Antifungal Activity of Lavandula angustifolia Essential Oil against Candida albicans: Time-Kill Study on Pediatric Sputum Isolates
Abstract
1. Introduction
2. Results
2.1. Microbial Identification
2.2. Chemical Composition of Essential Oil
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
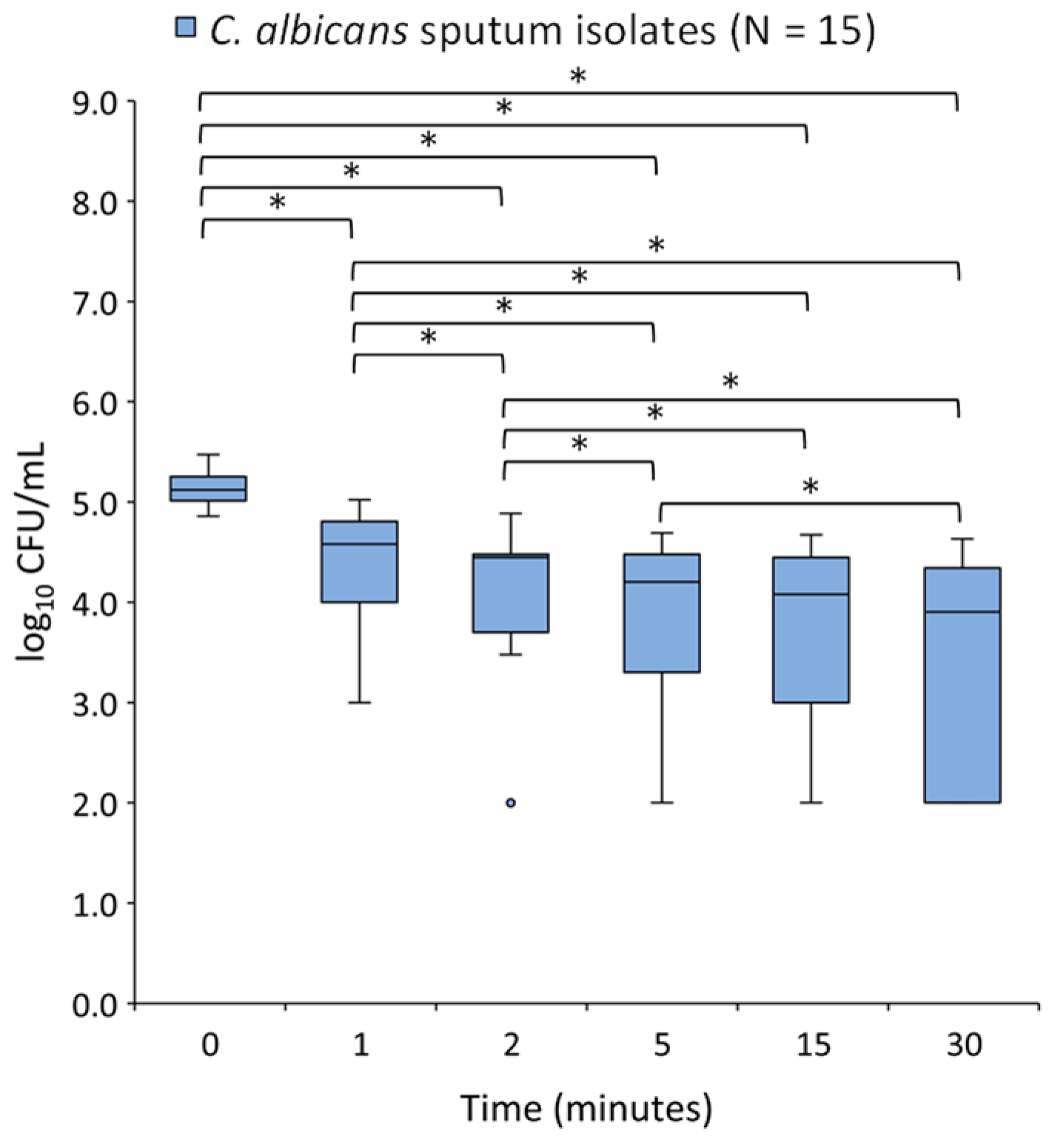
2.4. Time-Kill Assay
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Essential Oil
4.3. Chemical Identification of Essential Oil
4.4. Antifungal Agents
4.5. Antifungal Susceptibility Testing
4.5.1. Preparation of Inoculums
4.5.2. Determination of LAEO, Fluconazole and Caspofungin MIC and MFC Values
4.6. Time-Kill Essay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinbach, W.J. Pediatric Invasive Candidiasis: Epidemiology and Diagnosis in Children. J. Fungi 2016, 2, 5. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Lockhart, S. Current Epidemiology of Candida Infection. Clin. Microbiol. Newsl. 2014, 36, 131–136. [Google Scholar] [CrossRef]
- Palazzi, D.L.; Arrieta, A.; Castagnola, E.; Halasa, N.; Hubbard, S.; Brozovich, A.A.; Fisher, B.T.; Steinbach, W.J. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: Results from 441 infections in a prospective, multi-national study. Pediatr. Infect. Dis. J. 2014, 33, 1294–1306. [Google Scholar] [CrossRef]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 38–52. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Sheehan, D.; Puzniak, L.; Schlamm, H.; Ghannoum, M.A. Echinocandins: Are they all the same? J. Chemother. 2011, 23, 319–325. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- Uzun, E.; Sariyar, G.; Adsersen, A.; Karakoc, B.; Ötük, G.; Oktayoglu, E.; Pirildar, S. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. J. Ethnopharmacol. 2004, 95, 287–296. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Matailo, A.; Bec, N.; Salinas, M. Chemical composition and selective BuChE inhibitory activity of the essential oils from aromatic plants used to prepare the traditional Ecuadorian beverage horchata lojana. J. Ethnopharmacol. 2020, 263, 113162. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011, 10 (Suppl. S1), S11. [Google Scholar] [CrossRef]
- Carvalhinho, S.; Costa, A.M.; Coelho, A.C.; Martins, E.; Sampaio, A. Susceptibilities of Candida albicans mouth isolates to antifungal agents, essentials oils and mouth rinses. Mycopathologia 2012, 174, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Snoussi, M.; Hajlaoui, H.; Trabelsi, N.; Ksouri, R.; Valentin, E.; Bakhrouf, A. Chemical composition, antioxidant and antifungal potential of Melaleuca alternifolia (tea tree) and Eucalyptus globulus essential oils against oral Candida species. J. Med. Plants Res. 2011, 5, 4147–4156. [Google Scholar]
- Marcos-Arias, C.; Eraso, E.; Madariaga, L.; Quindós, G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement. Altern. Med. 2011, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Maroszyńska, M.; Dąbrowska, M. The effect of thyme and tea tree oils on morphology and metabolism of Candida albicans. Acta Biochim. Pol. 2014, 61, 305–310. [Google Scholar] [CrossRef]
- Mertas, A.; Garbusińska, A.; Szliszka, E.; Jureczko, A.; Kowalska, M.; Król, W. The influence of tea tree oil (Melaleuca alternifolia) on fluconazole activity against fluconazole-resistant Candida albicans strains. Biomed. Res. Int. 2015, 2015, 590470. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Fleming, T. PDR for Herbal Medicines, 2nd ed.; Medical Economics Company: Montvale, NJ, USA, 2000. [Google Scholar]
- D’Auria, F.D.; Tecca, M.; Strippoli, V.; Salvatore, G.; Battinelli, L.; Mazzanti, G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol. 2005, 43, 391–396. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report on Lavandula Angustifolia Miller, Aetheroleum and Lavandula Angustifolia Miller, Flos. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-lavandula-angustifolia-miller-aetheroleum-lavandula-angustifolia-miller-flos_en.pdf (accessed on 18 June 2022).
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Minooeianhaghighi, M.H.; Sepehrian, L.; Shokri, H. Antifungal effects of Lavandula binaludensis and Cuminum cyminum essential oils against Candida albicans strains isolated from patients with recurrent vulvovaginal candidiasis. J. Mycol. Med. 2017, 27, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [PubMed]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [PubMed]
- Mandras, N.; Roana, J.; Scalas, D.; Del Re, S.; Cavallo, L.; Ghisetti, V.; Tullio, V. The Inhibition of Non-albicans Candida Species and Uncommon Yeast Pathogens by Selected Essential Oils and Their Major Compounds. Molecules 2021, 26, 4937. [Google Scholar] [CrossRef]
- Singulani, J.L.; Pedroso, R.S.; Ribeiro, A.B.; Nicolella, H.D.; Freitas, K.S.; Damasceno, J.L.; Vieira, T.M.; Crotti, A.E.; Tavares, D.C.; Martins, C.H.; et al. Geraniol and linalool anticandidal activity, genotoxic potential and embryotoxic effect on zebrafish. Future Microbiol. 2018, 13, 1637–1646. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef]
- Perić, M.; Rajković, K.; Milić Lemić, A.; Živković, R.; Arsić Arsenijević, V. Development and validation of mathematical models for testing antifungal activity of different essential oils against Candida species. Arch. Oral Biol. 2019, 98, 258–264. [Google Scholar] [CrossRef]
- Santomauro, F.; Donato, R.; Sacco, C.; Pini, G.; Flamini, G.; Bilia, A.R. Vapour and Liquid-Phase Artemisia annua Essential Oil Activities against Several Clinical Strains of Candida. Planta Med. 2016, 82, 1016–1020. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement. Altern. Med. 2010, 10, 65. [Google Scholar] [CrossRef]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Manzoor, N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 2013, 48, 80–86. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Farooq, F.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J. Saudi Chem. Soc. 2013, 17, 237–243. [Google Scholar] [CrossRef]
- D’Auria, F.D.; Laino, L.; Strippoli, V.; Tecca, M.; Salvatore, G.; Battinelli, L.; Mazzanti, G. In vitro activity of tea tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J. Chemother. 2001, 13, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Muend, S.; Rao, R. Dysregulation of ion homeostasis by antifungal agents. Front Microbiol. 2012, 3, 133. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Hochmuth, D.H. MassFinder 3: Software for GC/MS Interpretation and Presentation, Mass Spectral Library Administration; Hochmuth Scientific Consulting: Hamburg, Germany, 2006. [Google Scholar]
- Espinel-Ingroff, A.; Alvarez-Fernandez, M.; Cantón, E.; Carver, P.L.; Chen, S.C.; Eschenauer, G.; Getsinger, D.L.; Gonzalez, G.M.; Govender, N.P.; Grancini, A.; et al. A multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob. Agents Chemother. 2015, 59, 6725–6732. [Google Scholar] [CrossRef]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of test conditions on antifungal timekill curve results: Proposal for standardized methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef]
- Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S. SCY-078 Is Fungicidal against Candida Species in Time-Kill Studies. Antimicrob. Agents Chemother. 2017, 61, e01961-16. [Google Scholar] [CrossRef]
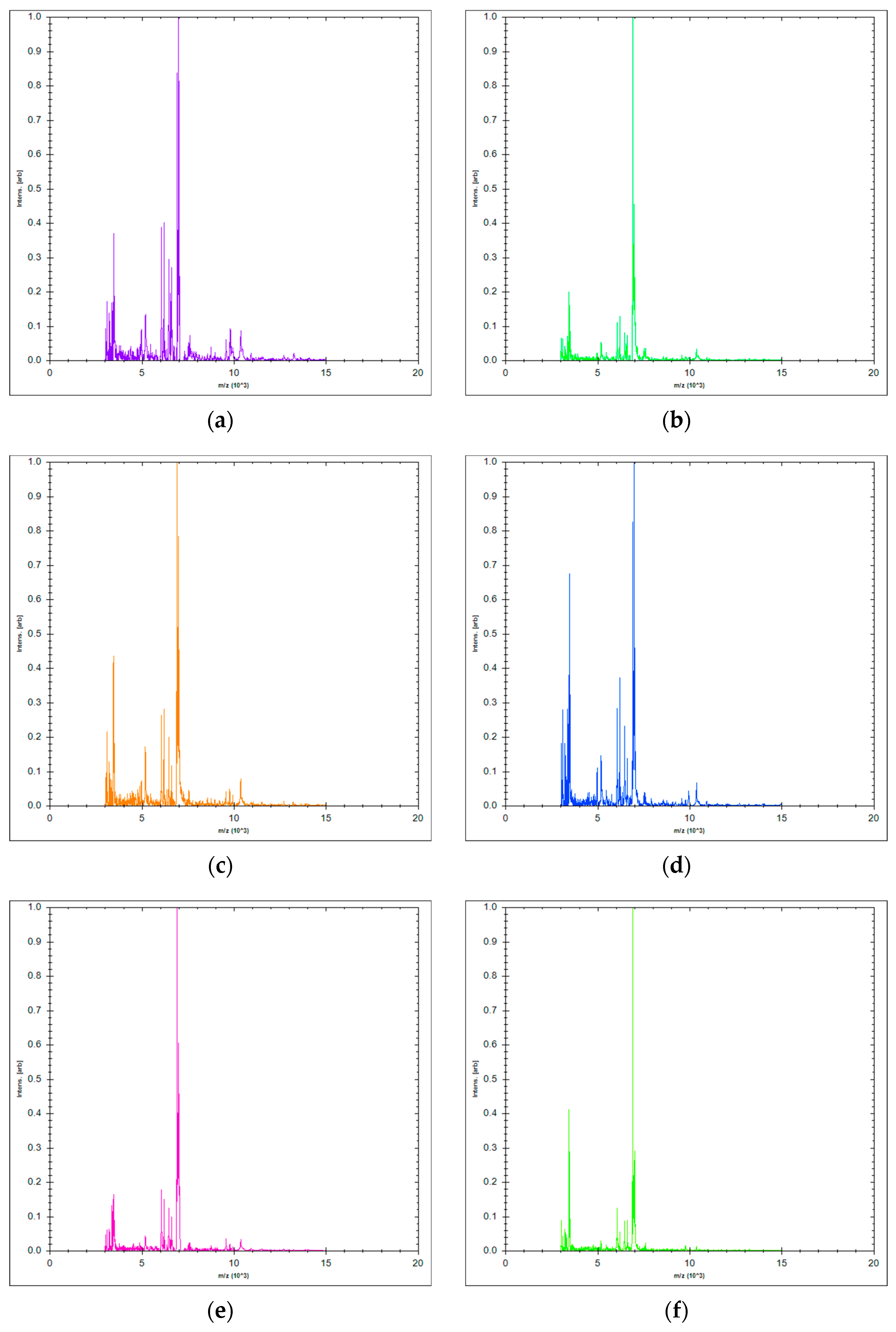



| Components | Contents (%) According to Ph. Eur 1 | Content (%) of LAEO 2 Sample |
|---|---|---|
| α-thujene | 0.1 | |
| α-pinene | 0.5 | |
| camphene | 0.3 | |
| octen-3-ol | 0.2 | |
| octanone-3 | 0.1–5 | 0.8 |
| myrcene | 0.7 | |
| α-phellandrene | 0.3 | |
| p-cymene | 0.3 | |
| limonene | Max 1 | 0.9 |
| 1,8-cineole | Max 2.5 | 5.0 |
| cis-β-ocimene | 0.7 | |
| trans-β-ocimene | 0.7 | |
| linalool oxide cis | 0.2 | |
| linalool oxide trans | 0.2 | |
| linalool | 20–45 | 33.4 |
| octenyl acetate | 0.5 | |
| camphor | Max 1.2 | 4.6 |
| borneol | 3.2 | |
| terpinene-4-ol | 0.1–8 | 3.9 |
| lavandulol | Min 0.1 | 0.4 |
| α-terpineol | Max 2 | 1.2 |
| hexyl isovalerate | 0.2 | |
| linalyl acetate | 25–47 | 30.5 |
| lavandulyl acetate | Min 0.2 | 3.5 |
| neryl acetate | 0.5 | |
| geranyl acetate | 0.8 | |
| β-caryophyllene | 2.3 | |
| trans-α-bergamotene | 0.2 | |
| trans-β-farnesene | 0.8 | |
| caryophyllene oxide | 0.6 |
| Strains | LAEO 1 | Fluconazole | Caspofungin | |||
|---|---|---|---|---|---|---|
| MIC 2 | MFC 3 | MIC | MFC | MIC | MFC | |
| C. albicans PED01 | 1 | 2 | 64 | >64 | 0.25 | 2 |
| C. albicans PED02 | 1 | 2 | 0.5 | >64 | 0.25 | 2 |
| C. albicans PED03 | 0.5 | 1 | 64 | >64 | 4 | 4 |
| C. albicans PED04 | 1 | 2 | 1 | >64 | 0.25 | 0.5 |
| C. albicans PED05 | 1 | 2 | 0.5 | >64 | 0.25 | 0.25 |
| C. albicans PED06 | 2 | 2 | 0.125 | >64 | 0.25 | 2 |
| C. albicans PED07 | 1 | 2 | 64 | >64 | 0.5 | 0.5 |
| C. albicans PED08 | 1 | 2 | 64 | >64 | 0.5 | 2 |
| C. albicans PED09 | 1 | 2 | 64 | >64 | 0.5 | 2 |
| C. albicans PED10 | 0.5 | 1 | 0.06 | >64 | 0.25 | 1 |
| C. albicans PED11 | 0.5 | 1 | 0.125 | >64 | 0.25 | 2 |
| C. albicans PED12 | 2 | 4 | 0.5 | >64 | 0.5 | 0.5 |
| C. albicans PED13 | 2 | 2 | 0.5 | >64 | 0.25 | 2 |
| C. albicans PED14 | 2 | 2 | 0.125 | >64 | 0.125 | 0.5 |
| C. albicans PED15 | 1 | 2 | 0.125 | >64 | 0.5 | 1 |
| C. albicans ATCC 10259 | 2 | 2 | 0.5 | >64 | 0.5 | 2 |
| Strains | 2% Solution of LAEO 1 | ||
|---|---|---|---|
| 50% Reduction 2 | 90% Reduction | 99.9% Reduction | |
| C. albicans PED01 | 0.2 | 0.7 | 5 |
| C. albicans PED02 | 1.4 | >30 | >30 |
| C. albicans PED03 | 1.1 | 2 | 29.7 |
| C. albicans PED04 | 3.5 | >30 | >30 |
| C. albicans PED05 | 0.2 | 0.6 | 2 |
| C. albicans PED06 | 0.2 | 0.5 | 1.8 |
| C. albicans PED07 | 0.4 | 3.3 | >30 |
| C. albicans PED08 | 0.2 | 0.7 | >30 |
| C. albicans PED09 | 1.1 | 15.2 | >30 |
| C. albicans PED10 | 1.3 | >30 | >30 |
| C. albicans PED11 | 0.6 | >30 | >30 |
| C. albicans PED12 | 0.6 | >30 | >30 |
| C. albicans PED13 | 0.7 | 18 | >30 |
| C. albicans PED14 | 0.4 | 3.1 | >30 |
| C. albicans PED15 | 0.5 | >30 | >30 |
| C. albicans ATCC 10259 | 0.8 | >30 | >30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijatovic, S.; Stankovic, J.A.; Calovski, I.C.; Dubljanin, E.; Pljevljakusic, D.; Bigovic, D.; Dzamic, A. Antifungal Activity of Lavandula angustifolia Essential Oil against Candida albicans: Time-Kill Study on Pediatric Sputum Isolates. Molecules 2022, 27, 6300. https://doi.org/10.3390/molecules27196300
Mijatovic S, Stankovic JA, Calovski IC, Dubljanin E, Pljevljakusic D, Bigovic D, Dzamic A. Antifungal Activity of Lavandula angustifolia Essential Oil against Candida albicans: Time-Kill Study on Pediatric Sputum Isolates. Molecules. 2022; 27(19):6300. https://doi.org/10.3390/molecules27196300
Chicago/Turabian StyleMijatovic, Stefan, Jelena Antic Stankovic, Ivana Colovic Calovski, Eleonora Dubljanin, Dejan Pljevljakusic, Dubravka Bigovic, and Aleksandar Dzamic. 2022. "Antifungal Activity of Lavandula angustifolia Essential Oil against Candida albicans: Time-Kill Study on Pediatric Sputum Isolates" Molecules 27, no. 19: 6300. https://doi.org/10.3390/molecules27196300
APA StyleMijatovic, S., Stankovic, J. A., Calovski, I. C., Dubljanin, E., Pljevljakusic, D., Bigovic, D., & Dzamic, A. (2022). Antifungal Activity of Lavandula angustifolia Essential Oil against Candida albicans: Time-Kill Study on Pediatric Sputum Isolates. Molecules, 27(19), 6300. https://doi.org/10.3390/molecules27196300








