.S.; Katsuno, N.; Nishizu, T. Formation of soluble protein complexes and yoghurt properties influenced by the addition of whey protein concentrate. Innov. Food Sci. Emerg. Technol. 2017, 44, 173–180. [Google Scholar] [CrossRef]
- Bierzuńska, P.; Cais-Sokolińska, D.; Yiğit, A. Storage stability of texture and sensory properties of yogurt with the addition of polymerized whey proteins. Foods 2019, 8, 548. [Google Scholar] [CrossRef]
- Fang, T.; Shen, X.; Hou, J.; Guo, M. Effects of polymerized whey protein prepared directly from cheese whey as fat replacer on physiochemical, texture, microstructure and sensory properties of low-fat set yogurt. LWT 2019, 115, 108268. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Guo, M. Impact of polymerized whey protein/pectin thickening (PP) system on physical properties and volatile compounds of goat milk kefir mild and kefir. J. Food Sci. 2021, 86, 1014–1021. [Google Scholar] [CrossRef]
- Li, J.; Guo, M. Effects of polymerized whey proteins on consistency and water-holding properties of goat’s milk yogurt. J. Food Sci. 2006, 71, C34–C38. [Google Scholar] [CrossRef]
- Sołowiej, B.G.; Nastaj, M.; Szafrańska, J.O.; Muszyński, S.; Gustaw, W.; Tomczyńska-Mleko, M.; Mleko, S. Effect of emulsifying salts replacement with polymerised whey protein isolate on textural, rheological and melting properties of acid casein model processed cheeses. Int. Dairy J. 2020, 105, 104694. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta potential of food matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Ravindran, S.; Williams, M.A.K.; Ward, R.L.; Gillies, G. Understanding how the properties of whey protein stabilized emulsions depend on pH, ionic strength and calcium concentration, by mapping environmental conditions to zeta potential. Food Hydrocoll. 2018, 79, 572–578. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, B.; Xie, F.; Hu, M.; Sun, Y.; Han, L.; Li, L.; Zhang, S.; Li, Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 2021, 113, 106391. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.N.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef]
- Tholstrup Sejersen, M.; Salomonsen, T.; Ipsen, R.; Clark, R.; Rolin, C.; Balling Engelsen, S. Zeta potential of pectin-stabilised casein aggregates in acidified milk drinks. Int. Dairy J. 2007, 17, 302–307. [Google Scholar] [CrossRef]
- Carter, B.G.; Drake, M.A. Influence of oral movement, particle size, and zeta potential on astringency of whey protein. J. Sens. Stud. 2021, 36, 12652. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Q. Effect of chitosan on the heat stability of whey protein solution as a function of pH. J. Sci. Food Agric. 2017, 97, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Wang, C.N.; Guo, M.R. Interactions between whey protein or polymerized whey protein and soybean lecithin in model system. J. Dairy Sci. 2018, 101, 9680–9692. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Xiong, Y.L. Electrical conductivity: A simple and sensitive method to determine emulsifying capacity of proteins. J. Food Sci. 2021, 86, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.A.; Leuliet, J.C.; Chopard, F.; Berthou, M.; Lebouché, M. Electrical conductivity of whey protein deposit: Xanthan gum effect on temperature dependency. Food Bioprod. Process. 2004, 82, 320–325. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.; Wang, C. Interactions between whey protein and inulin in a model system. J. Food Sci. Technol. 2018, 55, 4051–4058. [Google Scholar] [CrossRef]
- Arbab Sakandar, H.; Imran, M.; Huma, N.; Ahmad, S.; Khuram, H.; Aslam, W.; Azam, M.; Shoaib, M. Effects of polymerized whey proteins isolates on the quality of stirred yoghurt made from camel milk. J. Food Process Technol. 2014, 5, 350. [Google Scholar] [CrossRef]
- Ortega, L.; Romero, A.; Muro, C.; Riera, F. Antioxidant activity and functional properties of polymerized whey products by glycation process. Int. J. Polym. Sci. 2015, 2015, 154262. [Google Scholar] [CrossRef]
- Gustaw, W. Effect of addition of whey protein aggregates obtained by single and double heating method on the rheological properties of set yoghurts. Pol. J. Food Nutr. Sci. 2007, 57, 33–36. [Google Scholar]
- Glibowski, P.; Mleko, S.; Wesolowska-Trojanowska, M. Gelation of single heated vs. double heated whey protein isolate. Int. Dairy J. 2006, 16, 1113–1118. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Pikul, J.; Wójtowski, J.; Danków, R.; Teichert, J.; Czyżak-Runowska, G.; Bagnicka, E. Evaluation of quality of kefir from milk obtained from goats supplemented with a diet rich in bioactive compounds. J. Sci. Food Agric. 2015, 95, 1343–1349. [Google Scholar] [CrossRef]

| Parameters | Sample | Time of Heated (min) | MSE | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |||
| Zeta potential (mV) | SPWP | −16.3 ± 0.1 cB | −13.3 ± 0.3 aA | −17.0 ± 0.2 dA | −15.8 ± 0.2 bA | 0.03750 |
| DPWP | −15.8 ± 0.2 aA | −18.4 ± 0.1 cB | −18.4 ± 0.2 cB | −17.4 ± 0.3 bB | 0.03167 | |
| Conductivity (mS/cm−1) | SPWP | 2.989 ± 0.0 dB | 2.004 ± 0.0 cB | 1.421 ± 0.0 bB | 0.967 ± 0.2 aB | 0.01034 |
| DPWP | 0.967 ± 0.2 bA | 0.790 ± 0.0 bA | 0.449 ± 0.0 aA | 0.448 ± 0.0 aA | 0.01031 | |
| Z-average (d, nm) | SPWP | 301.5 ± 5.1 aA | 627.8 ± 142.7 bA | 768.5 ± 93.4 bB | 656.0 ± 61.3 bB | 8219.3 |
| DPWP | 656.0 ± 61.3 bB | 673.1 ± 79.4 bA | 525.2 ± 106.3 abA | 409.7 ± 67.4 aA | 6474.3 | |
| Assessment of Color | Sample | Time of Heated (min) | MSE | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |||
| L* | SPWP | 56.99 ± 0.2 aA | 78.25 ± 0.1 bA | 80.62 ± 0.1 cA | 83.09 ± 0.0 dA | 0.01127 |
| DPWP | 83.09 ± 0.0 aB | 85.47 ± 0.2 aB | 83.84 ± 0.4 aB | 83.59 ± 2.0 aA | 1.06600 | |
| WI | SPWP | 55.81 ± 0.2 aA | 72.35 ± 0.0 bA | 74.82 ± 0.0 cB | 74.87 ± 0.0 cA | 0.01519 |
| DPWP | 74.87 ± 0.0 aB | 74.60 ± 0.1 aB | 74.01 ± 0.1 aA | 73.41 ± 1.2 aA | 0.39021 | |
| YI | SPWP | 25.32 ± 0.6 aA | 30.13 ± 0.0 cA | 27.36 ± 0.3 bA | 30.63 ± 0.1 cA | 0.08141 |
| DPWP | 30.63 ± 0.1 aB | 33.27 ± 0.0 bB | 33.14 ± 0.1 bB | 34.17 ± 0.8 bB | 0.18144 | |
| C* | SPWP | 10.18 ± 0.2 aA | 17.08 ± 0.0 cA | 16.08 ± 0.0 bA | 18.59 ± 0.0 dA | 0.00962 |
| DPWP | 18.59 ± 0.0 aB | 20.84 ± 0.0 cB | 20.35 ± 0.1 bB | 20.88 ± 0.0 cB | 0.00485 | |
| BI | SPWP | 20.70 ± 0.5 aA | 27.38 ± 0.0 cA | 24.94 ± 0.0 bA | 28.39 ± 0.1 dA | 0.06402 |
| DPWP | 28.39 ± 0.1 aB | 31.34 ± 0.0 bB | 31.17 ± 0.1 bB | 32.19 ± 0.9 bB | 0.20496 | |
| Texture Parameters | Sample | Time of Heated (min) | MSE | |||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |||
| Firmness (g) | SPWP | 14.21 ± 0.5 aA | 87.29 ± 5.7 aA | 505.25 ± 90.3 bA | 842.18 ± 101.6 cA | 4623.8 |
| DPWP | 842.18 ± 101.6 aB | 1991.79 ± 751.4 aB | 1418.32 ± 739.7 aB | 1213.29 ± 494.7 aB | 3417 × 102 | |
| Consistency (g/s) | SPWP | 267.66 ± 16.3 aA | 2012.23 ± 296.3 aA | 6988.43 ± 1411.6 bA | 10,724.64 ± 1081.4 cA | 8125 × 102 |
| DPWP | 10,724.64 ± 1081.4 aB | 13,915.35 ± 3804.9 aB | 10,324.64 ± 3732.2 aB | 9577.46 ± 2836.3 aA | 9405 × 103 | |
| Cohesiveness (g) | SPWP | 8.68 ± 0.8 aA | 89.18 ± 10.8 aA | 265.71 ± 81.0 bB | 348.50 ± 19.1 bB | 1761.0 |
| DPWP | 348.50 ± 19.1 bB | 126.00 ± 87.2 aA | 64.71 ± 44.7 aA | 30.82 ± 8.5 aA | 2507.6 | |
| Index of viscosity (g/s) | SPWP | 22.53 ± 0.7 aA | 184.67 ± 13.8 abB | 246.00 ± 43.4 bB | 311.87 ± 83.6 bB | 2266.5 |
| DPWP | 311.87 ± 83.6 bB | 20.11 ± 14.3 aA | 12.22 ± 10.9 aA | 4.23 ± 1.7 aA | 1830.1 | |
| Parameters | Conductivity (mS/cm−1) | Zeta Potential (mV) | Z-Average (d, nm) | |
|---|---|---|---|---|
| Firmness (g) | r | −0.760 | −0.715 | 0.169 |
| p | 0 | 0 | 0.431 | |
| Consistency (g/s) | r | −0.847 | −0.579 | 0.375 |
| p | 0 | 0 | 0.071 | |
| Cohesiveness (g) | r | −0.236 | 0.199 | 0.639 |
| p | 0.266 | 0.352 | 0.001 | |
| Index of viscosity (g/s) | r | 0.035 | 0.544 | 0.567 |
| p | 0.870 | 0.006 | 0.004 | |
| L* | r | −0.902 | −0.249 | 0.574 |
| p | 0 | 0.241 | 0.003 | |
| WI | r | 0.824 | 0.125 | −0.671 |
| p | 0 | 0.561 | 0 | |
| YI | r | −0.869 | −0.387 | 0.103 |
| p | 0 | 0.062 | 0.632 | |
| C* | r | −0.944 | −0.363 | 0.349 |
| p | 0 | 0.081 | 0.094 | |
| BI | r | −0.912 | −0.386 | 0.205 |
| p | 0 | 0.063 | 0.338 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielska, P.; Cais-Sokolińska, D.; Dwiecki, K. Effects of Heat Treatment Duration on the Electrical Properties, Texture and Color of Polymerized Whey Protein. Molecules 2022, 27, 6395. https://doi.org/10.3390/molecules27196395
Bielska P, Cais-Sokolińska D, Dwiecki K. Effects of Heat Treatment Duration on the Electrical Properties, Texture and Color of Polymerized Whey Protein. Molecules. 2022; 27(19):6395. https://doi.org/10.3390/molecules27196395
Chicago/Turabian StyleBielska, Paulina, Dorota Cais-Sokolińska, and Krzysztof Dwiecki. 2022. "Effects of Heat Treatment Duration on the Electrical Properties, Texture and Color of Polymerized Whey Protein" Molecules 27, no. 19: 6395. https://doi.org/10.3390/molecules27196395
APA StyleBielska, P., Cais-Sokolińska, D., & Dwiecki, K. (2022). Effects of Heat Treatment Duration on the Electrical Properties, Texture and Color of Polymerized Whey Protein. Molecules, 27(19), 6395. https://doi.org/10.3390/molecules27196395