Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review
Abstract
1. Introduction
1.1. Anti-Cancer Marine Drugs
1.2. Fucoidan
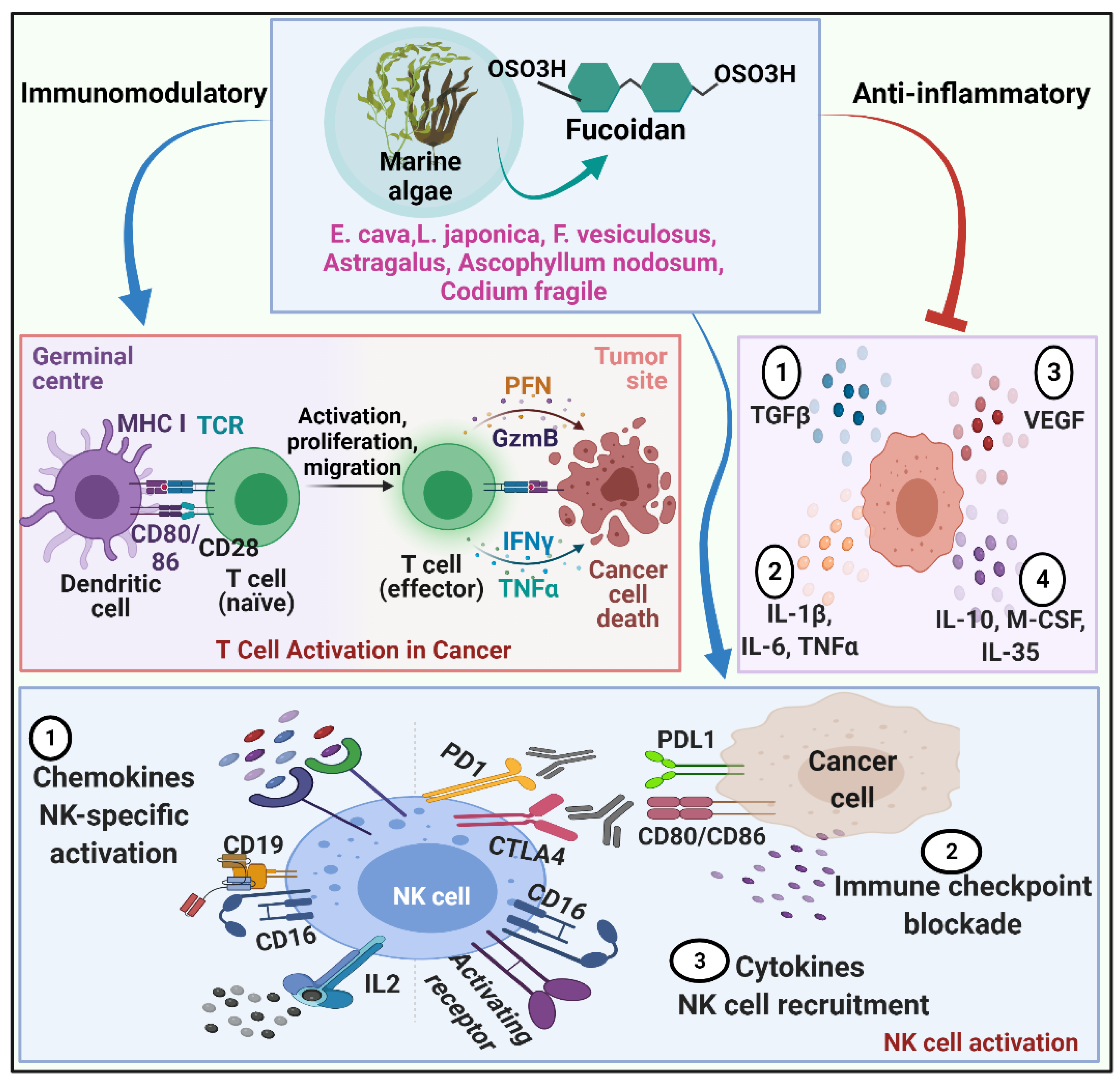
Fucoidan Sources and Structure
2. Pharmacokinetics of Fucoidan
2.1. Fucoidan and Cancer
2.1.1. Anticarcinogenic Mechanism of Fucoidan
2.1.2. Role of Fucoidan in Cell Cycle Arrest and Apoptosis
2.1.3. Fucoidan and Angiogenesis
2.1.4. Fucoidan and Immunomodulation
2.1.5. Fucoidan and Metastasis
2.1.6. Fucoidan and Gut Flora
3. Fucoidan from Different Sources of Marine Sea Weeds
3.1. Ecklonia cava
3.2. Laminaria japonica
3.3. Fucus vesiculosus
3.4. Astragalus membranaceus
3.5. Ascophyllum nodosum
3.6. Codium fragile
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Dobson, A.D.W.; Adams, C.; Gara, F. Emerging concepts promising new horizons for marine biodiscovery and synthetic biology. Mar. Drugs 2015, 13, 2924–2954. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef]
- Parmentier, E.; Michel, L. Boundary lines in symbiosis forms. Symbiosis 2013, 60, 1–5. [Google Scholar] [CrossRef]
- Hill, R.T.; Fenical, W. Pharmaceuticals from marine natural products: Surge or ebb? Curr. Opin. Biotechnol. 2010, 21, 777–779. [Google Scholar] [CrossRef]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching cancer pharmacology with drugs of marine origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Fuentes, B. Antidiabetic drugs for stroke prevention in patients with type-2 diabetes. The neurologist’s point of view. Med. Clínica 2018, 150, 275–281. [Google Scholar] [CrossRef]
- Friedman, A. Cancer as multifaceted disease. Math. Model. Nat. Phenom. 2012, 7, 3–28. [Google Scholar] [CrossRef]
- Stein, C.J.; Colditz, G.A. Modifiable risk factors for cancer. Br. J. Cancer 2004, 90, 299–303. [Google Scholar] [CrossRef]
- Iwamoto, T. Clinical application of drug delivery systems in cancer chemotherapy: Review of the efficacy and side effects of approved drugs. Biol. Pharm. Bull. 2013, 36, 715–718. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Marine seaweed polysaccharides-based engineered cues for the modern biomedical sector. Mar. Drugs 2020, 18, 7. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’Grady, M.N.; Lordan, S.; Stanton, C.; Kerry, J.P. Seaweed polysaccharides (laminarin and fucoidan) as functional ingredients in pork meat: An evaluation of anti-oxidative potential, thermal stability and bioaccessibility. Mar. Drugs 2015, 13, 2447. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Chen, B.-A.; Cai, X.-H.; Fu, R. Anticancer and multidrug-resistance reversing potential of traditional medicinal plants and their bioactive compounds in leukemia cell lines. Chin. J. Nat. Med. 2014, 12, 881–894. [Google Scholar] [CrossRef]
- Schwartsmann, G.; da Rocha, A.B.; Berlinck, R.G.S.; Jimeno, J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001, 2, 221–225. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Li, K.; Chung-Davidson, Y.-W.; Bussy, U.; Li, W. Recent advances and applications of experimental technologies in marine natural product research. Mar. Drugs 2015, 13, 2694–2713. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S.U. Marine sponges as a drug treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef]
- Cooper, E.L.; Hirabayashi, K.; Strychar, K.B.; Sammarco, P.W. Corals and their potential applications to integrative medicine. Evid. Based Complementary Altern. Med. 2014, 2014, 184959. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Bilela, L.L.; Moulin, C.; Taffin-De-Givenchy, E.; et al. Marine anticancer agents: An overview with a particular focus on their chemical classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Ahn, B.N.; Kong, C.S. Evaluation of effective mmp inhibitors from eight different brown algae in human fibrosarcoma ht1080 cells. Prev. Nutr. Food Sci. 2015, 20, 153–161. [Google Scholar] [CrossRef]
- Kusaykin, M.; Bakunina, I.; Sovo, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef]
- Jin, J.-O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The therapeutic potential of the anticancer activity of fucoidan: Current advances and hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. And Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Hauperich, S.; Keusgen, M. Phlorotannins from the brown algae cystophora torulosa and Sargassum spinuligerum. Nat. Toxins 1997, 5, 58–63. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, G.Y.; Nam, T.J.; Deuk Kim, N.; Hyun Choi, Y. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in ags human gastric cancer cells. J. Food Sci. 2011, 76, T77–T83. [Google Scholar] [CrossRef]
- Wang, S.K.; Li, Y.; White, W.L.; Lu, J. Extracts from new zealand Undaria pinnatifida containing fucoxanthin as potential functional biomaterials against cancer in vitro. J. Funct. Biomater. 2014, 5, 29–42. [Google Scholar] [CrossRef]
- Hye, S.K.; Hae, Y.C.; Ji, Y.K.; Byeng, W.S.; Hyun, A.J.; Jae, S.C. Inhibitory phlorotannins from the edible brown alga ecklonia stolonifera on total reactive oxygen species (ros) generation. Arch. Pharmacal Res. 2004, 27, 194–198. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Yadav, D.; Jin, J.O. Therapeutic potential of algal nanoparticles: A brief review. Comb. Chem. High Throughput Screen 2021, 25, 2443–2451. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Kylin, H. Zur biochemie der meeresalgen. Hoppe-Seyler. Z. Physiol. Chem. 1913, 83, 171–197. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural characterization of fucoidan from Laminaria hyperborea: Assessment of coagulation and inflammatory properties and their structure–function relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Bittkau, K.S.; Dörschmann, P.; Blümel, M.; Tasdemir, D.; Roider, J.; Klettner, A.; Alban, S. Comparison of the effects of fucoidans on the cell viability of tumor and non-tumor cell lines. Mar. Drugs 2019, 17, 441. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Dutot, M.; Grassin-Delyle, S.; Salvator, H.; Brollo, M.; Rat, P.; Fagon, R.; Naline, E.; Devillier, P. A marine-sourced fucoidan solution inhibits toll-like-receptor-3-induced cytokine release by human bronchial epithelial cells. Int. J. Biol. Macromol. 2019, 130, 429–436. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-derived bioactive compounds with anti-lung cancer potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef]
- Jankowski, V.; Vanholder, R.; Van Der Giet, M.; Henning, L.; Tölle, M.; Schönfelder, G.; Krakow, A.; Karadogan, S.; Gustavsson, N.; Gobom, J.; et al. Detection of angiotensin ii in supernatants of stimulated mononuclear leukocytes by matrix-assisted laser desorption ionization time-of-flight/time- of-flight mass analysis. Hypertension 2005, 46, 591–597. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- García-Ríos, V.; Ríos-Leal, E.; Robledo, D.; Freile-Pelegrin, Y. Polysaccharides composition from tropical brown seaweeds. Phycol. Res. 2012, 60, 305–315. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Chizhov, A.O.; Krupnova, T.N.; Sundukova, E.V.; Isakov, V.V. Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Biol. Ecol. 2003, 294, 1–13. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Nagamine, T.; Hayakawa, K.; Kusakabe, T.; Takada, H.; Nakazato, K.; Hisanaga, E.; Iha, M. Inhibitory effect of fucoidan on huh7 hepatoma cells through downregulation of cxcl12. Nutr. Cancer 2009, 61, 340–347. [Google Scholar] [CrossRef]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich elisa. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and tissue distribution of fucoidan from Fucus vesiculosus after oral administration to rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The pharmacokinetics of fucoidan after topical application to rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent tgfβ receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Hu, C.-H.; Shu, D.T.F.; Lu, M.-K. Fucoidan upregulates tlr4/chop-mediated caspase-3 and parp activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018, 432, 112–120. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Zdobnova, E.V.; Silchenko, A.S.; Kusaykin, M.I.; Ermakova, S.P. Radiosensitizing effect of the fucoidan from brown alga fucus evanescens and its derivative in human cancer cells. Carbohydr. Polym. 2019, 205, 465–471. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Park, A.Y.; Karpiniec, S.S. Therapies from fucoidan: New developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the okhotsk sea fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Fukahori, S.; Yano, H.; Akiba, J.; Ogasawara, S.; Momosaki, S.; Sanada, S.; Kuratomi, K.; Ishizaki, Y.; Moriya, F.; Yagi, M.; et al. Fucoidan, a major component of brown seaweed, prohibits the growth of human cancer cell lines in vitro. Mol. Med. Rep. 2008, 1, 537–542. [Google Scholar] [CrossRef][Green Version]
- Banafa, A.M.; Roshan, S.; Liu, Y.-Y.; Chen, H.-J.; Chen, M.-J.; Yang, G.-X.; He, G.-Y. Fucoidan induces g1 phase arrest and apoptosis through caspases-dependent pathway and ros induction in human breast cancer mcf-7 cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2013, 33, 717–724. [Google Scholar] [CrossRef]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan extracted from Cladosiphon okamuranus tokida induces apoptosis of human t-cell leukemia virus type 1-infected t-cell lines and primary adult t-cell leukemia cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Burz, C.; Berindan-Neagoe, I.; Balacescu, O.; Irimie, A. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009, 48, 811–821. [Google Scholar] [CrossRef]
- Ahn, G.; Lee, W.; Kim, K.N.; Lee, J.H.; Heo, S.J.; Kang, N.; Lee, S.H.; Ahn, C.B.; Jeon, Y.J. A sulfated polysaccharide of Ecklonia cava inhibits the growth of colon cancer cells by inducing apoptosis. EXCLI J. 2015, 14, 294–306. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.-Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human hs-sultan cells accompanied by activation of caspase-3 and down-regulation of erk pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Alonzo, R.; Athitsos, V. Experiments with Computer Vision Methods for Hand Detection. In Proceedings of the 4th International Conference on PErvasive Technologies Related to Assistive Environments, Crete, Greece, 25–27 May 2011; Association for Computing Machinery: New York, NY, USA, 2011; p. 21. [Google Scholar]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus tokida in u937 cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer mcf-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma smmc-7721 cells via the ros-mediated mitochondrial pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Jing, X.; Ding, X.; Yu, Y.; Zhao, Q. Fucoidan induces apoptosis and inhibits proliferation of hepatocellular carcinoma via the p38 mapk/erk and pi3k/akt signal pathways. Cancer Manag. Res. 2020, 12, 1713–1723. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chiu, Y.-H.; Chan, Y.-L.; Chiu, Y.-H.; Wang, H.; Huang, K.-C.; Li, T.-L.; Hsu, K.-H.; Wu, C.-J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (vegf) and matrix metalloproteinases (mmps) in lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Chang, A.K.; Liu, B.; Yang, L.; Li, Q.; Wang, P.; Zou, X. Fucoidan extract derived from Undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomedicine 2012, 19, 797–803. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Miao, Z.H. Marine-derived angiogenesis inhibitors for cancer therapy. Mar. Drugs 2013, 11, 903–933. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Liu, Q.; Thorlacius, H. Inhibition of selectin function and leukocyte rolling protects against dextran sodium sulfate-induced murine colitis. Scand. J. Gastroenterol. 2001, 36, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, L.; Bertholon, I.; Lavigne, D.; Vassy, R.; Jandrot-Perrus, M.; Chaubet, F.; Letourneur, D. Affinity of low molecular weight fucoidan for p-selectin triggers its binding to activated human platelets. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rouzet, F.; Bachelet-Violette, L.; Alsac, J.-M.; Suzuki, M.; Meulemans, A.; Louedec, L.; Petiet, A.; Jandrot-Perrus, M.; Chaubet, F.; Michel, J.-B.; et al. Radiolabeled fucoidan as a p-selectin targeting agent for in vivo imaging of platelet-rich thrombus and endothelial activation. J. Nucl. Med. 2011, 52, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-D.; Yao, C.-J.; Chow, J.-M.; Chang, C.-L.; Hwang, P.-A.; Chuang, S.-E.; Whang-Peng, J.; Lai, G.-M. Fucoidan elevates microrna-29b to regulate dnmt3b-mtss1 axis and inhibit emt in human hepatocellular carcinoma cells. Mar. Drugs 2015, 13, 6099–6116. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via pi3k-akt-mtor pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Z.-J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and potential antitumor activity of polysaccharide from gracilariopsis lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef]
- Busch, S.; Renaud, S.J.; Schleussner, E.; Graham, C.H.; Markert, U.R. Mtor mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of stat3. Exp. Cell Res. 2009, 315, 1724–1733. [Google Scholar] [CrossRef]
- Wu, W.-S.; Wu, J.-R.; Hu, C.-T. Signal cross talks for sustained mapk activation and cell migration: The potential role of reactive oxygen species. Cancer Metastasis Rev. 2008, 27, 303–314. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ermakova, S.P.; Choi, H.K.; Kusaykin, M.I.; Shevchenko, N.M.; Zvyagintseva, T.N.; Choi, H.S. Fucoidan from laminaria cichorioides inhibits ap-1 transactivation and cell transformation in the mouse epidermal jb6 cells. Mol. Carcinog. 2008, 47, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by lactobacillus brevis bj20 in individuals with high level of γ-gt: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012, 50, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from seaweeds: An ocean of opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The effect of Undaria pinnatifida fucoidan on the pharmacokinetics of letrozole and tamoxifen in patients with breast cancer. Integr. Cancer Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef]
- Tsai, H.-L.; Tai, C.-J.; Huang, C.-W.; Chang, F.-R.; Wang, J.-Y. Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef]
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.-I.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. Ask1 is required for sustained activations of jnk/p38 map kinases and apoptosis. EMBO Rep. 2001, 2, 222–228. [Google Scholar] [CrossRef]
- Azuma, K.; Ishihara, T.; Nakamoto, H.; Amaha, T.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Takashima, O.; Ifuku, S. Effects of oral administration of fucoidan extracted from Cladosiphon okamuranus on tumor growth and survival time in a tumor-bearing mouse model. Mar. Drugs 2012, 10, 2337–2348. [Google Scholar] [CrossRef]
- Suresh, V.; Anbazhagan, C.; Thangam, R.; Senthilkumar, D.; Senthilkumar, N.; Kannan, S.; Rengasamy, R.; Palani, P. Stabilization of mitochondrial and microsomal function of fucoidan from Sargassum plagiophyllum in diethylnitrosamine induced hepatocarcinogenesis. Carbohydr. Polym. 2013, 92, 1377–1385. [Google Scholar]
- Takeda, K.; Tomimori, K.; Kimura, R.; Ishikawa, C.; Nowling, T.K.; Mori, N. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. Int. J. Oncol. 2012, 40, 251–260. [Google Scholar]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of nk cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Bogen, B.; Fauskanger, M.; Haabeth, O.A.; Tveita, A. Cd4+ t cells indirectly kill tumor cells via induction of cytotoxic macrophages in mouse models. Cancer Immunol. Immunother. 2019, 68, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-H.; Yang, Y.-I.; Lee, K.-T.; Choi, J.-H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef]
- Zhang, W.; An, E.-K.; Park, H.-B.; Hwang, J.; Dhananjay, Y.; Kim, S.-J.; Eom, H.-Y.; Oda, T.; Kwak, M.; Lee, P.C.-W. Ecklonia cava fucoidan has potential to stimulate natural killer cells in vivo. Int. J. Biol. Macromol. 2021, 185, 111–121. [Google Scholar] [CrossRef]
- Wang, H.; Chiu, L.C.; Ooi, V.E.; Ang, P.O., Jr. A potent antitumor polysaccharide from the edible brown seaweed Hydroclathrus clathratus. Bot. Mar. 2010, 53, 265–274. [Google Scholar] [CrossRef]
- Dias, P.F.; Siqueira, J.M.; Vendruscolo, L.F.; Neiva, T.D.J.; Gagliardi, A.N.R.; Maraschin, M.; Ribeiro-do-Valle, R.M. Antiangiogenic and antitumoral properties of a polysaccharide isolated from the seaweed Sargassum stenophyllum. Cancer Chemother. Pharmacol. 2005, 56, 436–446. [Google Scholar] [CrossRef]
- Vinayak, R.; Puttananjaiah, S.; Chatterji, A.; Salimath, B. Anti-proliferative and angio-suppressive effect of stoechospermum marginatum (c. Agardh) kutzing extract using various experimental models. Nutr. Res. Pract. 2014, 8, 377–385. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 2012, 50, 695–700. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit vegf-a-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Numata, A.; Kanbara, S.; Takahashi, C.; Fujiki, R.; Yoneda, M.; Usami, Y.; Fujita, E. A cytotoxic principle of the brown alga Sargassum tortile and structures of chromenes. Phytochemistry 1992, 31, 1209–1213. [Google Scholar] [CrossRef]
- Ahn, M.H.; Shin, J.A.; Yang, S.O.; Choi, W.S.; Jang, S.; Kang, S.C.; Cho, S.D. Metabolite profiling of a Sargassum micracanthum methanol extract with in vitro efficacy against human head and neck squamous cell carcinoma aggressiveness. Arch. Oral Biol. 2022, 137, 105386. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in a549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-tumor and anti-angiogenic effects of fucoidan on prostate cancer: Possible jak-stat3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Yang, Y.; Wei, H.; Liu, Z.; Liu, Z.; Ma, Y.; Gao, Z.; Hou, L.; Zou, X. Fucoidan suppresses hypoxia-induced lymphangiogenesis and lymphatic metastasis in mouse hepatocarcinoma. Mar. Drugs 2015, 13, 3514–3530. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.; Lee, K.M.; Choi, Y.J.; Ye, B.R.; Kim, M.S.; Ko, S.G.; Lee, S.H.; Kang, D.H.; Heo, S.J. Tuberatolide b suppresses cancer progression by promoting ros-mediated inhibition of stat3 signaling. Mar. Drugs 2017, 15, 55. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). Biomed. Res. Int. 2013, 2013, 604787. [Google Scholar] [CrossRef]
- Vaseghi, G.; Sharifi, M.; Dana, N.; Ghasemi, A.; Yegdaneh, A. Cytotoxicity of Sargassum angustifolium partitions against breast and cervical cancer cell lines. Adv. Biomed. Res. 2018, 7, 43. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Somasundaram, S.N.; Shanmugam, S.; Subramanian, B.; Jaganathan, R. Cytotoxic effect of fucoidan extracted from Sargassum cinereum on colon cancer cell line hct-15. Int. J. Biol. Macromol. 2016, 91, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Silva Costa, L.; Silva Telles, C.B.; Medeiros Oliveira, R.; Duarte Barreto Nobre, L.T.; Dantas-Santos, N.; Barros Gomes Camara, R.; Santana Santos Pereira Costa, M.; Almeida-Lima, J.; Melo-Silveira, R.F.; Lopes Albuquerque, I.R.; et al. Heterofucan from Sargassum filipendula induces apoptosis in hela cells. Mar. Drugs 2011, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaykin, M.I.; Um, B.-H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Choi, E.O.; Lee, H.; Park, C.; Kim, G.-Y.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Jeon, Y.-J.; Hwang, H.J.; Choi, Y.H. Ethanol extracts of hizikia fusiforme induce apoptosis in human prostate cancer pc3 cells via modulating a ros-dependent pathway. Asian Pac. J. Trop. Biomed. 2020, 10, 78. [Google Scholar] [CrossRef]
- Wang, H.; Ooi, E.V.; Ang, P.O., Jr. Antiviral polysaccharides isolated from hong kong brown seaweed Hydroclathrus clathratus. Sci. China C Life Sci. 2007, 50, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Ahmed, E.F.; Abo-Zeid, M.A. In vitro cancer chemopreventive properties of polysaccharide extract from the brown alga, Sargassum latifolium. Food Chem. Toxicol. 2009, 47, 1378–1384. [Google Scholar] [CrossRef]
- Taheri, A.; Ghaffari, M.; Bavi, Z.; Sohili, F. Cytotoxic effect of the extract of seaweed Sargassum glaucescens against breast (mcf-7) and colorectal (ht-29) cancer cell lines. KAUMS J. (FEYZ) 2018, 22, 292–301. [Google Scholar]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.-N.; Jeon, Y.-J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Athukorala, Y.; Ahn, G.N.; Jee, Y.H.; Kim, G.Y.; Kim, S.H.; Ha, J.H.; Kang, J.S.; Lee, K.W.; Jeon, Y.J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the u-937 cell line. J. Appl. Phycol. 2009, 21, 307–314. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Boo, H.J.; Kwon, J.M.; Koh, Y.S.; Hyun, J.W.; Park, D.B.; Yoo, E.S.; et al. Apoptosis inducing activity of fucoidan in hct-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Hwang, J.; Lim, S.M.; Zhang, W.; Jin, J.O. Dendritic cell-mediated cancer immunotherapy with Ecklonia cava fucoidan. Int. J. Biol. Macromol. 2020, 159, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.; Delamarre, L. Dendritic cell-targeted vaccines. Front. Immunol. 2014, 5, 255. [Google Scholar] [CrossRef]
- Banchereau, J.; Palucka, A.K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 2005, 5, 296–306. [Google Scholar] [CrossRef]
- Zhang, W.; Park, H.B.; Yadav, D.; Hwang, J.; An, E.K.; Eom, H.Y.; Kim, S.J.; Kwak, M.; Lee, P.C.; Jin, J.O. Comparison of human peripheral blood dendritic cell activation by four fucoidans. Int. J. Biol. Macromol. 2021, 174, 477–484. [Google Scholar] [CrossRef]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.-O. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017, 8, 38554–38567. [Google Scholar] [CrossRef]
- Meng, J.; Cao, Y.; Meng, Y.; Luo, H.; Gao, X.; Shan, F. Maturation of mouse bone marrow dendritic cells (bmdcs) induced by Laminaria japonica polysaccharides (ljp). Int. J. Biol. Macromol. 2014, 69, 388–392. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Lu, W.J.; Tsai, G.J.; Chou, C.T.; Hsiao, H.I.; Hwang, P.A. Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using bacillus subtilis. Process. Biochem. 2016, 51, 1945–1953. [Google Scholar] [CrossRef]
- Geisen, U.; Zenthoefer, M.; Peipp, M.; Kerber, J.; Plenge, J.; Managò, A.; Fuhrmann, M.; Geyer, R.; Hennig, S.; Adam, D. Molecular mechanisms by which a Fucus vesiculosus extract mediates cell cycle inhibition and cell death in pancreatic cancer cells. Mar. Drugs 2015, 13, 4470–4491. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Lin, N.; Lu, W.; Huang, X.; Weng, J.; Sun, S.; Zhang, C.; Yang, Q.; Zhou, G.; et al. Fucoidan from Fucus vesiculosus attenuates doxorubicin-induced acute cardiotoxicity by regulating jak2/stat3-mediated apoptosis and autophagy. Biomed. Pharmacother. 2020, 130, 110534. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Singh, Y.; Sharma, K.; Shrivastav, A.; Sharma, A.; Singh, A.; Meher, J.G.; Singh, P.; Raval, K.; Kumar, A.; et al. Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int. J. Biol. Macromol. 2019, 122, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Jeong, D.; Na, K. Doxorubicin loading fucoidan acetate nanoparticles for immune and chemotherapy in cancer treatment. Carbohydr. Polym. 2013, 94, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Burney, M.; Mathew, L.; Gaikwad, A.; Nugent, E.K.; Gonzalez, A.O.; Smith, J.A. Evaluation fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus in combination with anticancer drugs in human cancer orthotopic mouse models. Integr. Cancer Ther. 2018, 17, 755–761. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in h22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of Astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C 2019, 98, 685–695. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Li, Y.; Song, K. Cytotoxicity and growth-inhibiting activity of Astragalus polysaccharides against breast cancer via the regulation of egfr and anxa1. J. Nat. Med. 2021, 75, 854–870. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Ke, Y.; Zeng, Y.-F.; Zhang, Y.-W.; Yu, H.-J. Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int. 2017, 17, 115. [Google Scholar] [CrossRef]
- Wu, J.; Yu, J.; Wang, J.; Zhang, C.; Shang, K.; Yao, X.; Cao, B. Astragalus polysaccharide enhanced antitumor effects of apatinib in gastric cancer ags cells by inhibiting akt signalling pathway. Biomed. Pharmacother. 2018, 100, 176–183. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Park, H.-B.; Lim, S.-M.; Go, S.; Kim, J.; Choi, I.; You, S.; Jin, J.-O. Human peripheral blood dendritic cell and t cell activation by Codium fragile polysaccharide. Mar. Drugs 2020, 18, 535. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ruan, Y.; Shen, T.; Huang, X.; Li, M.; Yu, W.; Zhu, Y.; Man, Y.; Wang, S.; Li, J. Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the pi3k/akt and p38mapk pathways. Oxidative Med. Cell. Longev. 2014, 2014, 674219. [Google Scholar] [CrossRef] [PubMed]
- Bamodu, O.A.; Kuo, K.-T.; Wang, C.-H.; Huang, W.-C.; Wu, A.T.H.; Tsai, J.-T.; Lee, K.-Y.; Yeh, C.-T.; Wang, L.-S. Astragalus polysaccharides (pg2) enhances the m1 polarization of macrophages, functional maturation of dendritic cells, and t cell-mediated anticancer immune responses in patients with lung cancer. Nutrients 2019, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qian, X.-H.; Zhao, D.-H.; Fu, S.-Z. Effects of Astragalus polysaccharide on the erythroid lineage and microarray analysis in k562 cells. J. Ethnopharmacol. 2010, 127, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-M.; Zhang, L.-S. effect of Astragalus polysaccharide on the function and maturation of plasmacytoid dendritic cells from chronic myelogenous leukemia before and after treatment. Zhonghua Xue Ye Xue Za Zhi = Zhonghua Xueyexue Zazhi 2010, 31, 740–743. [Google Scholar]
- Liu, A.-J.; Yu, J.; Ji, H.-Y.; Zhang, H.-C.; Zhang, Y.; Liu, H.-P. Extraction of a novel cold-water-soluble polysaccharide from Astragalus membranaceus and its antitumor and immunological activities. Molecules 2018, 23, 62. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of Astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Nakayasu, S.; Soegima, R.; Yamaguchi, K.; Oda, T. Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci. Biotechnol. Biochem. 2009, 73, 961–964. [Google Scholar] [CrossRef]
- Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on b16 melanoma. Biochem. Biophys. Res. Commun. 2015, 458, 727–732. [Google Scholar] [CrossRef]
- Zhang, W.; Kwak, M.; Park, H.B.; Okimura, T.; Oda, T.; Lee, P.C.; Jin, J.-O. Activation of human dendritic cells by ascophyllan purified from Ascophyllum nodosum. Mar. Drugs 2019, 17, 66. [Google Scholar] [CrossRef]
- Zhang, W.; Okimura, T.; Oda, T.; Jin, J.O. Ascophyllan induces activation of natural killer cells in mice in vivo and in vitro. Mar. Drugs 2019, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Kim, D.; Jiang, Z.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. Immunostimulatory activities of the sulfated polysaccharide ascophyllan from Ascophyllum nodosum in in vivo and in vitro systems. Biosci. Biotechnol. Biochem. 2012, 76, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zha, S.; Tentaku, M.; Okimura, T.; Jiang, Z.; Ueno, M.; Hirasaka, K.; Yamaguchi, K.; Oda, T. Suppressive effects of sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on the production of no and ros in lps-stimulated raw264.7 cells. Biosci. Biotechnol. Biochem. 2021, 85, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Okimura, T.; Xu, L.; Zhang, L.; Oda, T.; Kwak, M.; Yu, Q.; Jin, J.-O. Ascophyllan functions as an adjuvant to promote anti-cancer effect by dendritic cell activation. Oncotarget 2016, 7, 19284–19298. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Je, J.G.; Jayawardena, T.U.; Kim, Y.-S.; Ko, J.Y.; Fu, X.; Jeon, Y.-J. Protective effects of sulfated polysaccharides isolated from the enzymatic digest of Codium fragile against hydrogen peroxide-induced oxidative stress in in vitro and in vivo models. Algal Res. 2020, 48, 101891. [Google Scholar] [CrossRef]
- Park, H.B.; Hwang, J.; Zhang, W.; Go, S.; Kim, J.; Choi, I.; You, S.; Jin, J.-O. Polysaccharide from Codium fragile induces anti-cancer immunity by activating natural killer cells. Mar. Drugs 2020, 18, 626. [Google Scholar] [CrossRef]
- Harvey, A.L. Toxins and drug discovery. Toxicon 2014, 92, 193–200. [Google Scholar] [CrossRef]
- De Souza, J.M.; Goncalves, B.D.C.; Gomez, M.V.; Vieira, L.B.; Ribeiro, F.M. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front. Pharmacol. 2018, 9, 145. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and othermiscellaneousme. Mar. Drugs 2020, 18, 5. [Google Scholar] [CrossRef]
- Huang, L.-G.; Li, J.-P.; Pang, X.-M.; Chen, C.-Y.; Xiang, H.-Y.; Feng, L.-B.; Su, S.-Y.; Li, S.-H.; Zhang, L.; Liu, J.-L. Microrna-29c correlates with neuroprotection induced by fns by targeting both birc2 and bak1 in rat brain after stroke. CNS Neurosci. Ther. 2015, 21, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the hallmarks of cancer. J. Transl. Med. 2020, 18, 453. [Google Scholar] [CrossRef] [PubMed]
- Rivara, S.; Milazzo, F.M.; Giannini, G. Heparanase: A rainbow pharmacological target associated to multiple pathologies including rare diseases. Future Med. Chem. 2016, 8, 647–680. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.; Khurana, A.; Shridhar, V.; Dredge, K. The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front. Oncol. 2014, 4, 195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, H.; Chen, Y.; Xin, X.; Li, J.; Hou, Y.; Zhang, Z.; Zhang, X.; Xie, C.; Geng, M.; et al. Oligomannurarate sulfate, a novel heparanase inhibitor simultaneously targeting basic fibroblast growth factor, combats tumor angiogenesis and metastasis. Cancer Res. 2006, 66, 8779–8787. [Google Scholar] [CrossRef]
- Parish, C.R.; Coombe, D.R.; Jakobsen, K.B.; Bennett, F.A.; Underwood, P.A. Evidence that sulphated polysaccharides inhibit tumour metastasis by blocking tumour-cell-derived heparanases. Int. J. Cancer 1987, 40, 511–518. [Google Scholar] [CrossRef]
- Soeda, S.; Ishida, S.; Shimeno, H.; Nagamatsu, A. Inhibitory effect of oversulfated fucoidan on invasion through reconstituted basement membrane by murine Lewis lung carcinoma. Jpn. J. Cancer Res. 1994, 85, 1144–1150. [Google Scholar]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Bilan, M.I.; Usov, A.I. Structural analysis of fucoidans. Nat. Prod. Commun. 2008, 3, 1639–1648. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current status and future prospects of marine natural products (mnps) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef]
- Lear, M.J.; Hirai, K.; Ogawa, K.; Yamashita, S.; Hirama, M. A convergent total synthesis of the kedarcidin chromophore: 20-years in the making. J. Antibiot. 2019, 72, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U. Marine-derived pharmaceuticals–challenges and opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Lin, H.-T.V.; Lin, H.-Y.; Lo, S.-K. Dietary supplementation with low-molecular-weight fucoidan enhances innate and adaptive immune responses and protects against mycoplasma pneumoniae antigen stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Lee, O.-H.; Lee, H.-H.; Lee, B.-Y. A 4-week repeated oral dose toxicity study of fucoidan from the sporophyll of Undaria pinnatifida in sprague–dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a natural biopolymer in cancer combating: From edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808. [Google Scholar] [CrossRef]
- Kiruba, N.J.M.; Pradeep, M.A.; Thatheyus, A.J. Discovering promising anti-cancer drug candidates from marine algae. Sci. Int. 2018, 6, 44–50. [Google Scholar] [CrossRef]
- Mathew, L.; Burney, M.; Gaikwad, A.; Nyshadham, P.; Nugent, E.K.; Gonzalez, A.; Smith, J.A. Preclinical evaluation of safety of fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus for use in cancer treatment. Integr. Cancer Ther. 2017, 16, 572–584. [Google Scholar] [CrossRef]
- Bovet, L.; Samer, C.; Daali, Y. Preclinical evaluation of safety of fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus for use in cancer treatment. Integr. Cancer Ther. 2019, 18, 1–2. [Google Scholar] [CrossRef]

| Fucoidan Source | Cancer Type | Results | Risk of Clinically Significant Interactions | Reference |
|---|---|---|---|---|
| U. pinnatifida- | Breast cancer | No significant changes | Absent | [95] |
| Low-molecular-weight fucoidan (LMF) | Metastatic colorectal cancer (mcrc) | Improved disease control rate | Absent | [96] |
| HiQ-fucoidan from Laminaria japonica | Lung cancer | Survival rates increased by approx. 50% | Reduced the occurrence of general fatigue | [59] |
| Cladosiphon okamuranus | Colorectal cancer | Endure prolonged chemotherapy without fatigue | -Suppressed general fatigue -No suppression of diarrhea and neurotoxicity | [97] |
| Mozuku, Cladosiphon novae-caledoniae Kylin | Advanced cancer | Decreased level of proinflammatory cytokines | Insignificant quality of life score | [98] |
| Fucoidan Source | Cancer Cell Type | Action Mechanism | Reference |
|---|---|---|---|
| Cladosiphon okamuranus | Colon 26 | tumor growth↓ | [99] |
| (LMWF) | |||
| IMWF and HMWF | ↑survival time | ||
| Oral administration | ↑NK cells in the spleen | ||
| Fucus vesiculosus | 4T1 Lewis lung cancer cells B16 | -Inhibition of angiogenesis -Induction of apoptosis -Prevention of metastasis | [59,78,79] |
| Fucus evanescence | Lewis lung cancer cells | Antitumor and antimetastatic activities | [63] |
| Sargassum plagiophyllum | Diethylnitrosamine-induced hepatocellular | Inhibition of carcinogen metabolism | [100] |
| Cladosiphon okamuranus Tokida | Sarcoma 180 (S-180)-xenograft | ↑cytotoxicity via NO production by fucoidan-stimulated macrophages | [101] |
| Undaria pinnatifida | A20 | Cytolytic activity by Th1 and NK cell activation | [102] |
| Ascophyllum nodosum | MOPC-315 plasma cell tumor | Anti-angiogenesis | [103] |
| Sargassum mcclurei | colon cancer DLD-1 cells | Anti-tumorigenesis | [104] |
| Ecklonia cava | SKOV3 tumor xenograft | -↑ROS-mediated apoptosis -antitumoral | [105] |
| CT-26 carcinoma xenograft | ↑NK cell-mediated anticancer immunity | [106] | |
| Hydroclathrus clathratus | Sarcoma 180 xenograft | Suppressed tumor growth | [107] |
| Sargassum stenophyllum | B16F10 cells | Antiangiogenic and antitumoral | [108] |
| Stoechospermum marginatum | Ehrlich ascites tumor (EAT) cells | Angio-suppressive and antiproliferative activities | [109] |
| Sargassum fusiforme | A549 | Immunomodulatory activity | [110] |
| SPC-A-1 | Anti-angiogenesis | [111] |
| Fucoidan Source | Cell Type | Action Mechanism | Action Characteristic | Reference |
|---|---|---|---|---|
| Sargassum pallidum | HepG2, A549, and MGC-803 | Antitumor activity | -Antioxidant | [112] |
| Sargassum tortile | P-388 | Increases cytotoxicity | - | [113] |
| Sargassum micracanthum | Human head and neck squamous cell carcinoma (HNSCC) | Anticancer efficacy | - | [114] |
| Undaria pinnatifida | A549 SMMC-7721 NB4, KG1a, HL60, and K562 | -Induces apoptosis -Inhibit cell proliferation | Down-regulation of p38, PI3K/Akt, and the activation of the ERK1/2 MAPK pathway -NK-cell ↑ -Livin, XIAP mRNA ↓ Caspase-3,8,9 ↑ Bax-to-Bcl-2 ratio↑ Cytochrome c ↑ -ROS↑ | [40,115] |
| Fucus vesiculosus | HT-29 MCF-7 MDA-MB-231 Lewis lung A549 H1975 Huh-7 SNU-761 SNU-3085 HL-60 NB4 THP-1 SUDHL-4 OCI-LY8 NU-DUL-1 TMD8 U293 DB | -Inhibit cell proliferation -Induce cell apoptosis -Inhibit metastasis | -IRS-1/PI3K/AKT↓ -Ras/Raf/ERK↓ -Caspase-7,8,9 activation, cytochrome c, Bax ↑ Bcl-2↓ -Smad2/3,Smad4↓ -NF-κB↓ -Inhibit VEGF,MMPs -Caspase-3↑ PARP cleavage -ERK1/2, MEK1/2, JNK ↑ | [59,70,116,117] |
| Sargassum macrocarpum | MDA-MB-231, A549, and HCT116 | Induces ROS-mediated apoptosis | Inhibits STAT3 Signaling | [118] |
| Sargassum muticum | MCF-7 and MDA-MB-231 | Induce apoptosis, antioxidant, and antiangiogenesis effects | - | [119] |
| Sargassum angustifolium | HeLa and MCF-7 | Cytotoxic activity | - | [120] |
| Sargassum cinereum | Caco-2 and HCT-15 | Anticancer and apoptotic effect | Enhances ROS production | [121,122] |
| Sargassum filipendula | HeL | Induces apoptosis | Down-regulates Bcl-2 | [123] |
| Ecklonia cava | CT-26 | Induces apoptosis | Bcl-2/Bax signal pathway | [68] |
| Eisenia bicyclis | SK-MEL-28, DLD-1 | Inhibited the colony formation | - | [124,125] |
| Hizikia fusiformis | PC3 | Induces ROS-dependent apoptosis | Elevated expression of Fas, FasL, Bax and tBid, and decreased expression of Bcl-2 -reduced c-Flip expression and activated caspase-8, -9 and -3, leading to an increment of poly (ADP-ribose) polymerase (PARP) cleavage | [126] |
| Hydroclathrus clathratus | HL-60, MCF-7 | Antiproliferative activity | Induced sub-G1 arrest | [127] |
| Saccharina | DLD-1 | Inhibit cell proliferation | Inhibit the binding of EGF receptor with EGF | [38] |
| T-47D | ||||
| Sargassum | DLD-1 Huh6 Huh7 SK-Hep1 HepG2 | Inhibit cell proliferation | -Colony formation inhibition -TGF-β R1, 2↓ Phospho-Smad2/3↓ Smad 4 protein↓ | [38] |
| Cladosiphon | MCF-7 | Induce cell apoptosis | PARP cleavage Caspase-7,8,9 ↑ Cytochrome C, Bax, Bid↑ | [38] |
| Bifurcaria bifurcata | NSCLC-N6 | Inhibit cell proliferation | The growth arrest is irreversible | [38] |
| Turbinaria conoides | A549 | -Inhibit cell proliferation -Induce cell apoptosis | G0/G1 phase arrest | [38] |
| Sargassum latifolium | leukemia (1301 cells) | Chemopreventive activity | Antioxidant capacity | [128] |
| Sargassum glaucescens | MCF-7 HT-29 | Induce apoptosis | Fragmented the DNA of cancer cells | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.-O.; Yadav, D.; Madhwani, K.; Puranik, N.; Chavda, V.; Song, M. Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review. Molecules 2022, 27, 6032. https://doi.org/10.3390/molecules27186032
Jin J-O, Yadav D, Madhwani K, Puranik N, Chavda V, Song M. Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review. Molecules. 2022; 27(18):6032. https://doi.org/10.3390/molecules27186032
Chicago/Turabian StyleJin, Jun-O, Dhananjay Yadav, Kajal Madhwani, Nidhi Puranik, Vishal Chavda, and Minseok Song. 2022. "Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review" Molecules 27, no. 18: 6032. https://doi.org/10.3390/molecules27186032
APA StyleJin, J.-O., Yadav, D., Madhwani, K., Puranik, N., Chavda, V., & Song, M. (2022). Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug—A Review. Molecules, 27(18), 6032. https://doi.org/10.3390/molecules27186032











