Abstract
Flavonoids are polyphenols with broad known pharmacological properties. A series of 2,3-dihydroflavanone derivatives were thus synthesized and investigated for their anti-inflammatory activities. The target flavanones were prepared through cyclization of 2′-hydroxychalcone derivatives, the later obtained by Claisen–Schmidt condensation. Since nitric oxide (NO) represents an important inflammatory mediator, the effects of various flavanones on the NO production in the LPS-induced RAW 264.7 macrophage were assessed in vitro using the Griess test. The most active compounds were flavanone (4G), 2′-carboxy-5,7-dimethoxy-flavanone (4F), 4′-bromo-5,7-dimethoxy-flavanone (4D), and 2′-carboxyflavanone (4J), with IC50 values of 0.603, 0.906, 1.030, and 1.830 µg/mL, respectively. In comparison, pinocembrin achieved an IC50 value of 203.60 µg/mL. Thus, the derivatives synthesized in this work had a higher NO inhibition capacity compared to pinocembrin, demonstrating the importance of pharmacomodulation to improve the biological potential of natural molecules. SARs suggested that the use of a carboxyl-group in the meta-position of the B-ring increases biological activity, whereas compounds carrying halogen substituents in the para-position were less active. The addition of methoxy-groups in the meta-position of the A-ring somewhat decreased the activity. This study successfully identified new bioactive flavanones as promising candidates for the development of new anti-inflammatory agents.
1. Introduction
Inflammation is involved in many diseases, such as infectious diseases, chronic inflammation, asthma, diabetes, neurodegenerative diseases, coronaviruses, and cancer [1,2]. Anti-inflammatory treatments are mostly based on corticoids or non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin (2-acetyloxybenzoic acid) and ibuprofen (2-[4-(2-methylpropyl)phenyl]propanoic acid). However, long-term therapy involving these common pharmaceuticals leads to severe side effects such as gastrointestinal ulceration and bleeding, osteoporosis, hypertension, and glaucoma. Consequently, there is a need for new anti-inflammatory compounds with fewer or no side effect [3]. Macrophages are essential in the inflammatory process, and some bacterial endotoxins, such as lipopolysaccharide (LPS), allow their activation [4]. Nitric oxide (NO) is an inflammatory mediator that influences various biological processes [5]. At high levels, NO can exhibit cytotoxicity and tissue damage. Therefore, inhibition of this mediator can provide therapeutic effects and allow measurement of the degree of inflammation. Natural products such as the sesquiterpene yomogin isolated from Artemisia princeps or Ginkgo biloba extract are known to inhibit NO production [6,7].
Flavonoids are widely present in plants. A nutrition study revealed that diets rich in fruits and vegetables can help to prevent inflammatory diseases. These beneficial properties have been linked to synergetic effects of bioactive compounds, including flavonoids [8,9]. The latter compounds act as regulators of metabolic processes and have thus been investigated for the treatment of diseases. Consequently, flavonoids have been widely studied for their beneficial effects on human health, including antiallergenic, anti-inflammatory, vasodilating, anti-COVID-19, and antitumor capacities [1,2,10,11]. Flavonoids can furthermore act on important mechanisms in inflammatory processes by controlling regulatory enzymes and transcription factors [12]. Flavanones are a subgroup of flavonoids characterized by two aromatic rings (A and B) linked by a dihydropyrone ring C (Figure 1).

Figure 1.
General structure of flavanone.
Pinocembrin (5,7-dihydroxyflavanone) is a natural flavanone present in fruits, spices, propolis, tea, and red wine and has shown beneficial properties, including anti-inflammatory potential [13,14]. A recent in vivo study proved the ability of pinocembrin to inhibit the LPS-stimulated inflammatory response in macrophages and to regulate the TLR4-NF-κB signaling pathway [15]. Furthermore, pinocembrin is also found in pine species that are exploited for their wood [16]. The easy availability of biomass and low cost of co-products from these species may thus provide a renewable access to pinocembrin and its derivatives.
In order to obtain pinocembrin analogues, a two-step synthesis strategy was followed in this study. The chalcones obtained through initial Claisen–Schmidt condensation were subjected to subsequent cyclization to their corresponding flavanones [17,18]. The flavanone derivatives obtained were subsequently examined as regulators for the NO production on the LPS-induced murine macrophage. The aim of this work was to investigate the structural requirements on the A- and B-rings of flavanones for anti-inflammatory activity. Simple structure–activity relationship (SAR) analysis consequently determined the most effective candidates for future optimization studies.
2. Results and Discussion
2.1. Synthesis
A series of 2′-hydroxy chalcones (3A–3L) were synthesized by Claisen–Schmidt condensation from appropriately substituted 2′-hydroxyacetophenones and benzaldehydes. Various experimental conditions, such as acid catalysis [19], base catalysis [20], heat treatment [21], and light activation [22,23], have been reported to achieve the subsequent cyclization to flavanones. Two cyclization procedures were chosen in this work: basic and photochemical activation (Figure 2). The photochemical route was investigated as a milder approach [24]; however, the efficiency of photocyclization is known to depend on the irradiation wavelength [25]. Commercial pinocembrin (PC) was selected as a reference molecule.
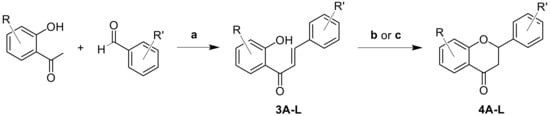
Figure 2.
Synthesis of chalcone (3A–3L) and flavanone (4A–4L) derivatives. Reaction conditions: (a) excess of NaOH in 25 mL of MeOH; (b) basic activation: 5 eq NaOAc in MeOH (20 mL) under reflux; (c) photochemical method: in EtOH at 419 nm, 30 °C for 7 days.
Following these synthetic approaches, a series of flavanones was prepared with or without methoxy-groups attached to the A-ring, while the B-ring carried various substituents in different positions (Table 1). The isolated yields of chalcones 3 ranged from 23–92%, while those of flavanones 4 varied widely between 7 and 74%, indicating a need for further optimization. After prolonged irradiation for 7 days, the photochemical cyclization gave a low isolated yield of just 7% for 4A, despite a near-complete conversion of 3A of 98%, suggesting significant losses during repeated purification by column chromatography. All other derivatives 4B–F were obtained by base-catalyzed cyclization instead. For the flavanones containing two methoxy-groups on the A-ring (A to F), the highest yield of flavanones overall was obtained with the carboxyl-group (4F), followed by the methoxy substituent (4B). For the series without substitution on the A-ring (G to K), the highest flavanone yield was achieved with the carboxyl-group (4J), followed by the parent flavanone 4G. Thus, when the corresponding benzaldehyde was substituted with an electron-withdrawing group at the 2-position (as in 4F and 4J), the formation of flavanones was effective. In contrast, the addition of electron-donating groups on the A-ring, as for derivatives 4A, G, and L, gave the desired flavanones in only low yields.

Table 1.
Synthesis of chalcones 3A–3L and flavanone derivatives 4A–4L.
Both cyclization methods were found to have limitations. The photochemical method did not require the addition of base and proceeded cleanly, but it demanded an exhaustive irradiation time to achieve a high conversion. The basic activation was found to operate faster but led to by-products that required subsequent purification steps.
2.2. Evaluation of Biological Activities
2.2.1. Biological Activities of Commercial Pinocembrin
Pinocembrin (PC) inhibitory activity was previously investigated on nitrite produced by LPS-induced RAW264.7 [26], and an IC50 of 203.60 µg/mL was determined. The anti-inflammatory bioactivity of commercially available PC was thus studied (Figure 3A), and the results showed a significant inhibitory response at 200 µg/mL (79.36 ± 7.30%) on LPS-dependent NO production, hence confirming the anti-inflammatory effect of PC in this concentration range. No inhibitory effect of PC was observed at 2 or 20 µg/mL (Figure 3A). The cytotoxicity was also analyzed (Figure 3B), and the results revealed a significant increase in percentage of cytotoxicity for PC at 200 µg/mL (94.23 ± 3.72%) compared to LPS-related cytotoxicity (32.28 ± 10.01%), while no significant difference in cytotoxicity was observed at 2 (25.76 ± 14.41%) and 20 µg/mL (34.45 ± 7.25%). A cytotoxic concentration of 200 µg/mL could possibly affect the determination of a reliable inhibitory response on NO production, as lower quantities of viable cells could result in inherently lower NO levels. These results thus highlight the importance of evaluating the cytotoxicity of natural compounds and their derivatives.

Figure 3.
(A) Anti-inflammatory activity. Nitrite representative of NO production was quantified using Griess reagent, and percent of the inhibitory response was calculated compared to the level of LPS-dependent nitrite production; (B) cytotoxicity of pinocembrin (PC) on LPS-induced RAW264.7. Murine macrophages RAW264.7 (150,000 cells/well in P96) were treated with LPS (E. coli 0111:B4) at 1 µg/mL and pinocembrin at 2, 20, and 200 µg/mL for 24 h. Cytotoxicity was measured by quantification of LDH production. Results are means ± SD (at least n = 3 well replicates). Mann–Whitney test was used to compare LPS and molecule treatments. ns, nonsignificant; **, p < 0.01.
2.2.2. Cytotoxicity of Flavanone Derivatives
Due to the cytotoxicity of PC, the cytotoxicity to LPS-induced RAW264.7 at 2 µg/mL was evaluated for all flavanone derivatives (Figure 4). Initial cytotoxicity of untreated cells was calculated at 32.41 ± 3.4%, which is consistent with previous observations [27]. No difference with LPS-related cytotoxicity was determined at 33.26 ± 10.61%, and no significant increase was observed for any flavanone at 2 µg/mL, with percentages of cytotoxicity ranging between 20 and 40%. Thus, the potential inhibitory effect of these compounds was investigated at this concentration and compared to PC.
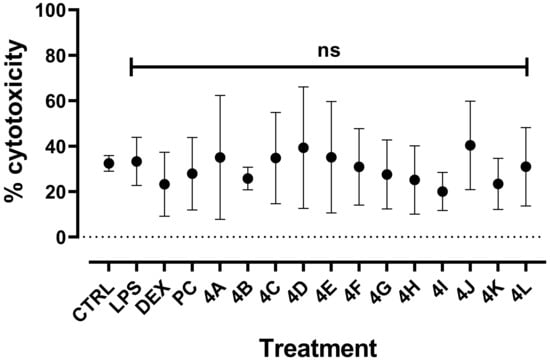
Figure 4.
Cytotoxicity of all derivatives on LPS-induced RAW264.7. Murine macrophages RAW264.7 (150,000 cells/well in P96) were treated with LPS (E. coli 0111:B4) at 1 µg/mL, analogues and pinocembrin (PC) at 2 µg/mL (in DMSO 0.1%), or anti-inflammatory dexamethasone (DEX) at 100 nM for 24 h. Cytotoxicity was measured by quantification of LDH production. Results are means ± SD of 4 independent experiments (with n = 3 well replicates/experiment). Mann–Whitney test was used to compare LPS and molecule treatments. ns, nonsignificant.
2.2.3. Inhibitory Activity on LPS-Induced NO Production
The anti-inflammatory responses of all synthetized flavanone compounds at 2 µg/mL were examined by evaluating their inhibitory effect on NO induction produced by murine macrophage RAW264.7 treated with bacterial LPS (Figure 5 and Table 2). The inhibitory response of the reference anti-inflammatory molecule dexamethasone at 100 nM was confirmed (100.4 ± 16.14%). As noted above, PC did not show an effective response at 2 µg/mL. In contrast, various inhibitory activities were determined for flavanones 4A–L at the same concentration.
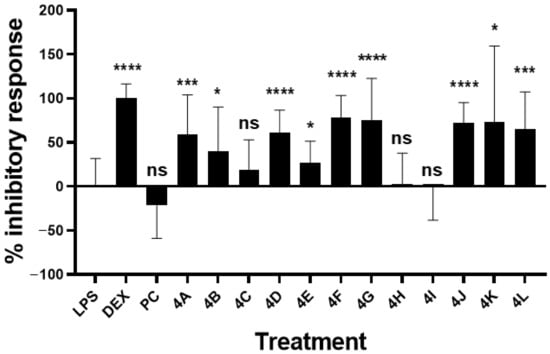
Figure 5.
Inhibitory effect of analogues on LPS-induced NO production by RAW264.7. Murine macrophages RAW264.7 (150,000 cells/well in P96) were treated with LPS (E. coli 0111:B4) at 1 µg/mL, analogues and pinocembrin (PC) at 2 µg/mL (in DMSO 0.1%), or anti-inflammatory dexamethasone (DEX) at 100 nM for 24 h. Nitrite representative of NO production was quantified using Griess reagent, and percent of the inhibitory response was calculated compared to the level of LPS-dependent nitrite production. Bars represent mean ± SD of 4 independent experiments (with n = 3 well replicates/experiment). Mann–Whitney test was used to compare LPS and molecule treatments. ns, nonsignificant; *, p < 0.05; ***, p < 0.0005; ****, p < 0.0001.

Table 2.
Inhibitory effect of analogues 4A–L on LPS-induced NO production by RAW264.7.
The most active compounds were 4F (78.65 ± 24.73%), 4G (75.65 ± 46.88%), 4J (72.56 ± 22.70%) and 4L (64.97 ± 42.37%), respectively, followed by 4D (61.10 ± 25.50%), and 4A (58.99 ± 45.19%). Due to their large SD variation, the following compounds were considered less effective but still showed a significant inhibitory response: 4K (73.29 ± 86.10%), 4B (40.45 ± 49.71%), and 4E (26.85 ± 24.43%). 4C (19.14 ± 33.83%), 4H (3.12 ± 34.64%), and 4I (−0.87 ± 37.54%) were not significantly bioactive (Table 2).
The IC50 values for the most effective molecules 4F, 4G, 4J, and 4D were subsequently calculated (Table 3). Dexamethasone presented an IC50 of 0.005 µg/mL (95% CI: 0.003–0.008). The most active flavanones were 4G, with the lowest IC50 of 0.603 µg/mL (95% CI: 0.366–1.003) and 4F with an IC50 of 0.906 µg/mL (95% CI: 0.550–1.765), while 4D and 4J furnished lower inhibitory activities with higher IC50 values of 1.030 µg/mL (95% CI: 0.675–1.382) and 1.830 µg/mL (95% CI: 1.467–2.677), respectively.

Table 3.
Inhibitory response of PC and selected derivatives on NO produced by LPS-induced RAW264.7.
2.3. Structure–Activity Relationship Study
The biological evaluation clearly indicated that the percentage of inhibition depended on the substitution pattern of flavanones 4A–L; Figure 6 summarizes the pharmacomodulation obtained from this study.

Figure 6.
Correlations between structures and anti-inflammatory activity.
For the first series with two methoxy-groups at the A-ring, the most active molecules were 4F (2′-carboxy-5,7-dimethoxy-flavanone) and 4D (4′-bromo-5,7-dimethoxy-flavanone). Hence, the presence of a carboxyl-group in the ortho-position or a bromo substituent in the para-position of the B-ring increased the anti-inflammatory activity. In addition, 4D (4′-bromo-5,7-dimethoxy-flavanone) was more active than the related 4E (4′-chloro-5,7-dimethoxy-flavanone). For the second series without substitution on the A-ring, the most active compounds were 4G (flavanone), 4J (2′-carboxyflavanone), and 4K (5′-bromo-2′-methoxy-flavanone). Overall, the presence of methoxy-groups on the flavanone skeleton impacted the NO inhibitory activity. Indeed, flavanones with a methoxy-group in the 5-position demonstrated strong inhibitory activity on NO production from LPS-stimulated macrophage cells [28]. For the functionalization on the A-ring, the comparison of 4G (flavanone), 4L (5-methoxyflavanone), and 4A (5,7-dimethoxyflavanone) showed that the addition of a methoxy-group contributes to a drop in activity. Additionally, the flavanone derivative with the methoxy-group in the 3-position (4B) was found more active than the corresponding analogue with the methoxy-substituent in the 4-position (4C). Pinocembrin (5,7-dihyodroxyflavanone) containing two hydroxyl-groups on the A-ring showed no significant effect at the same concentration if compared to the flavanone derivatives synthesized. These results are in agreement with Kim’s work, which demonstrated that naringenin (5,7,4′-trihydroxyflavanone) was inactive up to 100 µM [29]. It may thus be suggested that the presence of a hydroxy-group on the A-ring only weakly affects the NO inhibition.
This SARs clearly revealed that a 2′-carboxy-group is beneficial to achieve effective inhibition of NO production. This finding may be due to the electron-withdrawing and highly polar nature of the carboxyl-group. According to Shin’s study, a bulky and/or hydrophobic substituent in the meta-position of the B-ring results in the most active structure to inhibit NF-κB activation [30]. This study identified 4B (3’-methoxyflavanone) as more active than 4C (4’-methoxyflavanone) and revealed the importance of a carboxyl-group in the ortho-position of the B-ring instead, as in 4F and 4J.
The predictive analysis of the drug-like absorption of the most active compounds (Table 4) revealed that all compounds meet Lipinski’s rule of five [31]: hydrogen bond donors ≤ 5, hydrogen bond acceptors ≤ 10, molecular mass ≤ 500 daltons, an octanol-water partition coefficient (log p) ≤ 5, and a polar surface area ≤ 140 Å2. Subsequent future pharmacomodulations, however, may lead to compounds with further improved activity.

Table 4.
Predictive analysis of drug-like absorption.
3. Materials and Methods
3.1. Syntheses
3.1.1. General Information
All chemicals were purchased from Sigma–Aldrich (Saint-Louis, MO, USA) and were used as received. HPLC grade solvents were bought from Fischer Chemicals (Leicestershire, UK) and were used without further purification. Reactions were monitored by thin-layer chromatography (TLC) using silica gel-precoated aluminum sheets (60 F254, Merck KGaA, Darmstadt, Germany) with different solvent systems (cyclohexane/EtOAc or DCM/MeOH). Products were visualized with UV irradiation at 365 and 254 nm or by treatment with sulfuric acid-vanillin. Selected compounds were purified by column chromatography (CC) using silica gel 60 (0.015–0.040 mm, Merck) as a stationary phase or by LH-20 Sephadex column chromatography. Electrothermal and Gallenkamp melting point apparatuses (MP, OC, uncorrected) were used to determine the melting points in open capillaries. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker x400 (1H NMR: 400 MHz, 13C NMR: 100 MHz) spectrometer or Bruker DPX 500 NMR spectrometer (1H NMR: 500 MHz, 13C NMR: 125 MHz). The solvents used were CDCl3 or dimethyl sulfoxide (DMSO)-d6. Chemical shifts (δ) were recorded in parts per million (ppm) relative to residual solvent peaks (CDCl3: 1H NMR: δ = 7.26 ppm and 13C NMR: δ = 77.00 ppm; DMSO-d6: 1H NMR: δ = 2.50 ppm and 13C NMR: δ = 39.52 ppm). Coupling constants were reported in hertz (Hz). Multiplicities were reported as s (singlet), br s (broad singlet), d (doublet), t (triplet), br (broad), m (multiplet), dd (doublet of doublets), dt (doublet of triplets), ddt (doublet of doublet of triplet), ddd (doublet of doublets of doublets), app d (apparent doublet), and app t (apparent triplet). High resolution electrospray ionization mass spectrometry (HR ESI-MS) was performed at the ICOA/CBM platform (Orléans University) on a Bruker Q-TOF maXis mass spectrometer, coupled to an Ultimate 3000 RSLC chain (Dionex).
3.1.2. Synthetic Methods
For characterization of the molecular structures, the numbering of atoms shown in Figure 7 was followed.
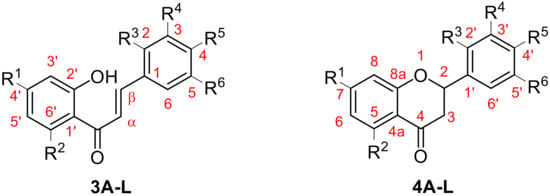
Figure 7.
Structures of chalcone and flavanone derivatives.
- General Procedure for the synthesis of chalcones (3A–L).
A solution of MeOH (25 mL), the corresponding acetophenones 1A–C (1 eq), the appropriate benzaldehyde 2A–G (1 eq), and excess of NaOH were stirred at room temperature, and the progress of the reaction was monitored by TLC. Upon completion, the excess of NaOH was neutralized by addition of HCl (1 M) with pH control. The solvent was evaporated, the residue was taken up in EtOAc, and the resulting solution was washed with distilled water. The organic layer was dried with MgSO4 and filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by crystallization from methanol (MeOH) or by column chromatography.
(E)-1-(2′-hydroxy-4,6-dimethoxyphenyl)-3-phenylprop-2-en-1-one or 2′-Hydroxy-4′,6′-dimethoxy-chalcone or flavokavain B (3A)
Prepared following the general procedure starting from 2-hydroxy-4,6-dimethoxy-acetophenone (1A, 0.1458 g, 0.74 mmol) and benzaldehyde (2A, 0.078 g, 0.74 mmol) with 10 eq of NaOH. Yellow crystals (0.057 g, 0.20 mmol), 27% yield, m.p. (Gallenkamp apparatus): 89 °C (85–86 °C, [32]).
1H-NMR (400 MHz, CDCl3): δ 14.20 (s, 1H, 2′-OH), 7.83 (d, J = 15.7 Hz, 1H, β-H), 7.72 (d, J = 15.7 Hz, 1H, α-H), 7.54 (dd, J = 7.5 Hz, 2.1 Hz, 2H, 2-H, 6-H), 7.30 (m, 3H, 3-H, 4-H, 5-H), 6.05 (d, J = 2.4 Hz, 1H, 3′-H), 5.90 (d, J = 2.4 Hz, 1H, 5′-H), 3.86 (s, 3H, 4′-OCH3), 3.77 (s, 3H, 6′-OCH3). 13C-NMR (100 MHz, CDCl3): δ 166.5 (4′-C), 162.6 (2′-C), 142.3 (β-C), 130 (1-C), 128.89 (3-C, 6-C), 128.4 (5-C), 128.3 (2-C), 127.6 (4-C), 102.7 (1′-C), 91.4 (3′-C), 91.1 (5′-C), 55.7 (4′-OCH3), 55.5 (6′-OCH3). 1H and 13C spectral data were consistent with the literature [32].
(E)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)-3-(3-methoxyphenyl)prop-2-en-1-one or 2′-hydroxy-3, 4′,6′-trimethoxy-chalcone (3B)
Prepared following the general procedure starting from 2-hydroxy-4,6-dimethoxy-acetophenone (1A, 0.50 g, 2.55 mmol) and 3-methoxy-benzaldehyde (2B, 0.346 g, 2.55 mmol) with 10 eq of NaOH. Yellow orange crystals (0.655 g, 2.09 mmol), 82% yield, m.p. (Electrothermal apparatus): 100 °C (100 °C, [33]).
1H-NMR (500 MHz, CDCl3): δ 14.25 (s, 1H, 2′-OH), 7.87 (d, J = 15.7 Hz, 1H, β-H), 7.73 (d, J = 15.7 Hz, 1H, α-H), 7.32 (t, J = 7.9 Hz, 1H, 5-H), 7.21 (d, J = 7.6 Hz, 1H, 6-H), 7.12 (d, J = 1.2 Hz, 1H, 2-H), 6.93 (dd, J = 8,2 Hz, 1H, 4-H), 6.11 (d, J = 2.4 Hz, 1H, 3′-H), 5.96 (d, J = 2.4 Hz, 1H, 5′-H), 3.91 (s, 3H, 4′-OCH3), 3.85 (s, 3H, 6′-OCH3), 3.83 (s, 3H, 3-OCH3). 13C-NMR (125 MHz, CDCl3): δ 192.6 (CO), 168.4 (4′-C), 166.3 (6′-C), 162.5 (2′-C), 159.9 (3-C), 142.2 (β-C), 137.0 (1-C),129.8 (5-C), 127.9 (6-C), 120.9 (α-C), 115.6 (4-C), 113.7 (2-C), 106.4 (1′-C), 93.8 (3′-C), 91.3 (5′-C), 55.8 (3-OCH3), 55.6 (5-OCH3), 55.3 (7-OCH3). 1H and 13C spectral data were consistent with the literature [33,34].
(E)-1-(2′-hydroxy-4′.6′-dimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one or 2′-hydroxy-4,4′,6′-trimethoxy-chalcone (3C) has been previously synthesized by our research group [33].
(E)-3-(4-bromophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one or 2′-hydroxy-4-bromo-4′,6′-dimethoxy-chalcone (3D)
Prepared following the general procedure starting from 2-hydroxy-4,6-dimethoxy-acetophenone (1A, 0.216 g, 1.10 mmol) and 4-bromo-benzaldehyde (2D, 0.204 g, 1.10 mmol) with 2.5 eq of NaOH. Yellow crystals (0.206 g, 0.57 mmol), 52% yield, m.p. (Gallenkamp apparatus): 166 °C (165 °C, [33], 150–151 °C, [32]).
1H NMR (400 MHz, CDCl3): δ 14.23 (s, 1H, 2′-OH), 7.90 (d, J = 15.6 Hz, 1H, β-H), 7.72 (d, J = 15.6 Hz, 1H, α-H), 7.58–7.53 (m, 2H, 3-H, 5-H), 7.51–7.46 (m, 2H, 2-H, 6-H), 6.14 (d, J = 2.4 Hz, 1H, 3′-H), 5.99 (d, J = 2.4 Hz, 1H, 5′-H), 3.94 (s, 3H, 4′-OCH3), 3.87 (s, 3H, 6′-OCH3). 13C NMR (100 MHz, CDCl3): δ 192.5 (CO), 166.3 (4′-C), 162.9 (6′-C), 162.6 (2′-C), 140.9 (β-C), 132.13 (5-C, 3-C) 131.6 (1-C), 129.69 (2-C, 6-C), 128.2 (4-C), 124.2 (α-C), 106.3 (1′-C), 93.8 (3′-C), 91.4 (5′-C), 55.9 (4′-OCH3), 55.6 (6′-OCH3). 1H and 13C spectra were consistent with the literature [32].
(E)-3-(4-chlorophenyl)-1-(2′-hydroxy-4′,6′-dimethoxyphenyl)prop-2-en-1-one or 4-chloro-2′-hydroxy-4′,6′-dimethoxy-chalcone (3E)
Prepared following the general procedure starting from 2-hydroxy-4,6-dimethoxy-acetophenone (1A, 0.50 g, 2.55 mmol) and 4-chloro-benzaldehyde (2E, 0.358 g, 2.55 mmol) with 5 eq of NaOH. Yellow crystals (0.189 g, 0.60 mmol), 23% yield, m.p. (Gallenkamp apparatus): 164 °C (173–175 °C, [32], 2006 and 158–168 °C, [33]).
1H NMR (400 MHz, CDCl3): δ 14.24 (s, 1H, 2′-OH), 7.88 (d, J = 15.6 Hz, 1H, β-H), 7.74 (d, J = 15.6 Hz, 1H, α-H), 7.55 (d, J = 8.6 Hz, 2H, 2-H, 6-H), 7.44–7.36 (m, 2H, 3-H, 5-H), 6.14 (d, J = 2.4 Hz, 1H, 3′-H), 5.99 (d, J = 2.4 Hz, 1H, 5′-H), 3.94 (s, 3H, 4′-OCH3), 3.87 (s, 3H, 6′-OCH3). 1H spectral data were consistent with the literature [32].
(E)-2-(3-(2′-hydroxy-4′,6′-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoic acid or 2′-hydroxy-2-carboxy 4′,6′-dimethoxychalcone (3F)
Prepared following the general procedure starting from 2-hydroxy-4,6-dimethoxy-acetophenone (1A, 0.369 g, 1.89 mmol) and 2-carboxy-benzaldehyde (2F, 0.283 g, 1.89 mmol) with 10 eq of NaOH. Yellow solid (0.220 g, 0.67 mmol), 36% yield, m.p. (Gallenkamp apparatus): 161 °C (160–161 °C, [32]).
1H NMR (400 MHz, CDCl3): δ 14.04 (s, 1H, 2′-OH), 13.74 (s, 1H, 2-COOH),8.57 (d, J = 15.4 Hz, 1H, β-H), 8.10 (dd, J = 7.1 Hz, 1.0 Hz, 1H, 3-H), 7.91 (d, J = 15.4 Hz, 1H, α-H), 7.78 (m, 1H, 5-H), 7.66 (m, 1H, 6-H), 7.56 (td, J = 8.1 Hz, 1.5 Hz, 1H, 4-H), 6.15 (d, J = 2.4 Hz, 1H, 3′-H), 6.12 (d, J = 2.4 Hz, 1H, 5′-H), 4.00 (s, 3H, 4′-OCH3), 3.90 (s, 3H, 6′-OCH3).1H spectral data were consistent with the literature [32]. HRMS (ESI): Calc. for C18H16O6, [M + H]+ m/z: 329.10196, found: 329.10204, [M + Na]+ m/z: 345.07334, found: 345.07320.
(E)-1-(2′-hydroxyphenyl)-3-phenylprop-2-en-1-one or 2′-hydroxy-chalcone (3G)
Prepared following the general procedure starting from 2-hydroxy-acetophenone (1B, 1 g, 7.34 mmol) and benzaldehyde (2A, 0.78 g, 7.34 mmol) with 10 eq of NaOH. Yellow solid (1.013 g, 4.52 mmol), 62% yield, m.p. (Gallenkamp apparatus): 77 °C (44–150 °C CAS: 1214-47-7 Aldrich).
1H NMR (400 MHz, CDCl3): δ 12.82 (s, 1H, 2′-OH), 7.96 (d, J = 15.6 Hz, 1H, β-H), 7.97–7.94 (dd, J = 8.1 Hz, 1.6 Hz, 1H, 6′-H), 7.72–7.67 (m, 3H, 2-H, 3-H, 5-H), 7.69 (d, J = 15.6 Hz, 1H, α-H), 7.53 (td, J = 8.6 Hz, 1.6 Hz, 1H, 5′-H), 7.49–7.45 (m, 4H, 2′-H, 3′-H, 4′-H, 4-H), 7.06 (dd, J = 8.5 Hz, 0.9 Hz, 1H, 6-H), 6.98 (ddd, J = 8.3 Hz, 7.4 Hz, 1.0 Hz, 1H, 4′-H). 13C NMR (100 MHz100 MHz, CDCl3): δ 193.7 (CO), 163.6 (2′-C), 145.5 (β-C), 1 36.1 (1-C), 130.9 (4′-C), 129.6 (5′-C), 129.08 (3-C), 129.07 (5-C), 128.68 (2-C, 128.67 (6-C), 127.7 (4-C), 120.2 (α-C), 118.9 (3′-C), 118.7 (1′-C). HRMS (ESI): Calc. for C15H12O2, [M + H]+ m/z: 225.09101, found: 225.09112, [M + Na]+ m/z: 247.07295, found: 247.07318.
(E)-3-(4-chlorophenyl)-1-(2′-hydroxyphenyl) prop-2-en-1-one or 4-chloro-2′-hydroxy-chalcone (3H)
Prepared following the general procedure starting from 2-hydroxy-acetophenone (1B, 2 g, 14.69 mmol) and 4-chloro-benzaldehyde (2E, 2.06 g, 14.69 mmol) with 5 eq of NaOH. Yellow solid (3.265 g, 12.62 mmol), 86% yield, m.p. (Gallenkamp apparatus): 135–140 °C (149–150 °C, [35]).
1H NMR (400 MHz, CDCl3): δ 12.76 (s, 1H, 2′-OH), 7.93 (dd, J = 8.1 Hz, 1.6 Hz, 1H, 6′-H), 7.89 (d, J = 15.6 Hz, 1H, β-H), 7.65 (d, J = 15.6 Hz, 1H, α-H), 7.65–7.60 (m, 2H, 3-H, 5-H), 7.54 (ddd, J = 8.8 Hz, 7.3 Hz, 1.6 Hz, 1H, 5′-H), 7.47–7.41 (m, 2H, 3′-H, 6′-H), 7.06 (dd, J = 8.4 Hz, 0.9 Hz, 1H, 6-H), 6.98 (ddd, J = 8.5 Hz, 7.2 Hz, 1.1 Hz, 1H, 4′-H). 13C NMR (100 MHz, CDCl3): δ 193.5 (CO), 163.7 (2′-C), 143.9 (β-C), 136.6 (4-C), 133.1 (1-C), 129.79 (3-C), 129.78 (5-C), 129.6 (4′-C), 129.38 (2-C), 129.37 (6-C), 120.7 (1′-C), 119.9 (α-C), 118.9 (3′-C), 118.7 (5′-C) [34]. HRMS (ESI): Calc. for C15H12ClO2, [M + H]+ m/z: 259.05203, found: 259.05249.
(E)-1-(2′-hydroxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one or 2′-hydroxy-4-methoxy-chalcone (3I)
Prepared following the general procedure starting from 2-hydroxy-acetophenone (1B, 2 g, 14.69 mmol) and 4-methoxy-benzaldehyde (2C, 2 g, 14.69 mmol) with 5 eq of NaOH. Orange solid (2.088 g, 8.21 mmol), 56% yield, m.p. (Gallenkamp apparatus): 84 °C (84–86 °C, [35]).
1H NMR (400 MHz, CDCl3): δ 12.95 (s, 1H, 2′-OH), 7.95 (dd, J = 8.1 Hz, 1.6 Hz, 1H, 6′-H), 7.93 (d, J = 15.4 Hz, 1H, β-H), 7.68–7.63 (m, 2H, 3-H, 5-H), 7.57 (d, J = 15.4 Hz, 1H, α-H), 7.51 (ddd, J = 8.6 Hz, 7.3 Hz, 1.6 Hz, 1H, 5′-H), 7.05 (dd, J = 8.4 Hz, 0.9 Hz, 1H, 6-H), 7.00–6.96 (m, 2H, 2-H, 3′-H), 6.99–6.93 (m, 1H, 4′-H), 3.89 (s, 3H, 4-OCH3). 13C NMR (100 MHz, CDCl3): δ 193.7 (CO), 163.6 (2′-C), 162.1 (4-C), 145.4 (β-C), 136.1 (6′-C), 130.55 (1-C), 130.54 (3-C), 130.53 (5-C), 129.55 (4′-C), 129.54 (2-C), 127.4 (6-C), 120.2 (1′-C), 118.8 (α-C), 118.6 (5′-C), 117.6 (3′-C), 55.5 (4-OCH3). HRMS (ESI): Calc. for C16H14O3, [M + H]+ m/z: 255.10157, found: 255.10208, [M + Na]+ m/z: 277.08352 found: 277.08381.
(E)-2-(3-(2′-hydroxyphenyl)-3-oxoprop-1-en-1-yl)benzoic acid or 2′-hydroxy-2-carboxy-chalcone (3J)
Prepared following the general procedure starting from 2-hydroxy-acetophenone (1B, 2.00 g, 14.69 mmol) and 2-carboxy-benzaldehyde (2F, 2.20 g, 14.69 mmol) with 5 eq of NaOH. Yellow solid (1.276 g, 4.76 mmol), 32% yield, m.p. (Gallenkamp apparatus): 144 °C.
1H NMR (400 MHz, CDCl3): δ 12.03 (s, 1H, 2′-OH), 7.96 (dd, J = 7.8 Hz, 2.3 Hz, 1H, 6′-H), 7.71 (ddd, J = 8.4 Hz, 7.4 Hz, 0.9 Hz, 1H, 5-H), 7.67 (dd, J = 8.1 Hz, 1.5 Hz, 1H, 6-H), 7.62 – 7.57 (m, 2H, 3-H, 4-H), 7.54 (ddd, J = 8.6 Hz, 7.3 Hz, 1.5 Hz, 1H, 5′-H), 7.05 (dd, J = 8.5 Hz, 0.7 Hz, 1H, 3′-H), 6.93 (ddd, J = 8.1 Hz, 7.1 Hz, 0.9 Hz, 1H, 4′-H), 6.19 (t, J = 6.4 Hz, 1H), 3.81 (dd, J = 17.6 Hz, 6.2 Hz, 1H, β-H), 3.46 (dd, J = 17.6 Hz, 6.8 Hz, 1H, α-H). 13C NMR (100 MHz, CDCl3): δ 201.7 (CO), 169.9 (2-C, COOH), 162.6 (2′-C), 149.3 (β-C), 137.2 (1-C), 129.84 (3-C), 129.83 (4′-C), 129.82 (5′-C), 129.63 (4-C), 129.62 (5-C), 125.93 (6′-C), 125.92 (6-C), 122.6 (2-C), 119.3 (1′-C), 119.1 (α-C), 118.8 (3′-C). HRMS (ESI): Calc. for C16H12O4, [M + H]+ m/z: 269.08084, found: 269.08139, [M + Na]+ m/z: 291.06278, found: 291.06338.
(E)-3-(5-bromo-2-methoxyphenyl)-1-(2′-hydroxyphenyl) prop-2-en-1-one or 5-bromo-2′-hydroxy-2-methoxy-chalcone (3K)
Prepared following the general procedure starting from 2-hydroxy-acetophenone (1B, 1.00 g, 7.34 mmol) and 5-bromo-2-methoxy-benzaldehyde (2G, 1.58 g, 7.34 mmol) with 10 eq of NaOH. Yellow solid (2.056 g, 6.17 mmol), 84% yield, m.p. (Gallenkamp apparatus): 121 °C.
1H NMR (400 MHz, CDCl3): δ 12.84 (s, 1H, 2′-OH), 8.16 (d, J = 15.7 Hz, 1H, β-H), 7.95 (dd, J = 8.1 Hz, 1.5 Hz, 1H, 6′-H), 7.78 (d, J = 2.4 Hz, 1H, 6-H), 7.74 (d, J = 15.7 Hz, 1H, α-H), 7.56–7.48 (m, 2H, 5′-H, 3′-H), 7.06 (dd, J = 8.4 Hz, 0.9 Hz, 1H, 4-H), 6.98 (td, J = 8.2 Hz, 7.2 Hz, 1.2 Hz, 1H, 4′-H), 6.87 (d, J = 8.9 Hz, 1H, 3-H), 3.94 (s, 3H, 2-OCH3). 13C NMR (100 MHz, CDCl3): δ 193.9 (CO), 163.6 (2′-C), 157.9 (2-C), 139.2 (β-C), 136.4 (1-C), 134.4 (4-C), 131.4 (6-C), 129.75 (4′-C), 129.74 (5′-C), 125.7 (6′-C), 121.7 (α-C), 120.1 (1′-C), 118.9 (5-C), 118.6 (3′-C), 113.1 (3-C), 55.9 (2-OCH3). HRMS (ESI): Calc. for C16H13BrO3, [M + H]+ m/z: 333.01208, found: 333.01247, [M + Na]+ m/z: 354.99403, found: 354.99467.
(E)-1-(2′-hydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one or 2′-hydroxy-6′-methoxy-chalcone (3L)
Prepared following the general procedure starting from 2-hydroxy-6-methoxy-acetophenone (1C, 0.5 g, 3.0 mmol) and benzaldehyde (2A, 0.32 g, 3.0 mmol) with 10 eq of NaOH. Yellow crystals (0.657 g, 2.58 mmol), 30% yield, m.p. (Electrothermal apparatus): 100 °C.
1H NMR (500 MHz, CDCl3): δ 13.13 (s, 1H, 2′-OH), 7.86 (d, J = 15.6 Hz, 1H, β-H), 7.80 (d, J = 15.6 Hz, 1H, α-H), 7.62–7.58 (m, 2H, 3-H, 5-H), 7.43–7.36 (m, 3H, 2-H, 4-H, 6-H), 7.34 (t, J = 8.1 Hz, 1H, 4′-H), 6.60 (d, J = 8.2 Hz, 1H, 5′-H), 6.41 (d, J = 8.3 Hz, 1H, 3′-H), 3.93 (s, 3H, 6′-OCH3). 13C NMR (125 MHz, CDCl3): δ 194.5 (4-C, CO), 164.9 (2′-C), 161.0 (6′-C), 142.9 (β-C),136.0 (4′-C), 135.3 (1-C), 130.3 (4′-C), 128.45 (3-C, 5-C), 127.6 (1′-C), 126.8 (2-C, 6-C), 112.1 (3′-C), 112.0 (5′-C),101.6 (α-C), 79.4 (β-H), 55.9 (6′-OCH3).
- Flavanone 3A synthesis by photochemical activation.
Chalcone 3A (85 mg, 1 eq) was dissolved in 70 mL of ethanol in a Pyrex vessel. The solution was purged with purified nitrogen gas for 10 min before the reaction vessel was sealed. Irradiation was conducted in an RPR-200 photochemical reactor equipped with 16 fluorescent tubes (8 W each, 419 ± 25 nm) at approximately 30 °C [36]. The progress of the reaction was followed by TLC analysis, and after 7 days, almost complete conversion to the desired cyclized product was observed. The solution was evaporated to dryness, and the residue was purified on a chromatographic column.
5,7-dimethoxy-2-phenyl-2,3-dihydro-4H-benzopyran-4-one or 5,7-dimethoxy-2-phenylchroman-4-one or 5,7-dimethoxy-flavanone (4A)
This product was purified by repeated column chromatography (C6H12/EtOAc 90:10, 70:30 and 50:50). White solid (0.006 g, 0.02 mmol), 7% yield, m.p. (Gallenkamp apparatus): 144–146 °C.
1H NMR (400 MHz, CDCl3): δ 7.41–7.26 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H, 6′-H), 6.10 (d, J = 2.3 Hz, 1H, 6-H), 6.03 (d, J = 2.3 Hz, 1H, 8-H), 5.35 (dd, J = 13.1 Hz, 3.0 Hz, 1H, 2-H), 3.83 (s, 3H,5-OCH3), 3.76 (s, 3H, 7-OCH3), 2.96 (dd, J = 16.6 Hz, 13.1 Hz, 1H, 3ax-H), 2.74 (dd, J = 16.6 Hz, 3.1 Hz, 1H, 3eq-H). 13C NMR (100 MHz, CDCl3): δ 192.7 (4-C, CO), 166.26 (7-C), 165.9 (5-C), 162.5 (8a-C), 135.6 (1′-C), 128.45 (3′-C, 5′-C), 127.7 (4′-C), 126.85 (2′-C, 6′-C), 106.4 (4a-C), 93.8 (8-C), 93.7 (6-C), 79.5 (2-C), 56.8 (7-OCH3), 55.9 (4-OCH3), 38.9 (3-C). Calc. for C17H16O4, [M + H]+ m/z: 285.11214 Da, found 285.11258 Da, [M + Na]+ m/z: 307.09408 found : 307.09413.
- General Procedure for flavanones synthesis by base activation (4B–L).
To a solution of chalcones 3B–L (1 eq) in methanol (20 mL) was added 5 eq of sodium acetate. The mixture was heated to reflux for 24 h and was monitored by TLC. The solvent was evaporated, EtOAc (20 mL) was added, and the mixture was washed with distilled water (3 × 20 mL). The solution was dried over anhydrous MgSO4 and filtered. The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography (CC) using silica gel 60, 0.2–0.5 mm C35-70mesh ASTH CAS: 7631-86-9 (Scharlau Spain) as stationary phase to give flavanones 4B–L.
5,7-dimethoxy-2-(3-methoxyphenyl)-2,3-dihydro-4H-benzopyran-4-one or 5,7,3′-trimethoxy-flavanone (4B)
Prepared following the general procedure starting from chalcone 3B (0.603 g, 1.92 mmol). This product was purified by column chromatography (C6H12/EtOAc 90:10, 70:30, 50:50). Pale yellow solid (0.445 g, 1.41 mmol), 74% yield, m.p. (Electrothermal apparatus): 89–91 °C.
1H NMR (500 MHz, CDCl3): δ 7.32 (t, J = 7.9 Hz, 1H, 5′-H), 7.02 (m, 2H, 2′-H, 6′-H), 6.90 (dd, J = 8,3 Hz, 2.1 Hz, 1H, 4′-H), 6.16 (d, J = 2.3 Hz, 1H, 6-H), 6.09 (d, J = 2.3 Hz, 1H, 8-H), 5.38 (dd, J = 13.3 Hz, 2.8 Hz, 1H, 2-H), 3.89 (s, 3H, 7-OCH3), 3.83 (s, 3H, 5-OCH3), 3.82 (s, 3H, 3′-OCH3), 3.00 (dd, J = 16.5 Hz, 13.3 Hz, 1H, 3eq-H), 2.79 (dd, J = 16.50 Hz, 2.80 Hz, 1H, 3ax-H), 13C NMR (125 MHz, CDCl3): δ 189.2 (4-C, CO), 166.0 (7-C), 164.9 (5-C), 162.3 (8a-C), 159.9 (3′-C), 129.89 (1′-C), 129.88 (5′-C), 118.3 (6′-C), 114.0 (4′-C), 111.8 (4a-C), 106.1 (2′-C), 93.6 (6-C), 93.2 (8-C), 79.1 (2-C), 56.2 (7-OCH3), 55.6 (5-OCH3), 55.3 (3′-OCH3), 45.6 (3-C). HRMS (ESI): Calc. for C18H18O5, [M + H]+ m/z: 315.12270 Da, found 315.12292, [M + Na]+ m/z: 337.10464, found: 337.10455.
5,7-dimethoxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-benzopyran-4-one or 5,7,4′-trimethoxy-flavanone (4C)
Prepared following the general procedure starting from chalcone 3C (0.32 g, 1.08 mmol). This product was purified by column chromatography (C6H12/EtOAc 70:30). Pale yellow solid (0.213 g, 0.68 mmol), 66% yield, m.p. (Electrothermal apparatus): 60–70 °C.
1H-NMR (400MHz, CDCl3): δ 7.44–7.34 (m, 2H, 2′-H, 6′-H), 7.01–6.91 (m, 2H, 3′-H, 5′-H), 6.16 (d, J = 2.3 Hz, 1H, 6-H), 6.11 (d, J = 2.3 Hz, 1H, 8-H), 5.38 (dd, J = 13.1 Hz, 2.9 Hz, 1H, 2-H), 3.92 (s, 3H, 7-OCH3), 3.85 (s, 3H, 5-OCH3), 3.84 (s, 3H, 4′-OCH3), 3.06 (dd, J = 16.5 Hz, 13.1 Hz, 1H, 3eq-H), 2.79 (dd, J = 16.5 Hz, 2.9 Hz, 1H, 3ax-H). 13C NMR (100 MHz, CDCl3): δ 189.5 (4-C, CO), 165.9 (7-C), 165.1 (5-C), 162.3 (8a-C), 159.9 (4′-C), 130.8 (1′-C), 127.71 (2′-C), 127.70 (6′-C), 114.17 (3′-C), 114.16 (5′-C), 106.0 (4a-C), 93.6 (6-C), 93.1 (8-C), 79.0 (2-C), 56.2 (7-OCH3), 55.6 (5-OCH3), 55.4 (4′-OCH3), 45.4 (3-C). HRMS (ESI): Calc. for C18H18O5, [M + H]+ m/z: 315.12270 Da, found: 315.12282, [M + Na]+ m/z: 337.10464, found: 337.10436.
2-(4-bromophenyl)-5,7-dimethoxy-)-2,3-dihydro-4H-benzopyran-4-one or 4′-bromo-5,7-dimethoxy-flavanone (4D)
Prepared following the general procedure starting from chalcone 3D (0.1033 g, 0.28 mmol). This product was purified by column chromatography (C6H12/EtOAc 70:30). Pale yellow solid (0.0719 g, 0.20 mmol), 69% yield, m.p. (Electrothermal apparatus): 60–70 °C.
1H NMR (500 MHz, CDCl3): δ 7.62–7.54 (m, 2H, 2′-H, 6′-H), 7.38–7.33 (d, J = 8.3 Hz, 2H, 3′-H, 5′-H), 6.17 (d, J = 2.3 Hz, 1H, 6-H), 6.13 (d, J = 2.3 Hz, 1H, 8-H), 5.40 (dd, J = 12.8 Hz, 3.2 Hz, 1H, 2-H), 3.92 (s, 3H, 5-OCH3), 3.86 (s, 3H, 7-OCH3), 2.99 (dd, J = 16.5 Hz, 12.8 Hz, 1H, 3ax-H), 2.81 (dd, J = 16.5 Hz, 3.2 Hz, 1H, 3eq-H). 13C NMR (125 MHz, CDCl3): δ 192.3 (4-C, CO), 168.5 (7-C), 166.4 (5-C), 162.5 (8a-C), 134.5 (1′-C), 132.11 (3′-C), 132.10 (5′-C), 129.69 (2′-C), 129.68 (6′-C), 124.2 (4′-C), 106.3 (4a-C), 93.8 (8-C), 91.3 (6-C), 77.3 (2-C), 55.9 (7-OCH3), 55.6 (5-OCH3). Calc. for C17H15BrO4, [M + H]+ m/z: 363.02265 Da, found 363.02329, [M + Na]+ m/z: 385.00459 found: 385.00514.
2-(4-chlorophenyl)-5,7-dimethoxy-)-2,3-dihydro-4H-benzopyran-4-one or 4′-chloro-5,7-dimethoxy-flavanone (4E)
Prepared following the general procedure starting from chalcone 3E (0.127 g, 0.40 mmol). This product was purified by column chromatography (C6H12/EtOAc 90:10, 70:30, 50:50). Colorless solid (0.084g, 0.26 mmol), 66% yield, m.p. (Gallenkamp apparatus): 120 °C.
1H NMR (400 MHz, DMSO): δ 7.57–7.53 (m, 2H, 2′-H, 6′-H), 7.52–7.47 (m, 2H, 3′-H, 5′-H), 6.25 (d, J = 2.3 Hz, 1H, 6-H), 6.22 (d, J = 2.3 Hz, 1H, 8-H), 5.57 (dd, J = 12.5 Hz, 3.0 Hz, 1H, 2-H), 3.82 (s, 3H, 7-OCH3), 3.79 (s, 3H, 5-OCH3), 3.02 (dd, J = 16.3 Hz, 12.5 Hz, 1H, 3eq-H), 2.67 (dd, J = 16.3 Hz, 3.0 Hz, 1H, 3ax-H). 13C NMR (100 MHz, CDCl3): δ 187.9 (4-C, CO), 165.9 (7-C), 164.5 (5-C), 162.3 (8a-C), 138.5 (1′-C), 133.4 (4′-C), 128.99 (3′-C), 128.98 (5′-C), 128.86 (2′-C), 128.85 (6′-C), 105.9 (4a-C), 94.2 (6-C), 93.5 (8-C), 77.9 (2-C), 56.2 (7-OCH3), 56.4 (5-OCH3), 45.1 (3-C). HRMS (ESI): Calc. for C17H15ClO4, [M + H]+ m/z : 319.07316 Da, found 319.07330, [M+ Na]+ m/z: 341.05511, found: 341.05529.
2-(5,7-dimethoxy-4-oxo-3,4-dihydro-2H-benzopyran-2-yl)benzoic acid or 2′-carboxy-5,7-dimethoxy-flavanone (4F)
Prepared following the general procedure starting from chalcone 3F (0.220g, 0.67 mmol). This product was purified by column chromatography (C6H12/EtOAc 70:30). White solid (0.159 g, 0.48 mmol), 72% yield, m.p. (Gallenkamp apparatus): 168 °C.
1H NMR (400 MHz, CDCl3): δ 13.75 (s, 1H, 2′-OH), 7.93 (d, J = 7.7 Hz, 1H, 3′-H), 7.70–7.65 (m, 1H, 4′-H), 7.63–7.53 (m, 2H, 5′-H, 6′-H), 6.18 (dd, J = 7.9 Hz, 5.4 Hz, 1H, 2-H), 6.13 (d, J = 2.3 Hz, 1H, 6-H), 5.94 (d, J = 2.3 Hz, 1H, 8-H), 3.89 (dd, J = 18.5 Hz, 5.3 Hz, 1H, 3ax-H), 3.43 (dd, J = 18.5 Hz, 7.9 Hz, 1H, 3eq-H). 13C NMR (125 MHz, CDCl3): δ 200.2 (4-C, CO), 170.5 (2′-COOH), 167.8 (7-C), 166.7 (5-C), 162.7 (8a-C), 150.3 (2′-C), 134.2 (4′-C), 129.2 (3′-C), 126.0 (1′-C), 125.6 (6′-C), 123.1 (5′-C), 105.7 (4a-C), 93.8 (6-C), 91.1 (8-C), 77.48 (2-C), 55.68 (7-OCH3), 55.67 (5-OCH3), 48.94 (3-C). Calc. for C18H16O6, [M + H]+ m/z: 329.10197 Da, found: 329.10205, [M + Na]+ m/z: 351.08391, found: 351.08389.
2,3-dihydro-2-phenyl-4H-1-benzopyran-4-one or flavanone (4G)
Prepared following the general procedure starting from chalcone 3G (0.5g, 2.23 mmol). This product was purified by column chromatography (C6H12/EtOAc 90:10, 70:30, 50:50). Colorless solid (0.294 g, 1.31 mmol), 59% yield, m.p. (Gallenkamp apparatus): 70 °C (75–76 °C, [37]).
1H NMR (400 MHz, CDCl3): δ 7.97 (dd, J = 8.1 Hz, 1.7 Hz, 1H, 5-H), 7.58–7.37 (m, 6H, 2′-H, 3′-H, 4′-H, 5′-H, 6′-H, 6-H), 7.11–7.05 (m, 2H, 7-H, 8-H), 5.52 (dd, J = 13.3 Hz, 2.9 Hz, 1H, 2-H), 3.13 (dd, J = 16.9 Hz, 13.3 Hz, 1H, 3ax-H), 2.93 (dd, J = 16.9 Hz, 2.9 Hz, 1H, 3eq-H). 13C NMR (125 MHz, CDCl3): δ 191.9 (4-C, CO), 161.6 (8a-C), 138.8 (1′-C), 136.2 (7-C), 128.86 (3′-C, 5′-C), 128.8 (4′-C), 127.1 (5-C), 126.16 (2′-C, 6′-C), 121.6 (6-C), 120.9 (4a-C), 118.1 (8-C), 79.6 (2-C), 44.7 (3-C) [37].
2-(4-chlorophenyl)-2,3-dihydro-4H-benzopyran-4-one or 4′-chloro-flavanone (4H)
Prepared following the general procedure starting from chalcone 3H (1.5 g, 5.80 mmol). This product was purified by column chromatography (C6H12/EtOAc 70:30). Colorless solid (0.556 g, 2.15 mmol), 37% yield, m.p. (Gallenkamp apparatus): 80–90 °C (93-95 °C, [37]).
1H NMR (400 MHz, CDCl3): δ 7.96 (ddd, J = 7.8 Hz, 1.6 Hz, 0.4 Hz, 1H, 5-H), 7.55 (ddd, J = 8.7 Hz, 7.2 Hz,1.8 Hz, 1H, 2′-H), 7.48–7.41 (m, 4H, 3′-H, 5′-H, 6′-H, 6-H), 7.12–7.04 (m, 2H, 7-H, 8-H), 5.50 (dd, J = 13.1 Hz, 2.9 Hz, 1H, 2-H), 3.07 (dd, J = 16.8 Hz, 13.1 Hz, 1H, 3ax-H), 2.91 (dd, J = 16.8 Hz, 2.9 Hz, 1H, 3eq-H). 13C NMR (100 MHz, CDCl3): δ 191.5 (4-C, CO), 161.6 (8a-C), 137.3 (1′-C), 136.3 (4′-C), 133.86 (6-C), 133.85 (7-C), 129.1 (3′-C, 5′-C), 127.52 (2′-C), 127.51 (6′-C), 127.1 (5-C), 121.8 (4a-C), 118.1 (8-C), 78.8 (2-C), 44.6 (3-C) ([37]).
2,3-dihydro-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one or 4′-methoxy-flavanone (4I)
Prepared following the general procedure starting from chalcone 3I (1 g, 3.93 mmol). This product was purified by column chromatography (C6H12/EtOAc 90:10, 70:30, 50:50). Pale yellow solid (0.374 g, 1.47 mmol), 37% yield, m.p. (Gallenkamp apparatus): 85 °C (87–88 °C, [37]).
1H NMR (400 MHz, CDCl3): δ 7.95 (dd, J = 8.0 Hz, 1.5 Hz, 1H, 5-H), 7.55–7.50 (m, 1H, 2′-H), 7.46–7.41 (m, 2H, 3′-H, 5′-H), 7.01–6.95 (m, 2H, 6′-H, 6-H), 7.10–7.03 (m, 2H, 7-H, 8-H), 5.46 (dd, J = 13.3 Hz, 2.9 Hz, 1H, 2-H), 3.86 (s, 3H, 4′-OCH3), 3.13 (dd, J = 16.8 Hz, 13.3 Hz, 1H, 3ax-H), 2.89 (dd, J = 16.7 Hz, 2.9 Hz, 1H, 3eq-H). 13C NMR (100 MHz, CDCl3): δ 162.6 (4′-C), 137.2 (1′-C), 129.84 (6-C), 129.83 (7-C), 129.65 (3′-C), 129.64 (5′-C), 125.9 (5-C), 125.95 (2′-C), 125.94 (6′-C), 122.7 (4a-C), 118.8 (8-C), 76.6 (2-C), 43.3 (3-C) [37].
2-(2’-Carboxyphenyl)benzopyran-4-one o r 2′-carboxyflavanone (4J)
Prepared following the general procedure starting from chalcone 3J (0.6 g, 2.24 mmol). This product was purified by column chromatography (C6H12/EtOAc 70:30). Colorless solid (0.486 g, 1.81 mmol), 81% yield, m.p. (Gallenkamp apparatus): 130 °C.
1H NMR (500 MHz, CDCl3): δ 12.01 (br s, 2′-COOH) 7.94 (d, J = 7.8 Hz, 1H, 5-H), 7.69 (ddd, J = 7.5 Hz, 7.5 Hz, 0.8 Hz, 1H, 3′-H), 7.65 (dd, J = 8.1 Hz, 1.4 Hz, 1H, 6′-H), 7.60–7.55 (m, 2H, 4′-H, 5′-H), 7.51 (dd, J = 8.1 Hz, 1.4 Hz, 1H, 6-H), 7.06 (dd, J = 8.4 Hz, 0.7 Hz, 1H, 7-H), 6.91 (ddd, J = 7.9 Hz, 7.3 Hz, 0.9 Hz, 1H, 8-H), 6.16 (app t, J = 6.5 Hz, 1H, 2-H), 3.80 (dd, J = 17.6 Hz, 6.2 Hz, 1H, 3ax-H), 3.47 (dd, J = 17.6 Hz, 6.8 Hz, 1H, 3eq-H).13C NMR (100 MHz, CDCl3): δ 201.6 (4-C, CO), 169.9 (2′-COOH), 162.6 (8a-C), 134.4 (1′-C), 129.6 (4′-C), 129.8 (6-C), 129.6 (7-C), 129.81 (3′-C), 129.80 (5′-C), 125.9 (5-C), 122.68 (2′-C), 122.67 (6′-C), 119.3 (4a-C), 118.4 (8-C), 76.6 (2-C), 43.3 (3-C). Calc. for C16H12O4, [M + H]+ m/z: 269.08084 Da, found: 269.08049, [M + Na]+ m/z: 291.06278, found: 291.06275.
2-(5-bromo-2-methoxyphenyl)-2,3-dihydro-4H-benzopyran-4-one or 5′-bromo-2′-methoxy-flavanone (4K)
Prepared following the general procedure starting from chalcone 3K (1.5 g, 4.50 mmol). This product was purified by column chromatography (hexane/dichloromethane 90:10, 70:30, 50:50, 70:30 then ethyl acetate and methanol). White solid (0.846 g, 0.25 mmol), 56% yield, m.p. (Gallenkamp apparatus): 130–135 °C.
1H NMR (400 MHz, CDCl3): δ 7.97 (dd, J = 7.8 Hz, 1.8 Hz, 1H, 5-H), 7.80 (dd, J = 2.5 Hz, 0.6 Hz, 1H, 6′-H), 7.54 (ddd, J = 8.9 Hz, 7.1 Hz, 1.7 Hz, 1H, 8-H), 7.46 (dd, J = 8.9 Hz, 2.5 Hz, 1H, 4′-H), 7.13–7.06 (m, 2H, 6-H, 7-H), 6.82 (d, J = 8.8 Hz, 1H, 3′-H), 5.80 (dd, J = 13.4 Hz, 2.8 Hz, 1H, 2-H), 3.85 (s, 3H, 2′-OCH3), 3.00 (dd, J = 16.8 Hz, 13.4 Hz, 1H, 3ax-H), 2.85 (dd, J = 16.8 Hz, 2.8 Hz, 1H, 3eq-H). 13C NMR (100 MHz, CDCl3): δ 192.2 (4-C,CO), 161.7 (8a-C), 154.7 (2′-C), 136.1 (4′-C), 131.9 (6′-C), 129.8 (6-C), 129.3 (7-C), 127.1 (5-C), 121.7 (4a-C), 120.9 (1′-C), 118.1 (5′-C), 112.3 (3′-C, 8-C), 74.2 (2-C), 55.6 (2′-OCH3), 43.7 (3-C). Calc. for C16H13BrO3, [M + H]+ m/z: 333.01208 Da, found: 333.01236, [M + Na]+ m/z: 354.99403, found: 354.99396.
5-methoxy-2-phenyl-2,3-dihydro-4H-benzopyran-4-one or 5-methoxy-flavanone (4L)
Prepared following the general procedure starting from chalcone 3L (0.657 g, 2.58 mmol). This product was purified by column chromatography (C6H12/EtOAc 90:10, 70:30 and 50:50). Colorless solid (0.056 g, 0.2 mmol), 9% yield, m.p. (Electrothermal apparatus): 95–98.7 °C.
1H NMR (500 MHz, CDCl3): δ 7.49–7.34 (m, 6H, 2′-H,3′-H, 4′-H, 5′-H, 6′-H, 7-H), 6.66 (dd, J = 8.4 Hz, 0.6 Hz, 1H, 8-H), 6.55 (d, J = 8.3 Hz, 1H, 6-H), 5.44 (dd, J = 13.2 Hz, 2.9 Hz, 1H, 2-H), 3.93 (s, 3H, 5-OCH3), 3.07 (dd, J = 16.4 Hz, 13.2 Hz, 1H, 3ax-H), 2.86 (dd, J = 16.4 Hz, 2.9 Hz, 1H, 3eq-H). 13C NMR (125 MHz, CDCl3): δ 190.7 (4-C, CO), 163.2 (5-C), 160.8 (8a-C), 138.7 (1′-C), 136.1 (7-C), 128.81 (3′-C, 5′-C), 128.68 (4′-C), 126.11 (2′-C, 6′-C), 111.4 (4a-C), 110.2 (8-C), 104.1 (6-C), 78.9 (2-C), 56.2 (5-OCH3), 45.9 (3-C). Calc. for C16H14O3, [M + H]+ m/z: 255.10157 Da, found: 255.10199 Da, [M + Na]+ m/z: 277.08352, found: 277.08392.
3.2. Biological Assays
3.2.1. Cell Culture and Treatments
The murine macrophage RAW 264.7 cell line (ATCC, TIB-71) was used as an in vitro model for studying the immunomodulatory properties of the molecules. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) GlutaMAX™ (Gibco; 10566016) containing 4.5 g/L D-glucose, HEPES buffer at 25 mM, and supplemented with 10% fetal bovine serum (FBS; Gibco), antibiotics, and antifungal solution (penicillin, 10,000 U; streptomycin, 10 mg/mL; amphotericin B, 25 µg/mL). Cell cultures were maintained by cyclic resuspension in 75 cm2 culture flasks in a humidified atmosphere with 5% CO2 at 37 °C. For biological assays, cells were seeded at 150,000 cells/well in 96-well plates and left to recover for 24 h before treatments. The cells were incubated for an additional 24 h with LPS at 1µg/mL and/or pinocembrin (Sigma–Aldrich, USA) or molecules in dimethyl sulfoxide (DMSO; Sigma Aldrich) at 0.1% and at the final concentration, as indicated in Figure 3, Figure 4 and Figure 5. Control cells were incubated under the same conditions with or without DMSO at 0.1%, without LPS or molecules. Dexamethasone (Sigma–Aldrich; D4902-100MG) at 100 nM was used as the reference anti-inflammatory molecule inhibiting NO production ([38]). Cell culture supernatants were collected for nitrite quantification and cytotoxicity analyses.
3.2.2. Determination of Cell Mortality
Cytotoxicity was evaluated by quantifying the release of lactate dehydrogenase (LDH) in the culture supernatant that correlates with the amount of cell death and membrane damage, providing an accurate measurement of cellular toxicity [39]. LDH was quantified using the commercial CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega; G1780) following the manufacturer’s specifications. Absorbance at 450 nm (A450) was read using a microplate spectrophotometer (Multiskan™ FC, Thermo Fisher Scientific). LDH in the supernatants was normalized against absorbance obtained for total lysed cells, and results were expressed as percent of cytotoxicity, as recommended for LDH-based assays [40].
3.2.3. Quantification of Nitric Oxide (NO)
Nitrite (NO2™) production was determined as an indicator of Nitric Oxide (NO) synthesis in cell culture supernatant, as previously described [41], using the Griess Reagent System (Promega; G2930) and following the supplier’s recommendations. Briefly, 50 µL of Griess reagent A (1% sulfanilamide in 5% phosphoric acid) was added to 50 µL of cellular supernatant and incubated for 5–10 min at room temperature, protected from light, before additional dispensing of Griess reagent B (0.1% N-1-napthylethylenediamine dihydrochloride in water). Absorbance at 570 nm (A570) was read using a microplate spectrophotometer (Multiskan™ FC, Thermo Fisher Scientific). Standard calibration curves were prepared using the provided sodium nitrite and after serial dilutions (0–100 µM). Due to the good correlation factor of this linear regression (R2 = 0.98), the nitrite concentrations accumulated in the cellular supernatants was determined. The reduction or increase in nitrite levels was assessed, and results for molecules are expressed as the percentage of inhibitory response of nitrite production in the target sample relative to the LPS-induced nitrite production level, calculated as the inhibition of NO produced according to the following equation:
where [NO]Blank, [NO]LPS, and [NO]M+LPS are the concentrations of NO quantified when cells were not treated, induced with LPS, and treated with the molecules and LPS, respectively.
% Inhibitory response = 100 × [1 − ([NO]M+LPS − [NO]Blank)/([NO]LPS − [NO]Blank)]
3.2.4. Predictive Analysis of Drug-Like Absorption
Log P predictions were performed using the software ACD/ChemSketch (ACD/Labs). Molecular properties were determined using the Molinspiration Cheminformatics website (http://www.molinspiration.com/cgi-bin/properties, accessed on 26 February 2022).
3.2.5. Statistical Analysis
Results are provided as mean +/− standard deviation (SD). Statistical analyses were performed using the software statistical package Prism 9.0 (GraphPad Software LLC, USA). Cytotoxicity and anti-inflammatory inhibitory response were evaluated using a Mann–Whitney nonparametric test to compare distribution between treatments and LPS induction. p values ≤ 0.05 were considered significant. The concentrations of molecules leading to 50% of inhibitory activity (IC50) were also calculated using the software Prism 9.0 from a logarithm-transformed (inhibitor) vs. normalized response equation.
4. Conclusions
Twelve flavanone derivatives were successfully synthesized and were evaluated for their ability to inhibit NO production against commercial pinocembrin. Six of these derivatives were found to be highly active, with percentage inhibition responses greater than 50%. The most active flavanone derivatives were characterized by the presence of a carboxyl group in the ortho-position or a bromo-group in the para-position of the B-ring. The importance of the presence of methoxy-groups on the inhibition of NO production was noted, in line with findings by Lee in 2015. In particular, the incorporation of a methoxy group on ring A decreased the biological activity. In contrast, the least active molecules were 4’-chloro flavanone, 4’-methoxyflavanone, and pinocembrin. SAR identified flavanone 4F carrying a carboxyl group in the ortho-position of the B-ring as a potential new lead compound for anti-inflammatory activity.
Author Contributions
Conceptualization, C.S., V.S. and N.L.; methodology, C.S., V.S., N.L., M.O. and M.M.; validation, V.S., N.L. and M.O.; formal analysis, C.S., M.M., M.O., F.B. and V.C.; investigation, C.S., M.M. and N.L.; resources, N.L., M.O., M.M. and V.S.; writing—original draft preparation, C.S.; writing—review and editing, N.L., V.S., M.O. and M.M.; visualization, C.S. and M.M.; supervision, M.O., V.S. and N.L.; project administration, N.L.; funding acquisition, N.L. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the foundation of University of New Caledonia (Project NORAS), the French Ministry of Higher Education, Research and Innovation (MESRI), the Institut Pasteur of New Caledonia, and the Pasteur Network. S.C., and M.M. thanks the Government of New Caledonia for scholarships.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the foundation of University of New Caledonia (Project NORAS) and by the Government of New Caledonia for the PhD thesis of S.C. and the position of M.M. The authors are thankful to the College of Science and Engineering, Discipline of Chemistry, at James Cook University in Townsville for technical support, providing analysis equipment, and NMR experiments. The cell culture facility and analytical platform at the Institut Pasteur of New Caledonia are supported by the French Ministry of Higher Education, Research and Innovation (MESRI) and the Pasteur Network. We are thankful for the use of the analysis equipment of PEIRENE Laboratory (Limoges University, France), BISCEm Platform (Unité Mixte de Services, Inserm 042, CNRS 2015, CHU of Limoges), for NMR experiments and grateful to Cyril Colas (ICOA, Orléans University, France) for HRMS experiments.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Abbreviations
The following abbreviations are used in this manuscript.
| NMR. | Nuclear magnetic resonance |
| 1H | Proton nuclear magnetic resonance |
| 13C | Carbon nuclear magnetic resonance |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MeOH | Methanol |
| EtOAc | Ethyl acetate |
| EtOH | Ethanol |
| TLC | Thin Layer chromatography |
| PC | Pinocembrin |
| IC50 | Half maximal inhibitory concentration |
| LDH | Lactate dehydrogenase |
| SD | Standard deviation |
| DMSO | Dimethyl sulfoxide |
| DEX | Dexamethasone |
| DCM | Dichloromethane |
| CC | Column chromatography |
| m.p. | Melting point |
| Hz | Hertz |
| HRMS | High-resolution mass spectrometry |
| ESI | Electrospray ionization |
| M | Mass |
| CAS | Chemical abstracts service |
| Da | Dalton |
| W | Watt |
| C6H12 | Cyclohexane |
| SAR | Structure–activity relationship |
| CI | Confidence Interval |
References
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a Flavanone with Antiviral and Anti-Inflammatory Effects: A Promising Treatment Strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent Developments in Anti-Inflammatory Natural Products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Ialenti, A.; Moncada, S.; Di Rosa, M. Modulation of Adjuvant Arthritis by Endogenous Nitric Oxide. Br. J. Pharmacol. 1993, 110, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stichtenoth, D.O.; Frolin, J.C. Nitric Oxide and Inflammatory Joint Diseases. Br. J. Rheumatol. 1998, 37, 246–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.H.; Lee, H.J.; Jeong, Y.S.; Ryu, S.Y.; Han, Y.N. Yomogin, an Inhibitor of Nitric Oxide Production in LPS-Activated Macrophages. Arch. Pharmacal Res. 1998, 21, 481–484. [Google Scholar] [CrossRef]
- Kobuchi, H.; Droy-lefaix, M.T.; Christen, Y.; Packer, L. Ginkgo Biloba Extract (Egb 761): Inhibitory Effect on Nitric Oxide Production in the Macrophage Cell Line RAW 264.7. Biochem. Pharm. 1997, 53, 897–903. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Preterre, A.; Iqbal, K.; Bechthold, A. Food Groups and Risk of Colorectal Cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Van Breda, S.G.; De Kok, T.M.C.M. Smart Combinations of Bioactive Compounds in Fruits and Vegetables May Guide New Strategies for Personalized Prevention of Chronic Diseases. Mol. Nutr. Food Res. 2018, 62, 1700597. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Kanadaswami, C.; Lee, L.T.; Lee, P.H.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The Antitumor Activities of Flavonoids. Vivo 2005, 19, 895–910. [Google Scholar]
- Lolli, G.; Cozza, G.; Mazzorana, M.; Tibaldi, E.; Cesaro, L.; Donella-Deana, A.; Meggio, F. Inhibition of Protein Kinase CK2 by Flavonoids and Tyrphostins. A Structural Insight. Biochemistry 2012, 51, 6097–6107. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z. Advances in Biosynthesis, Pharmacology, and Pharmacokinetics of Pinocembrin, a Promising Natural Small-Molecule Drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soromou, L.W.; Chu, X.; Jiang, L.; Wei, M.; Huo, M.; Chen, N.; Guan, S.; Yang, X.; Chen, C.; Feng, H.; et al. In Vitro and in Vivo Protection Provided by Pinocembrin against Lipopolysaccharide-Induced Inflammatory Responses. Int. Immunopharmacol. 2012, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Li, X.; Cui, Y.; Xiao, T.; Liu, R.; Wang, M.; Wei, Y. Pinocembrin Relieves Lipopolysaccharide and Bleomycin Induced Lung Inflammation via Inhibiting TLR4-NF-ΚB-NLRP3 Inflammasome Signaling Pathway. Int. Immunopharmacol. 2021, 90, 107230. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Pinto, A.P.; Dufour, M.; Corio-Costet, M.; Mérillon, J. Pinus Pinaster Knot: A Source of Polyphenols against Plasmopara Viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef]
- French, D.; Schifano, P.; Cortés-Concepcion, J.; Hargrove-Leak, S. Li-Al Layered Double Hydroxides as Catalysts for the Synthesis of Flavanone. Catal. Commun. 2010, 12, 92–94. [Google Scholar] [CrossRef]
- Shi, L.; Feng, X.E.; Cui, J.R.; Fang, L.H.; Du, G.H.; Li, Q.S. Synthesis and Biological Activity of Flavanone Derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 5466–5468. [Google Scholar] [CrossRef]
- Brennan, C.M.; Hunt, I.; Jarvis, T.C.; Johnson, D.; McDonnell, P.D. Stereoelectronic effects in ring closure reactions: The 2′-hydroxychalcone—flavanone equilibrium, and related systems. Can. J. Chem. 1990, 1, 1780–1785. [Google Scholar] [CrossRef] [Green Version]
- Keane, D.D.; Marathe, K.G.; O’Sullivan, W.I.; Philbin, E.M.; Simons, R.M.; Teague, P.C. Configuration and Conformation of 3-Arylideneflavanones. J. Org. Chem. 1970, 35, 2286–2290. [Google Scholar] [CrossRef]
- Hoshino, Y.; Takeno, N. Thermal Isomerization Equilibrium between 2′-Hydroxychalcones and Flavanones. Bull. Chem. Soc. Jpn. 1986, 59, 2903–2904. [Google Scholar] [CrossRef]
- Pandey, G.; Krishna, A.; Kumaraswamy, G. Photosensitized (Set) Conversion of 2′-Hydroxychalcones to Flavonoids a Probable Biogenetic Pathway. Tetrahedron Lett. 1987, 28, 4615–4616. [Google Scholar] [CrossRef]
- Geresh, S.; Levy, O.; Markovits, Y.; Shani, A. On the Mechanism of Intramolecular Photocycloaddition of Substituted O-Allylphenols to Cyclic Ethers. Tetrahedron 1975, 31, 2803–2807. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N. Studies in Organic and Physical Photochemistry-an Interdisciplinary Approach. Org. Biomol. Chem. 2016, 14, 7392–7442. [Google Scholar] [CrossRef] [Green Version]
- Matsushima, R.; Hiroyuki, K. Photochemical Cyclization of 2′-Hydroxychalcones. J. Chem. Soc. Perkin Trans. 2 1985, 6, 743–748. [Google Scholar] [CrossRef]
- Tundis, R.L.; Frattaruolo, G.C.; Armentano, B.; Badolato, M.; Loizzo, M.; Aiello, F.; Cappello, A. An ancient remedial repurposing: Synthesis of new pinocembrin fatty acid acyl derivatives as potential antimicrobial/anti-inflammatory agents. Nat. Prod. Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Forest, V.; Figarol, A.; Boudard, D.; Cottier, M.; Grosseau, P.; Pourchez, J. Adsorption of lactate dehydrogenase enzyme on carbon nanotubes: How to get accurate results about the cytotoxicity of these nanomaterials. Langmuir 2015, 31, 3635–3643. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H. In-Vitro Evaluation for Antioxidant and Anti-Inflammatory Property of Flavanone Derivatives. Food Biosci. 2015, 11, 1–7. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of Naturally Occurring Flavonoids on Nitric Oxide Production in the Macrophage Cell Line RAW 264.7 and Their Structure-Activity Relationships. Biochem. Pharm. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Shin, S.Y.; Woo, Y.; Hyun, J.; Yong, Y.; Koh, D.; Lee, Y.H.; Lim, Y. Relationship between the Structures of Flavonoids and Their NF-ΚB-Dependent Transcriptional Activities. Bioorganic Med. Chem. Lett. 2011, 21, 6036–6041. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Boeck, P.; Bandeira Falcão, C.A.; Leal, P.C.; Yunes, R.A.; Filho, V.C.; Torres-Santos, E.C.; Rossi-Bergmann, B. Synthesis of Chalcone Analogues with Increased Antileishmanial Activity. Bioorganic Med. Chem. 2006, 14, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Thieury, C.; Lebouvier, N.; Le Guével, R.; Barguil, Y.; Herbette, G.; Antheaume, C.; Hnawia, E.; Asakawa, Y.; Nour, M.; Guillaudeux, T. Mechanisms of Action and Structure-Activity Relationships of Cytotoxic Flavokawain Derivatives. Bioorganic Med. Chem. 2017, 25, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2’-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorganic Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Chen, X.; Hughes, R.A.; Williams, S.J.; Woodman, O.L. Understanding the Cardioprotective Effects of Flavonols: Discovery of Relaxant Flavonols without Antioxidant Activity. J. Med. Chem. 2008, 51, 1874–1884. [Google Scholar] [CrossRef]
- Krüger, K.; Lüdke, V.; Pettinger, J.; Ashton, L.; Bonnet, L.; Motti, C.A.; Lex, J.; Oelgemöller, M. Photochemical synthesis of cyclic peptide models from phthalimido acetamides and phthaloyl dipeptide esters. Tetrahedron Lett. 2018, 59, 1427–1430. [Google Scholar] [CrossRef]
- Kavala, V.; Lin, C.; Kuo, C.W.; Fang, H.; Yao, C.F. Iodine Catalyzed One-Pot Synthesis of Flavanone and Tetrahydropyrimidine Derivatives via Mannich Type Reaction. Tetrahedron 2012, 68, 1321–1329. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Li, N.; Bauer, D.; Mendonca, P.; Taka, E.; Darb, M.; Thomas, L.; Williams, H.; Soliman, K.F.A. Natural product HTP screening for antibacterial (E.coli 0157:H7) and anti-inflammatory agents in (LPS from E. coli O111:B4) activated macrophages and microglial cells; focus on sepsis. BMC Complementary Altern. Med. 2016, 16, 467. [Google Scholar] [CrossRef] [Green Version]
- Haslam, G.; Wyatt, D.; Kitos, P.A. Estimating the Number of Viable Animal Cells in Multi-Well Cultures Based on Their Lactate Dehydrogenase Activities. Cytotechnology 2000, 32, 63–75. [Google Scholar] [CrossRef]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity Assays: In Vitro methods to measure dead cells. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Kumar-Roiné, S.; Matsui, M.; Reybier, K.; Darius, H.T.; Chinain, M.; Pauillac, S.; Laurent, D. Ability of Certain Plant Extracts Traditionally Used to Treat Ciguatera Fish Poisoning to Inhibit Nitric Oxide Production in RAW 264.7 Macrophages. J. Ethnopharmacol. 2009, 123, 369–377. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).