In Vitro Antimelanoma Properties of Verbena officinalis Fractions
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Antimelanoma Studies of Different Fractions
2.2. Phytochemical Analysis of V. officinalis by HR-ESI-MS
3. Materials and Methods
3.1. Chemicals, Instruments, and Software
3.2. Collection of Plant Materials
3.3. Extraction and Fractionation
3.3.1. Hexane Fraction
3.3.2. Isolation of EA3 from the Ethyl Acetate Fraction
3.3.3. Isolation of VO79 from the Chloroform Fraction with Column Chromatography
3.3.4. N-Butanol Fraction
3.3.5. Acetone Fraction
3.4. Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akther, Y.; Nabi, J.; Tabassum, N. Comprehensive Overview of Some Edible Medicinal Plants from Kashmir Valley: Cultural, Economic, and Pharmacological Importance. In Edible Plants in Health and Diseases; Springer: Singapore, 2022; pp. 137–159. [Google Scholar]
- Aljohny, B.O.; Rauf, A.; Anwar, Y.; Naz, S.; Wadood, A. Antibacterial, Antifungal, Antioxidant, and Docking Studies of Potential Dinaphthodiospyrols from Diospyros lotus Linn Roots. ACS Omega 2021, 6, 5878–5885. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shen, Q.; Zhang, L.H.; Okumura, M.; Kawakami, A.; Ambrose, J.; Sigoillot, F.; Miller, H.R.; Gleim, S.; Cobos-Correa, A. CYP27A1-dependent anti-melanoma activity of limonoid natural products targets mitochondrial metabolism. Cell Chem. Biol. 2021, 28, 1407–1419.e6. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Khan, A.-U.; Ahmed, T. Anticonvulsant, anxiolytic, and sedative activities of Verbena officinalis. Front. Pharmacol. 2016, 7, 499. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Costa, S.; Guerra, M.; Utan, A.; Govoni, P.; Berger, A.; Müller, A.; Stuppner, H. Effects of differential extraction of Verbena officinalis on rat models of inflammation, cicatrization and gastric damage. Planta Med. 2007, 73, 227–235. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef]
- Vitalini, S.; Tomè, F.; Fico, G. Traditional uses of medicinal plants in Valvestino (Italy). J. Ethnopharmacol. 2009, 121, 106–116. [Google Scholar] [CrossRef]
- Lai, S.-W.; Yu, M.-S.; Yuen, W.-H.; Chang, R.C.-C. Novel neuroprotective effects of the aqueous extracts from Verbena officinalis Linn. Neuropharmacology 2006, 50, 641–650. [Google Scholar] [CrossRef]
- Schönbichler, S.; Bittner, L.; Pallua, J.; Popp, M.; Abel, G.; Bonn, G.; Huck, C. Simultaneous quantification of verbenalin and verbascoside in Verbena officinalis by ATR-IR and NIR spectroscopy. J. Pharm. Biomed. Anal. 2013, 84, 97–102. [Google Scholar] [CrossRef]
- Calvo, M. Anti-inflammatory and analgesic activity of the topical preparation of Verbena officinalis L. J. Ethnopharmacol. 2006, 107, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Vilalta, N.; San Julian, A.; Fernandez, M. Anti-inflammatory activity of leaf extract of Verbena officinalis L. Phytomedicine 1998, 5, 465–467. [Google Scholar] [CrossRef]
- Bilia, A.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F. HPLC-DAD-ESI-MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.; GarcAa-Mina, J.; Calvo, M. Antioxidant and antifungal activity of Verbena officinalis L. leaves. Plant Foods Hum. Nutr. 2008, 63, 93–97. [Google Scholar] [CrossRef]
- Hernández, N.E.; Tereschuk, M.; Abdala, L. Antimicrobial activity of flavonoids in medicinal plants from Tafı del Valle (Tucuman, Argentina). J. Ethnopharmacol. 2000, 73, 317–322. [Google Scholar] [CrossRef]
- Mengiste, B.; Lulie, S.; Getachew, B.; Gebrelibanos, M.; Mekuria, A.; Masresha, B. In vitro antibacterial activity of extracts from aerial parts of Verbena officinalis. Adv. Biol. Res. 2015, 9, 53–57. [Google Scholar]
- Martino, L.D.; D’Arena, G.; Minervini, M.M.; Deaglio, S.; Sinisi, N.P.; Cascavilla, N.; Feo, V.D. Active caspase-3 detection to evaluate apoptosis induced by Verbena officinalis essential oil and citral in chronic lymphocytic leukaemia cells. Rev. Bras. Farmacogn. 2011, 21, 869–873. [Google Scholar] [CrossRef]
- Martino, L.d.; D’Arena, G.; Minervini, M.; Deaglio, S.; Fusco, B.; Cascavilla, N.; Feo, V.D. Verbena officinalis essential oil and its component citral as apoptotic-inducing agent in chronic lymphocytic leukemia. Int. J. Immunopathol. Pharmacol. 2009, 22, 1097–1104. [Google Scholar] [CrossRef]
- Bekara, A.; Amazouz, A.; Douma, T.B. Evaluating the antidepressant Effect of Verbena officinalis L.(Vervain) aqueous extract in adult rats. Basic Clin. Neurosci. 2020, 11, 91. [Google Scholar] [CrossRef]
- Encalada, M.A.; Rehecho, S.; Ansorena, D.; Astiasaran, I.; Cavero, R.Y.; Calvo, M.I. Antiproliferative effect of phenylethanoid glycosides from Verbena officinalis L. on colon cancer cell lines. LWT-Food Sci. Technol. 2015, 63, 1016–1022. [Google Scholar] [CrossRef]
- Grases, F.; Melero, G.; Costa-Bauza, A.; Prieto, R.; March, J. Urolithiasis and phytotherapy. Int. Urol. Nephrol. 1994, 26, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Kou, W.-Z.; Yang, J.; Yang, Q.-H.; Wang, Y.; Wang, Z.-F.; Xu, S.-L.; Liu, J. Study on in-vivo anti-tumor activity of Verbena officinalis extract. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 512–517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calvo, M.; San Julian, A.; Fernandez, M. Identification of the major compounds in extracts of Verbena officinalis L. (Verbenaceae) by HPLC with post-column derivatization. Chromatographia 1997, 46, 241–244. [Google Scholar] [CrossRef]
- Deepak, M.; Handa, S.S. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother. Res. 2000, 14, 463–465. [Google Scholar] [CrossRef]
- Rimpler, H.; Schafer, B. Hastatoside, a new iridoid from Verbena hastata L. and Verbena officinalis L. Z. Far Nat. C 1979, 34, 311–318. [Google Scholar]
- Kaur, J.; Kumar, D.; Madaan, R.; Kumar, S. Estimation of isolated triterpenoid-ursolic acid in Verbena officinalis L. aerial parts using TLC densitometry. J. Pharm. Technol. Res. Manag. 2014, 2, 121–135. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Kokotkiewicz, A.; Miceli, N.; Taviano, M.F.; Maugeri, A.; Cirmi, S.; Synowiec, A.; Gniewosz, M.; Elansary, H.O. Production of Verbascoside, Isoverbascoside and phenolic acids in callus, suspension, and bioreactor cultures of Verbena officinalis and biological properties of biomass extracts. Molecules 2020, 25, 5609. [Google Scholar] [CrossRef]
- AlQathama, A.; Prieto, J. Natural products with therapeutic potential in melanoma metastasis. Nat. Prod. Rep. 2015, 32, 1170–1182. [Google Scholar] [CrossRef]
- Jin, S.; Kim, K.C.; Kim, J.-S.; Jang, K.-I.; Hyun, T.K. Anti-melanoma activities and phytochemical compositions of sorbus commixta fruit extracts. Plants 2020, 9, 1076. [Google Scholar] [CrossRef]
- Chambers, S.A.; Newman, M.; Frangie, M.M.; Savenka, A.V.; Basnakian, A.G.; Alam, M.A. Antimelanoma activities of chimeric thiazole–androstenone derivatives. R. Soc. Open Sci. 2021, 8, 210395. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Stat Facts: Melanoma of the Skin. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 15 August 2022).
- Salama, Y.; Jaradat, N.; Hattori, K.; Heissig, B. Aloysia Citrodora Essential Oil Inhibits Melanoma Cell Growth and Migration by Targeting HB-EGF-EGFR Signaling. Int. J. Mol. Sci. 2021, 22, 8151. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, E.; Archer, R.; Tucknott, M.; Colston, K.; Pirianov, G.; Ramanthan, D.; Dhillon, R.; Sinclair, A.; Skinner, G.A. The synthesis and anticancer effects of a range of natural and unnatural hop β-acids on breast cancer cells. Phytochem. Lett. 2012, 5, 144–149. [Google Scholar] [CrossRef]
- Habib, M.R.; Karim, M.R. Antitumour evaluation of di-(2-ethylhexyl) phthalate (DEHP) isolated from Calotropis gigantea L. flower. Acta Pharm. 2012, 62, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Yu, Z.-P.; Wang, Y.-Y.; Bao, J.; Zhu, K.-K.; Yuan, T.; Zhang, H. Triterpenoids and triterpenoid saponins from Dipsacus asper and their cytotoxic and antibacterial activities. Phytochemistry 2019, 162, 241–249. [Google Scholar] [CrossRef]
- Kao, T.-C.; Wu, C.-H.; Yen, G.-C. Bioactivity and Potential Health Benefits of Licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef]
- Woo, H.J.; Lee, J.Y.; Woo, M.H.; Yang, C.H.; Kim, Y.H. Apoptogenic activity of 2α,3α-dihydroxyurs-12-ene-28-oic acid from Prunella vulgaris var. lilacina is mediated via mitochondria-dependent activation of caspase cascade regulated by Bcl-2 in human acute leukemia Jurkat T cells. J. Ethnopharmacol. 2011, 135, 626–635. [Google Scholar] [CrossRef]
- Shu, J.-C.; Liu, J.-Q.; Chou, G.-X. A new triterpenoid from Verbena officinalis L. Nat. Prod. Res. 2013, 27, 1293–1297. [Google Scholar] [CrossRef]
- Attaur, R.; Ansari, A.A.; Kenne, L. Hederagenin, Ursolic Acid, and Pinatol from Fagonia indica. J. Nat. Prod. 1984, 47, 186–187. [Google Scholar]
- Li, N.; Wu, C.-F.; Xu, X.-Y.; Liu, Z.-Y.; Li, X.; Zhao, Y.-Q. Triterpenes possessing an unprecedented skeleton isolated from hydrolyzate of total saponins from Gynostemma pentaphyllum. Eur. J. Med. Chem. 2012, 50, 173–178. [Google Scholar] [CrossRef]
- Aftab, Z.; Afzal, M.; Bushra; Khan, H.; Badshah, S.; Khan, D.; Ullah, H.; Khan, S. Fistuloates A–C: New antioxidative aromatic compounds isolated from Cassia fistula. J. Chem. Res. 2019, 43, 516–521. [Google Scholar] [CrossRef]
- Serala, K.; Steenkamp, P.; Mampuru, L.; Prince, S.; Poopedi, K.; Mbazima, V. In vitro antimetastatic activity of Momordica balsamina crude acetone extract in HT-29 human colon cancer cells. Environ. Toxicol. 2021, 36, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Lenora, L.; Kumar, J.S.; Murugesan, S.; Senthilkumar, N. GGC-MS-MS analysis of alien invasive aquatic weed, Eichhornia crassipes (Mart.) Solms. Chem. Sin. 2016, 7, 48–52. [Google Scholar]
- Van der Doelen, G.A.; van den Berg, K.J.; Boon, J.J.; Shibayama, N.; De La Rie, E.R.; Genuit, W.J.L. Analysis of fresh triterpenoid resins and aged triterpenoid varnishes by high-performance liquid chromatography–atmospheric pressure chemical ionisation (tandem) mass spectrometry. J. Chromatogr. A 1998, 809, 21–37. [Google Scholar] [CrossRef]
- Rios, M.Y.; Salinas, D.; Villarreal, M.L. Cytotoxic Activity of Moronic Acid and Identification of the New Triterpene 3,4-seco-Olean-18-ene-3,28-dioic Acid from Phoradendron reichenbachianum. Planta Med. 2001, 67, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Peláez, G.L.M.; Sierra, J.A.; Alzate, F.; Holzgrabe, U.; Ramirez-Pineda, J.R. Pentacyclic triterpenes from Cecropia telenitida with immunomodulatory activity on dendritic cells. Rev. Bras. Farmacogn. 2013, 23, 754–761. [Google Scholar] [CrossRef]
- Alkhaibari, I.S.; Raj, K.C.H.; Alnufaie, R.; Gilmore, D.; Alam, M.A. Synthesis of Chimeric Thiazolo-Nootkatone Derivatives as Potent Antimicrobial Agents. ChemMedChem 2021, 16, 2628–2637. [Google Scholar] [CrossRef]
- Hansa, R.K.C.; Khan, M.M.K.; Frangie, M.M.; Gilmore, D.F.; Shelton, R.S.; Savenka, A.V.; Basnakian, A.G.; Shuttleworth, S.L.; Smeltzer, M.S.; Alam, M.A. 4-4-(Anilinomethyl)-3-[4-(trifluoromethyl)phenyl]-1H-pyrazol-1-ylbenzoic acid derivatives as potent anti-gram-positive bacterial agents. Eur. J. Med. Chem. 2021, 219, 113402. [Google Scholar] [CrossRef]


| Fraction | IC50 (µg/mL) | |||
|---|---|---|---|---|
| B16 | SK MEL 28 | LOX IMVI | SK MEL 5 | |
| HA | 7.6± 1.1 | 10.6 ± 1.0 | 2.8 ± 0.2 | 8.0 ± 0.1 |
| VO79 | 7.0 ± 0.8 | 7.2 ± 0.7 | 6.2 ± 0.1 | 9.6 ± 0.0 |
| EA3 | 6.2 ± 0.8 | 4.8 ± 1.0 | 3.3 ± 0.0 | 6.2 ± 0.1 |
| Taxol (µM) | 27.4 ± 4.5 | 27.3 ± 2.81 | 32.1 ± 1.1 | 19.8 ± 3.1 |
| Cisplatin | 24.3 ± 1.0 | 5.4 ± 0.3 | 27.1 ± 3.1 | |
| No | Probable Compounds | MF | m/z (found) | Class | Structure | m/z (cald) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| HA | V1 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 391.2812 | Phthalate | 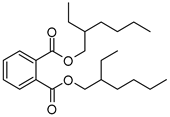 | 391.2842 | [35] |
| V2 | Lupulone A | C26H36O4 | 413.2665 | β-Bitter acids | 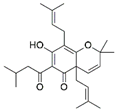 | 413.2686 | [34] | |
| Vo79 | V3 | Unknown | C26H30O3 | 391.23 | - | - | - | - |
| V4 | Hederagonic acid or Glycyrrhetinic acid | C30H46O4 | 471.34 | Triterpenoids | 471.3468 | [36,37] | ||
| V5 | (2α,3β)-2,3-Dihydroxyurs-12-en-28-oic acid or Hederagenin | C30H48O4 | 473.36 | Triterpenoids | 473.3625 | [38] [39,40] | ||
| EA3 | V6 | Gypensapogenin A | C30H42O2 | 435.32 | Triterpenes | 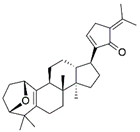 | 435.3257 | [41] |
| V7 | Fistuloate A | C30H44O2 | 437.34 | Aromatic compounds | 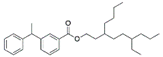 | 436.67 | [42] | |
| V8 | Momordicinin | C30H46O2 | 439.35 | Triterpenoids |  | 439.3570 | [43] | |
| V9 | Camarolide | C30H44O3 | 453.33 | Triterpenoids |  | 453.3363 | [44] | |
| V10 | Ursonic acid or Moronic acid | C30H46O3 | 455.35 | Triterpenoids | 455.3519 | [45,46] | ||
| V11 | Unknown | C31H48O4 | 485.36 | - | - | - | - | |
| V12 | Serjanic acid | C31H48O5 | 501.36 | Pentacyclic triterpenes | 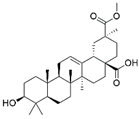 | 501.3574 | [47] | |
| V13 | Unknown | C34H46O5 | 511.34 | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, R.; Adhikary, S.; Ahmad, S.; Alam, M.A. In Vitro Antimelanoma Properties of Verbena officinalis Fractions. Molecules 2022, 27, 6329. https://doi.org/10.3390/molecules27196329
Nisar R, Adhikary S, Ahmad S, Alam MA. In Vitro Antimelanoma Properties of Verbena officinalis Fractions. Molecules. 2022; 27(19):6329. https://doi.org/10.3390/molecules27196329
Chicago/Turabian StyleNisar, Rabia, Sanjay Adhikary, Saeed Ahmad, and Mohammad Abrar Alam. 2022. "In Vitro Antimelanoma Properties of Verbena officinalis Fractions" Molecules 27, no. 19: 6329. https://doi.org/10.3390/molecules27196329
APA StyleNisar, R., Adhikary, S., Ahmad, S., & Alam, M. A. (2022). In Vitro Antimelanoma Properties of Verbena officinalis Fractions. Molecules, 27(19), 6329. https://doi.org/10.3390/molecules27196329







