Integrating a Luminescent Porous Aromatic Framework into Indicator Papers for Facile, Rapid, and Selective Detection of Nitro Compounds
Abstract
:1. Introduction
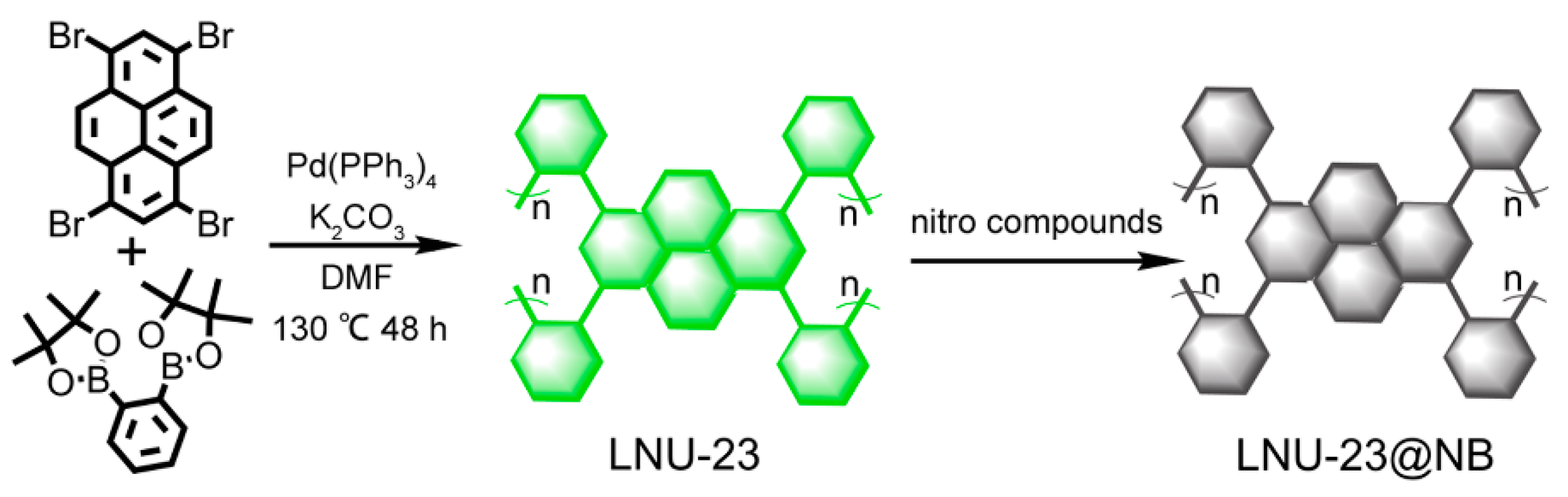
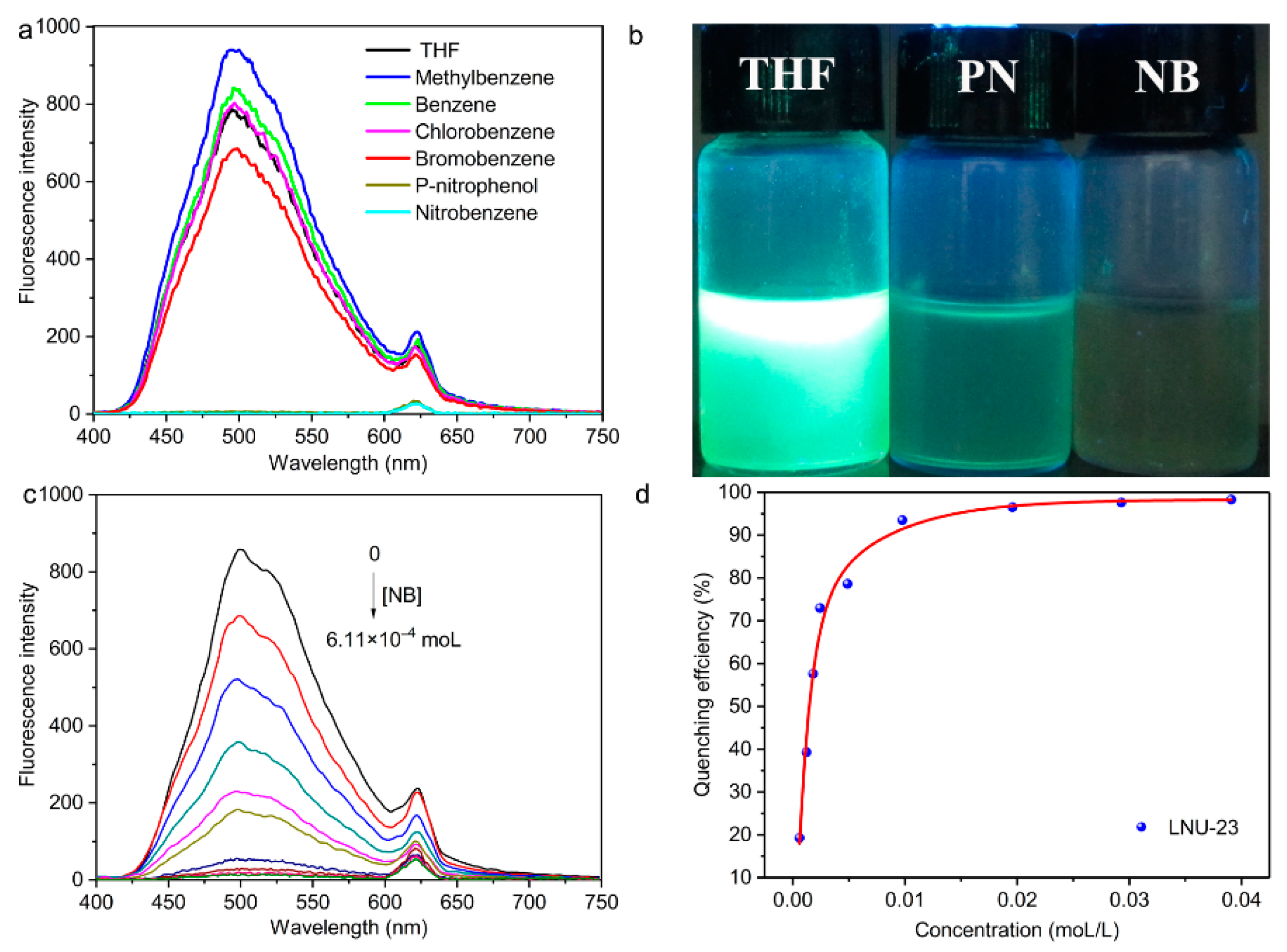
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of LNU-23
3.3. Preparation of LNU-23 Indicator Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xie, W.; Yuan, Y.; Zhou, T.Y.; Wang, J.J.; Nie, Z.B.; Xu, Y.H.; Su, Z.M. Stable zinc metal-organic framework as efficient bifunctional fluorescent probe for selective detection of nitrobenzene and Fe(III). J. Solid State Chem. 2022, 310, 123093–123099. [Google Scholar] [CrossRef]
- Wang, X.F.; Bian, J.Y.; Xu, L.C.; Wang, H.; Feng, S.Y. Thiophene functionalized silicon-containing aggregation-induced emission enhancement materials: Applications as fluorescent probes for the detection of nitroaromatic explosives in aqueous-based solutions. Phys. Chem. Chem. Phys. 2015, 17, 32472–324878. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, J.; Xi, L.; Xie, J.; Wang, X.; Ma, Y.; Li, L. Two novel lanthanide metal-organic frameworks: Selective luminescent sensing for nitrobenzene, Cu2+, and MnO4−. Cryst. Growth Des. 2020, 20, 5225–5234. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z.G. Creation of carbazole-based fluorescent porous polymers for recognition and detection of various pesticides in water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef]
- Lei, S.N.; Cong, H. Fluorescence detection of perfluorooctane sulfonate in water employing a tetraphenylethylene-derived dual macrocycle BowtieCyclophane. Chinese Chem. Lett. 2022, 33, 1493–1496. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Li, P.; Qu, B.; Mu, Z.; Liu, Y.; Qu, Y.; Kong, D.; Chang, Q.; Jing, L. Highly sensitive fluorescence detection of chloride ion in aqueous solution with Ag-modified porous g-C3N4 nanosheets. Chinese Chem. Lett. 2020, 31, 2725–2729. [Google Scholar] [CrossRef]
- Yan, Z.J.; Ren, H.; Ma, H.P.; Yuan, R.R.; Yuan, Y.; Zou, X.Q.; Sun, F.X.; Zhu, G.S. Construction and sorption properties of pyrene-based porous aromatic frameworks. Micropor. Mesopor. Mat. 2013, 173, 92–98. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, L.; Shen, J.; Qi, L. Enzyme immobilization on a pH-responsive porous polymer membrane for enzymatic kinetics study. Chin. Chem. Lett. 2021, 32, 3195–3198. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Kong, J.; Shen, B.; Xu, J. Superhydrophobic hierarchical porous divinylbenzene polymer for BTEX sensing and toluene/water selective detection. Chin. Chem. Lett. 2020, 31, 2125–2128. [Google Scholar] [CrossRef]
- Gao, W.; Li, M.; Fa, Y.; Zhao, Z.; Cai, Y.; Liang, X.; Yu, Y.; Jiang, G. Porous covalent organic frameworks-improved solid phase microextraction ambient mass spectrometry for ultrasensitive analysis of tetrabromobisphenol-A analogs. Chin. Chem. Lett. 2021, 33, 3849–3852. [Google Scholar] [CrossRef]
- He, Q.; Zhang, C.; Li, X.; Wang, X.; Mu, P.; Jiang, J.X. Pyrene-based conjugated microporous polymer as high performance electrode for lithium-ion batteries. Acta Chim. Sin. 2018, 76, 202–208. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Yuan, Y.; Xu, Y.M.; Zheng, G.Y.; Zhang, Q.; Jiang, Y.Q.; Wang, Z.Y.; Bu, N.S.; Xia, L.X.; Yan, Z.J. Fine-regulating ultramicropores in porous carbon via a self-sacrificial template route for high-performance supercapacitors. Nanoscale 2021, 13, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, G. Porous aromatic frameworks as a platform for multifunctional applications. ACS Cent. Sci. 2019, 5, 409–418. [Google Scholar] [CrossRef]
- Han, Z.Y.; Li, H.K.; Zhu, Q.Q.; Yuan, R.; He, H. An intriguing electrochemical impedance aptasensor based on a porous organic framework supported silver nanoparticles for ultrasensitively detecting theophylline. Chin. Chem. Lett. 2021, 32, 2865–2868. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Sun, F.-X.; Zhu, G.S. Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2. Chinese Chem. Lett. 2014, 25, 1407–1410. [Google Scholar] [CrossRef]
- Cui, P.; Jing, X.F.; Yuan, Y.; Zhu, G.S. Synthesis of porous aromatic framework with Friedel-Crafts alkylation reaction for CO2 separation. Chin. Chem. Lett. 2016, 27, 1479–1484. [Google Scholar] [CrossRef]
- Bai, M.G.M.; Babu, H.V.; Lakshmi, V.; Rao, M.R. Structure-property-function relationship of fluorescent conjugated microporous polymers. Mater. Chem. Front. 2021, 5, 2506–2551. [Google Scholar] [CrossRef]
- Finden, J.; Kunz, T.K.; Branda, N.R.; Wolf, M.O. Reversible and amplified fluorescence quenching of a photochromic polythiophene. Adv. Mater. 2008, 20, 1998–2000. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, Y.W. Tetraphenylethylene-interweaving conjugated macrocycle polymer materials as two-photon fluorescence sensors for metal ions and organic molecules. Adv. Mater. 2018, 30, 1800177–1800183. [Google Scholar] [CrossRef]
- Li, Z.P.; Li, H.; Xia, H.; Ding, X.S.; Luo, X.L.; Liu, X.M.; Mu, Y. Triarylboron-linked conjugated microporous polymers: Sensing and removal of fluoride ions. Chem. Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Sigen, A.; Zou, Y.C.; Luo, X.L.; Li, Z.P.; Xia, H.; Liu, X.M.; Mu, Y. Gas uptake, molecular sensing and organocatalytic performances of a multifunctional carbazole-based conjugated microporous polymer. J. Mater. Chem. A 2014, 2, 13422–13430. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, H.; Sun, F.X.; Jing, X.F.; Cai, K.; Zhao, X.J.; Wang, Y.; Wei, Y.; Zhu, G.S. Sensitive detection of hazardous explosives via highly fluorescent crystalline porous aromatic frameworks. J. Mater. Chem. 2012, 22, 24558–24562. [Google Scholar] [CrossRef]
- Ma, H.P.; Li, B.; Zhang, L.M.; Han, D.; Zhu, G.S. Targeted synthesis of core-shell porous aromatic frameworks for selective detection of nitro aromatic explosives via fluorescence two-dimensional response. J. Mater. Chem. A 2015, 3, 19346–19352. [Google Scholar] [CrossRef]
- Xi, E.P.; Zhao, Y.; Xie, Y.L.; Gao, N.; Bian, Z.; Zhu, G.S. Biological application of porous aromatic frameworks: State of the art and opportunities. J. Phys. Chem. Lett. 2021, 12, 11050–11060. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.X.; Zhang, H.C.; Feng, B.; Yang, D.Q.; Bu, N.S.; Zhao, Y.B.; Yan, Z.J.; Li, Z.N.; Yuan, Y.; Zhao, X.J. Facile strategy to prepare fluorescent porous aromatic frameworks for sensitive detection of nitroaromatic explosives. Chem. J. Chin. Univ. 2019, 40, 2456–2464. [Google Scholar]
- Xu, Y.H.; Jin, S.B.; Xu, H.; Nagai, A.; Jiang, D.L. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Echeverri, M.; Gámez-Valenzuela, S.; González-Cano, R.C.; Guadalupe, J.; Cortijo-Campos, S.; Navarrete, J.T.L.; Iglesias, M.; Delgado, M.C.R.; Gómez-Lor, B. Effect of the linkage position on the conjugation length of truxene-based porous polymers: Implications for their sensing performance of nitroaromatics. Chem. Mater. 2019, 31, 6971–6978. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhang, C.; Hu, C.; Liu, M.; Fei, Y.T.; Xia, H.Y. Synthesis of 1,6-disubstituted pyrene-based conjugated microporous polymers for reversible adsorption and fluorescence sensing of iodine. New J. Chem. 2020, 44, 2312–2320. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.J.; Zhu, G.S. Multifunctional porous aromatic frameworks: State of the art and opportunities. EnergyChem 2020, 2, 100037–100056. [Google Scholar] [CrossRef]
- Geng, T.M.; Zhu, Z.M.; Zhang, W.Y.; Wang, Y. A nitrogen-rich fluorescent conjugated microporous polymer with triazine and triphenylamine units for high iodine capture and nitro aromatic compound detection. J. Mater. Chem. A 2017, 5, 7612–7617. [Google Scholar] [CrossRef]
- Sun, L.B.; Zou, Y.C.; Liang, Z.Q.; Yu, J.H.; Xu, R.R. A one-pot synthetic strategy via tandem Suzuki-Heck reactions for the construction of luminescent microporous organic polymers. Polym. Chem. 2014, 5, 471–478. [Google Scholar] [CrossRef]
- Shen, X.S.; Faheem, M.; Matsuo, Y.; Aziz, S.; Zhang, X.; Li, Y.H.; Song, J.; Tian, Y.-Y.; Zhu, G.-S. Polarity engineering of porous aromatic frameworks for specific water contaminant capture. J. Mater. Chem. A 2019, 7, 2507–2512. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Cao, D.P. Porous organic polymer nanotubes as luminescent probe for highly selective and sensitive detection of Fe3+. Sci. China Chem. 2017, 60, 1090–1097. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Shang, Y.Q.; Wang, Z.G. Synthesis of fluorescent micro- and mesoporous polyaminals for detection of toxic pesticides. Macromolecules 2018, 51, 1769–1776. [Google Scholar] [CrossRef]
- Majeed, S.; Junaid, H.M.; Waseem, M.T.; Mahmood, T.; Farooq, U.; Shahzad, S.A. Receptor free fluorescent and colorimetric sensors for solution and vapor phase detection of hazardous pollutant nitrobenzene; a new structural approach to design AIEE active and piezofluorochromic sensors. J. Photoch. Photobiol. A 2022, 431, 114022–114033. [Google Scholar] [CrossRef]
- Jiang, D.Y.; Fang, H.F.; Li, G.; Zheng, G.L. A responsive supramolecular-organic framework: Functionalization with organic laser dye and lanthanide ions for sensing of nitrobenzene. J. Solid State Chem. 2020, 284, 121171–121177. [Google Scholar] [CrossRef]
- Li, H.H.; Han, Y.B.; Shao, Z.C.; Li, N.; Huang, C.; Hou, H.W. Water-stable Eu-MOF fluorescent sensors for trivalent metal ions and nitrobenzene. Dalton Trans. 2017, 46, 12201–12208. [Google Scholar] [CrossRef]
- He, H.; Chen, S.H.; Zhang, D.Y.; Yang, E.C.; Zhao, X.J. A luminescent metal-organic framework as an ideal chemosensor for nitroaromatic compounds. RSC Adv. 2017, 7, 38871–38876. [Google Scholar] [CrossRef]
- Wang, X.; Yan, P.; Li, Y.; An, G.; Yao, X.; Li, G. Highly efficient white-light emission and UV-Visible/NIR Luminescence sensing of lanthanide metal-organic frameworks. Cryst. Growth Des. 2017, 17, 2178–2185. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, Y.; Lin, H.Y.; Xu, N.; Liu, G.C.; Wang, X.; Chang, Z.H.; Li, J.R. A novel cadmium metal-organic framework-based multiresponsive fluorescent sensor demonstrating outstanding sensitivities and selectivities for detecting NB, Fe3+ ions and Cr2O72− anions. CrystEngComm 2020, 22, 6626–6631. [Google Scholar] [CrossRef]
- Xie, H.L.; Wang, H.; Xu, Z.; Qiao, R.J.; Wang, X.F.; Wang, X.M.; Wu, L.F.; Lu, H.F.; Feng, S.Y. A silicon-cored fluoranthene derivative as a fluorescent probe for detecting nitroaromatic compounds. J. Mater. Chem. C 2014, 2, 9425–9430. [Google Scholar] [CrossRef]
- Quan, X.P.; Xu, X.; Yan, B. Facile fabrication of Tb3+-functionalized COF mixed-matrix membrane as a highly sensitive platform for the sequential detection of oxolinic acid and nitrobenzene. J. Hazard. Mater. 2022, 427, 127869–127878. [Google Scholar] [CrossRef] [PubMed]
- Haug, W.K.; Moscarello, E.M.; Wolfson, E.R.; McGrier, P.L. The luminescent and photophysical properties of covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 839–864. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.M.; Zhu, Z.M.; Wang, X.; Xia, H.Y.; Wang, Y.; Li, D.-K. Poly{tris[4-(2-Thienyl)phenyl]amine} fluorescent conjugated microporous polymer for selectively sensing picric acid. Sensor. Actuat. B 2017, 244, 334–343. [Google Scholar] [CrossRef]
- Yang, T.; Feng, C.Y.; Zhao, P.; Wu, Y.S.; Ding, Y.; Wang, G.-Y.; Hu, A.G. Fluorescent electronic tongue supported with water-borne polyurethane for the discrimination of nitroaromatics in aqueous solution. J. Mater. Chem. C 2020, 8, 2500–2506. [Google Scholar] [CrossRef]
- Kumar, S.; Venkatramaiah, N.; Patil, S. Fluoranthene based derivatives for detection of trace explosive nitroaromatics. J. Phys. Chem. C 2013, 117, 7236–7245. [Google Scholar] [CrossRef]
- Dou, A.N.; Yang, L.B.; Fang, X.D.; Yin, Q.; Li, M.D.; Li, J.; Wang, M.Y.; Zhu, A.X.; Xu, Q.Q. Two luminescent lanthanide-organic frameworks containing bithiophene groups for the selective detection of nitrobenzene and Fe3+. CrystEngComm 2018, 20, 3609–3619. [Google Scholar] [CrossRef]
- Xie, W.Z.; Zhang, H.C.; Zheng, Y.S. Tetraphenylethylene tetracylhydrazine macrocycle with capability for discrimination of n-propanol from i-propanol and highly sensitive/selective detection of nitrobenzene. J. Mater. Chem. C 2017, 5, 10462–10468. [Google Scholar] [CrossRef]
- Lu, Y.B.; Liao, Y.Q.; Dong, L.; Zhu, S.D.; Wen, H.R.; Huang, J.; Dai, X.X.; Lian, P.; Jiang, X.M.; Li, R.; et al. Ultra-stable metal-organic framework with concurrent high proton conductivity and fluorescence sensing for nitrobenzene. Chem. Mater. 2021, 33, 7858–7868. [Google Scholar] [CrossRef]
- Yi, D.; Yang, H.; Liu, R.; Shao, C.; Yang, L. A multi-responsive chemosensor for highly sensitive and selective detection of Fe3+, Cu2+, Cr2O72− and nitrobenzene based on a luminescent lanthanide metal-organic framework. Dalton. Trans. 2020, 49, 13003–13016. [Google Scholar]
- Wang, L.B.; Wang, J.J.; Yue, E.L.; Li, J.F.; Tang, L.; Wang, X.; Hou, X.Y.; Zhang, Y.Q.; Ren, Y.X.; Chen, X.L. Luminescent Zn (II) coordination polymers for highly selective detection of triethylamine, nitrobenzene and tetracycline in water systems. Dye. Pigment. 2022, 197, 109863–109872. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, Y.; Feng, M.; Wu, L.; Wang, D.; Li, C. Two isostructural Ln3+-based heterometallic MOFs for the detection of nitro-aromatics and Cr2O72−. New J. Chem. 2020, 44, 12748–12754. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Gao, C.; Fan, J.; Liu, J.; Feng, B.; Ruan, X.; Yang, Y.; Yuan, Y.; Chu, K.; Yan, Z.; et al. Integrating a Luminescent Porous Aromatic Framework into Indicator Papers for Facile, Rapid, and Selective Detection of Nitro Compounds. Molecules 2022, 27, 6252. https://doi.org/10.3390/molecules27196252
Cui B, Gao C, Fan J, Liu J, Feng B, Ruan X, Yang Y, Yuan Y, Chu K, Yan Z, et al. Integrating a Luminescent Porous Aromatic Framework into Indicator Papers for Facile, Rapid, and Selective Detection of Nitro Compounds. Molecules. 2022; 27(19):6252. https://doi.org/10.3390/molecules27196252
Chicago/Turabian StyleCui, Bo, Changyuan Gao, Jiating Fan, Jinni Liu, Bin Feng, Xianghui Ruan, Yajie Yang, Ye Yuan, Kuo Chu, Zhuojun Yan, and et al. 2022. "Integrating a Luminescent Porous Aromatic Framework into Indicator Papers for Facile, Rapid, and Selective Detection of Nitro Compounds" Molecules 27, no. 19: 6252. https://doi.org/10.3390/molecules27196252
APA StyleCui, B., Gao, C., Fan, J., Liu, J., Feng, B., Ruan, X., Yang, Y., Yuan, Y., Chu, K., Yan, Z., & Xia, L. (2022). Integrating a Luminescent Porous Aromatic Framework into Indicator Papers for Facile, Rapid, and Selective Detection of Nitro Compounds. Molecules, 27(19), 6252. https://doi.org/10.3390/molecules27196252








