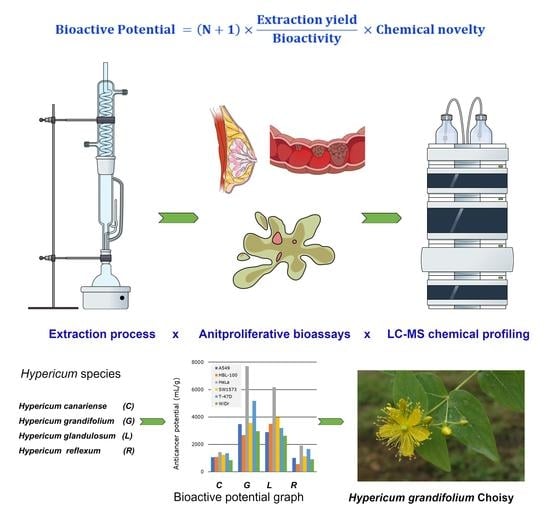
Bioactive Potential: A Pharmacognostic Definition through the Screening of Four Hypericum Species from the Canary Islands
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Plants and Microextraction
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity
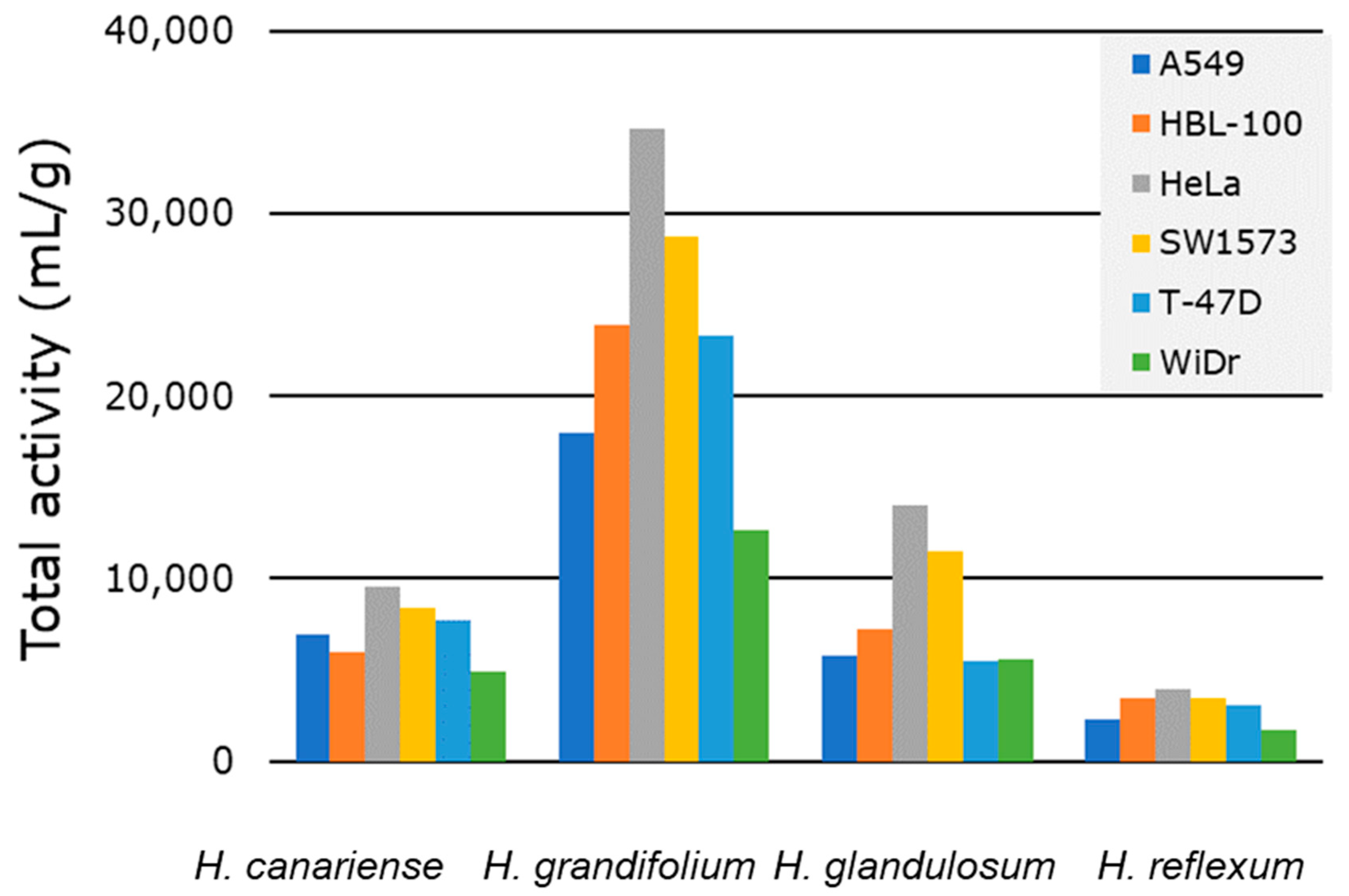
2.2.2. Total Activity
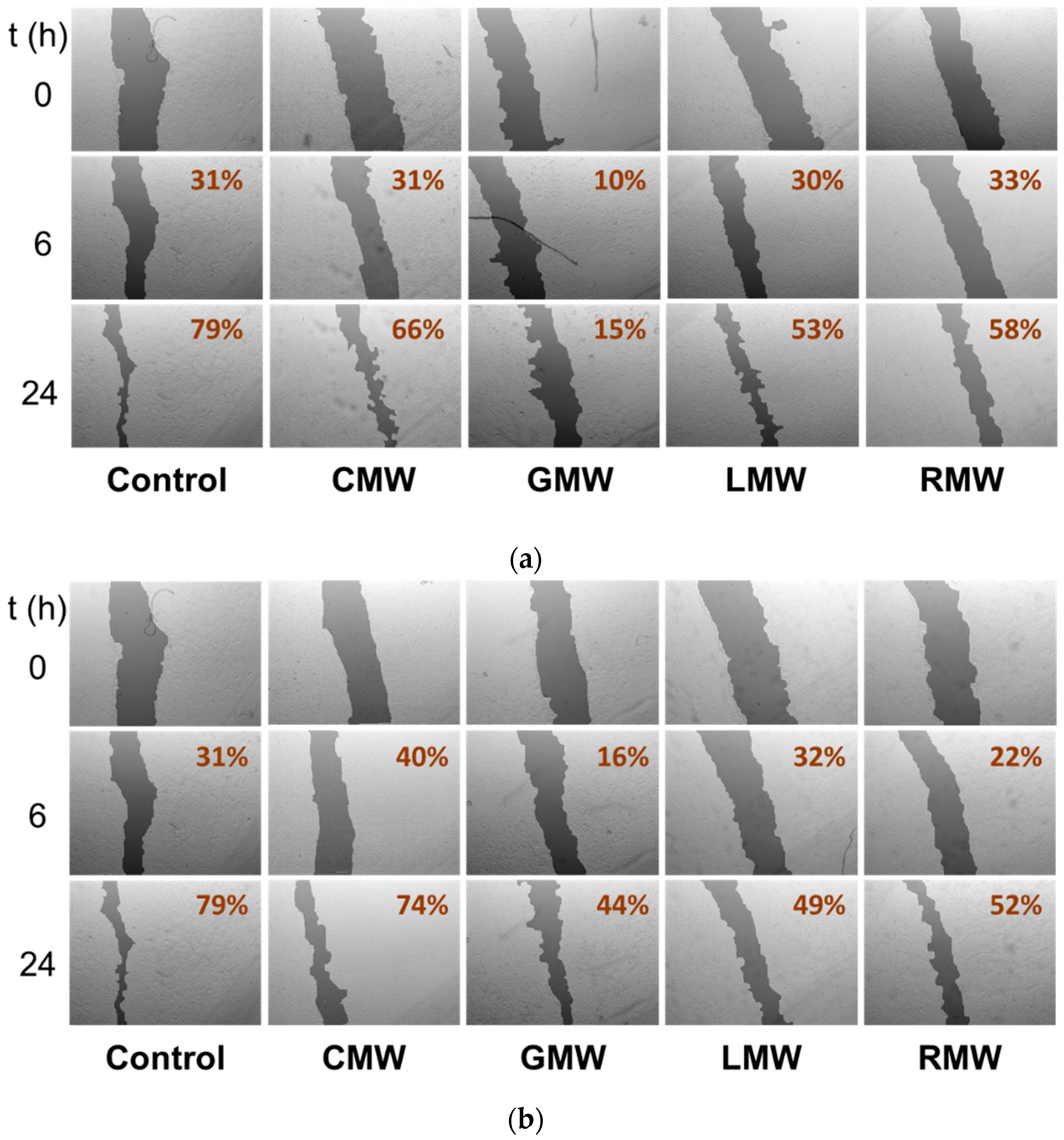
2.2.3. Cell Migration Disturbances in A549 Cells
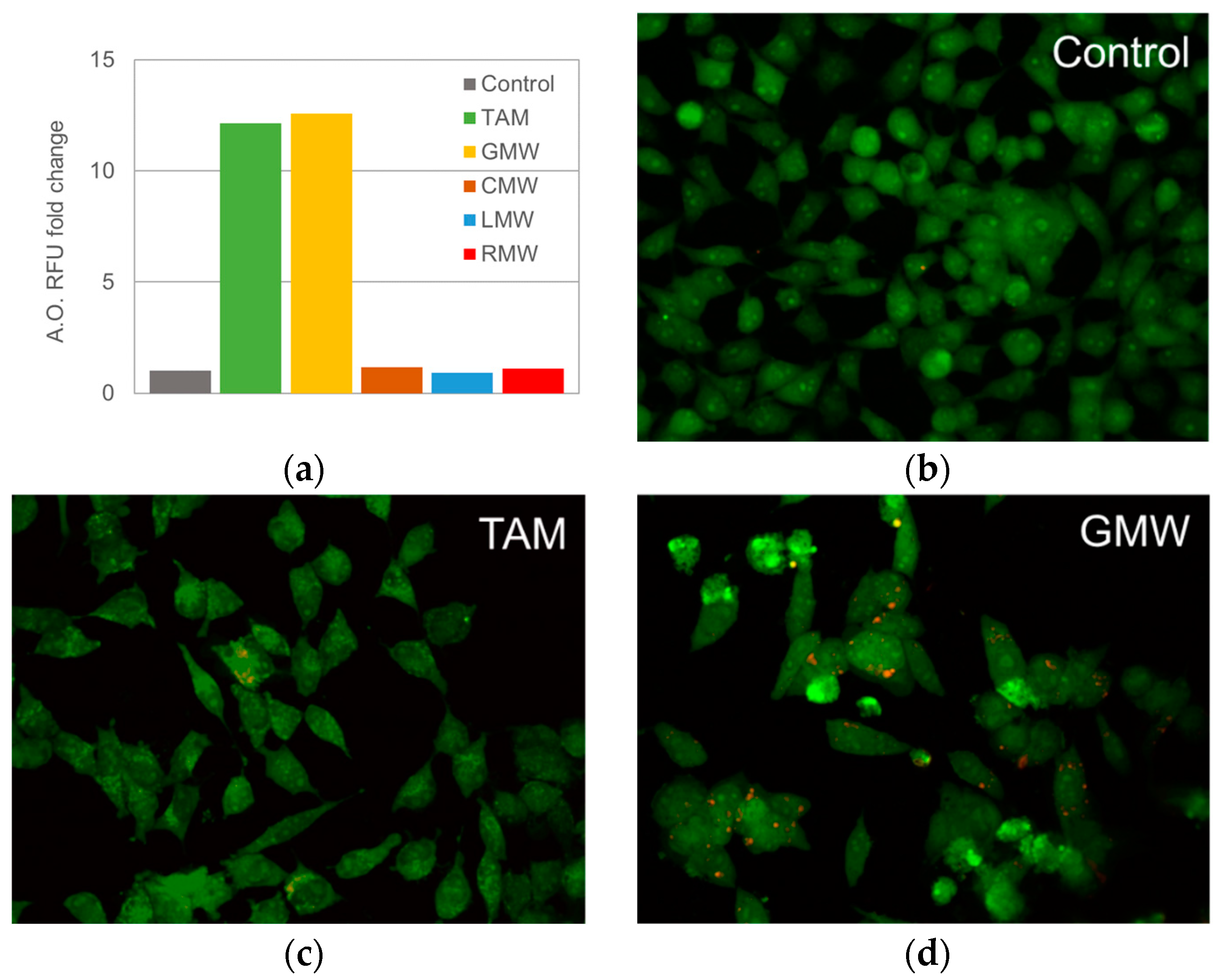
2.2.4. Formation of Acidic Vacuoles in HeLa Cells
2.3. Early Chemical Profiling of MW Microextracts
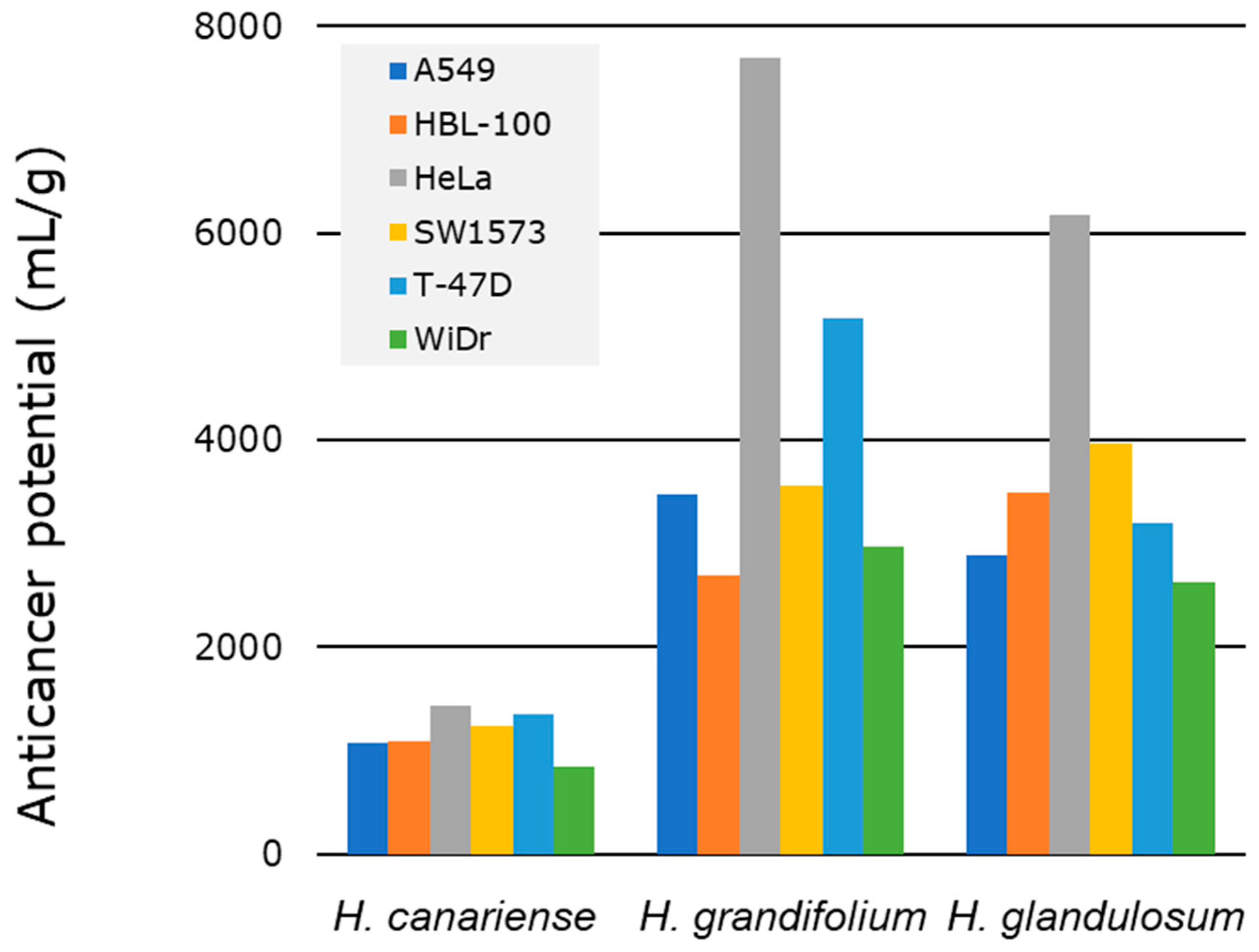
2.4. Bioactive and Anticancer Potential
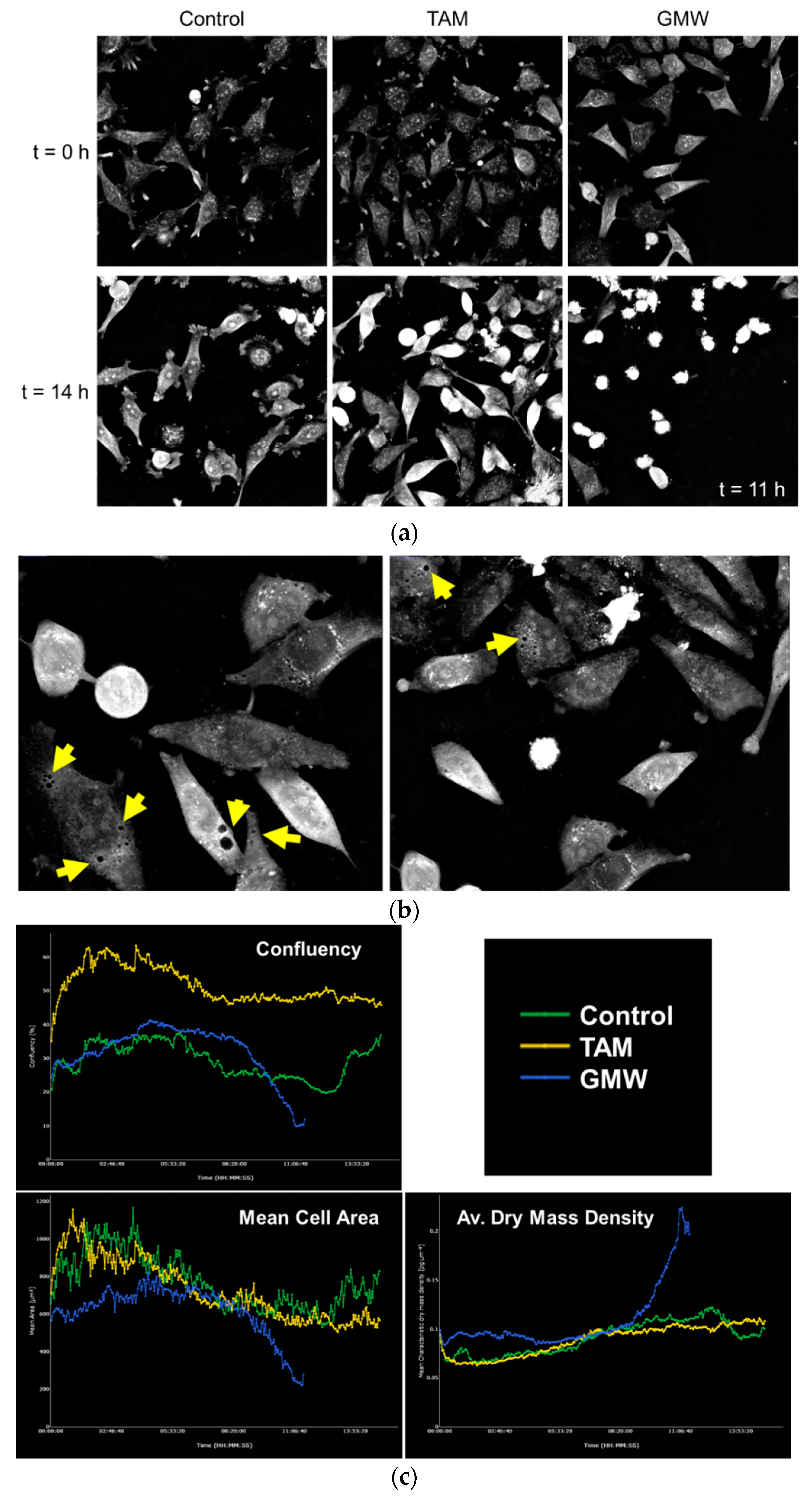
2.5. MW Microextracts of H. Grandifolium Induce Cell Death in HeLa Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction of Plant Material
3.4. UHPLC–DAD–MS3 Analysis
3.5. Cell, Culture and Plating
3.6. Antiproliferative Tests
3.7. Cell Migration Assay
3.8. Acridine Orange Staining Assay
3.9. Live-Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aburjai, T.; Natsheh, F.M. Plants Used in Cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Chugh, A. Role of Marine Bioprospecting Contracts in Developing Access and Benefit Sharing Mechanism for Marine Traditional Knowledge Holders in the Pharmaceutical Industry. Glob. Ecol. Conserv. 2015, 3, 176–187. [Google Scholar] [CrossRef]
- Dixit, S.; Shukla, A.; Singh, V.; Upadhyay, S.K. Bioprospecting of Natural Compounds for Industrial and Medical Applications. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Wiley: Hoboken, NJ, USA, 2021; pp. 53–71. [Google Scholar]
- Kewalanand; Prajapati, B. Cultivation and Bioprospecting of Medicinal Plants; Springer: Singapore, 2018; ISBN 9789811082917. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.O.; Ochoa-Pacheco, A.; Daniel, M.; Ortiz-Beat, E.; Font-salmo, O.; Guisado-bourzac, F.; Molina-bertr, S.; Monzote, L.; Cos, P.; Foubert, K.; et al. Cultivars of Plectranthus Neochilus Schltr. Plants 2022, 11, 134. [Google Scholar] [CrossRef]
- Schultz, F.; Osuji, O.F.; Nguyen, A.; Anywar, G.; Scheel, J.R.; Caljon, G.; Pieters, L.; Garbe, L.A. Pharmacological Assessment of the Antiprotozoal Activity, Cytotoxicity and Genotoxicity of Medicinal Plants Used in the Treatment of Malaria in the Greater Mpigi Region in Uganda. Front. Pharmacol. 2021, 12, 678535. [Google Scholar] [CrossRef]
- Amoo, S.O.; Finnie, J.F.; Van Staden, J. In Vitro Pharmacological Evaluation of Three Barleria Species. J. Ethnopharmacol. 2009, 121, 274–277. [Google Scholar] [CrossRef]
- Eloff, J.N. Quantification the Bioactivity of Plant Extracts during Screening and Bioassay Guided Fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef]
- Gado, D.A.; Abdalla, M.A.; Ahmed, A.S.; Madikizela, B.; Nkadimeng, S.M.; Ehlers, M.M.; McGaw, L.J. In Vitro Antibacterial Activity of Loxostylis Alata Extracts and Isolated Compounds against Salmonella Species. BMC Complement. Med. Ther. 2021, 21, 121. [Google Scholar] [CrossRef]
- Bisi-Johnson, M.A.; Obi, C.L.; Hattori, T.; Oshima, Y.; Li, S.; Kambizi, L.; Eloff, J.N.; Vasaikar, S.D. Evaluation of the Antibacterial and Anticancer Activities of Some South African Medicinal Plants. BMC Complement. Altern. Med. 2011, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Hellio, C.; Patel, A.V.; Abbas, G. Phytochemical Profile, Antioxidant and Antibacterial Activity of Four Hypericum Species from the UK. South African J. Bot. 2020, 133, 45–53. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-Infective Potential of Natural Products: How to Develop a Stronger in Vitro “Proof-of-Concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N. Hypericum Perforatum (St John’s Wort) beyond Depression: A Therapeutic Perspective for Pain Conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, W.; Wang, J.C. Recent Advances Regarding Constituents and Bioactivities of Plants from the Genus Hypericum. Chem. Biodivers. 2015, 12, 309–349. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated Phloroglucinols from Hypericum Scruglii, an Endemic Species of Sardinia (Italy), as New Dual HIV-1 Inhibitors Effective on HIV-1 Replication. PLoS ONE 2018, 13, e0195168. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Y.; Morcol, T.; Lin, F.; Gu, R.; Kennelly, E.J.; Long, C. UPLC-QTof-MS Chemical Profiling and Characterization of Antiproliferative and Anti-Inflammatory Compounds from Seven Hypericum Species in China. Ind. Crops Prod. 2021, 173, 114156. [Google Scholar] [CrossRef]
- Cenić-Miloŝević, D.; Tambur, Z.; Ivanĉajić, S.; Stanojković, T.; Grozdanić, N.; Kuliŝić, Z.; Juranić, Z. Antiproliferative Effects of Tanaceti Partheni, Hypericum Perforatum and Propolis on HeLa Cells. Arch. Biol. Sci. 2014, 66, 705–712. [Google Scholar] [CrossRef]
- Gaid, M.; Füller, J.; Müller-Goymann, C. The Petroleum Ether Extract from Hypericum Perforatum Root Cultures Exhibits Potent Antiproliferative Activity in Human Keratinocytes and Fibroblasts. Planta Med. 2019, 85, 591–598. [Google Scholar] [CrossRef]
- Mokoro Vincent, O.; Mwanzia Nguta, J.; Mutie Musila, F.; Matara Nyak, D.; Hashim Mohammed, A.; Apiri Gervason, M.; Simon Mitema, E. Ethnopharmacology, Pharmacological Activities, and Chemistry of the Hypericum Genus. J. Phytopharm. 2021, 10, 105–113. [Google Scholar] [CrossRef]
- De Paz, P.L.P.; Padrón, C.E.H. Plantas Medicinales o Útiles En La Flora Canaria: Aplicaciones Populares; Francisco Lemus: La Laguna, Spain, 1999; ISBN 8487973124. [Google Scholar]
- Pérez de Paz, P.L.; Medina Medina, I. Catálogo de Las Plantas Medicinales de La Flora Canaria: Aplicaciones Populares, 1st ed.; Viceconsejería de Cultura y Deportes del Gobierno de Canarias, Instituto de Estudios Canarios: Santa Cruz de Tenerife, Spain, 1988. [Google Scholar]
- Pardo de Santayana, M.; Morales, R.; Tardío, J.; Aceituna, L.; Molina, M. Conocimientos Tradicionales Relativos a La Biodiversidad; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2018; Volume Segunda Fa, ISBN 9788449114724. [Google Scholar]
- Herrera, R.M.; Pérez, M.; Martín-Herrera, D.A.; López-García, R.; Rabanal, R.M.; Arias, A. Antimicrobial Activity of Extracts from Plants Endemic to the Canary Islands. Phyther. Res. 1996, 10, 364–366. [Google Scholar] [CrossRef]
- Rabanal, R.M.; Arias, A.; Prado, B.; Hernández-Pérez, M.; Sánchez-Mateo, C.C. Antimicrobial Studies on Three Species of Hypericum from the Canary Islands. J. Ethnopharmacol. 2002, 81, 287–292. [Google Scholar] [CrossRef]
- Luz Cardona, M.; Pedro, J.R.; Seoane, E.; Vidal, R. Xanthone Constituents of Hypericum Canariensis. J. Nat. Prod. 1985, 48, 467–469. [Google Scholar] [CrossRef]
- Cardona, M.L.; Fernandez, M.I.; Pedro, J.R.; Seoane, E.; Vidal, R. Additional New Xanthones and Xanthonolignoids from Hypericum Canariensis. J. Nat. Prod. 1986, 49, 95–100. [Google Scholar] [CrossRef]
- Cardona, M.L.; Fernandez, M.I.; Pedro, J.R.; Serrano, Á. Xanthones From Hypericum Reflexum. Phytochemestry 1990, 29, 3003–3006. [Google Scholar] [CrossRef]
- Cardona, L.; Pedro, J.R.; Serrano, A.; Muñoz, M.C.; Solans, X. Spiroterpenoids from Hypericum Reflexum. Phytochemistry 1993, 33, 1185–1187. [Google Scholar] [CrossRef]
- Díaz, J.G.; De Paz, P.P.; Herz, W. New Water Soluble Flavone and Xanthone Glycosides from Hypericum Canariense L. Phytochem. Lett. 2010, 3, 171–175. [Google Scholar] [CrossRef]
- Díaz, J.G. Chemical Composition of Hypericum Coadunatum Chr. from the Canary Islands. J. Mol. Struct. 2022, 1248, 131447. [Google Scholar] [CrossRef]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F.; et al. Phytochemical Analysis and in Vitro Biological Activity of Three Hypericum Species from the Canary Islands (Hypericum Reflexum, Hypericum Canariense and Hypericum Grandifolium). Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef]
- Prado, B.; Rabanal, R.M.; Sanchez-Mateo, C.C. Evaluation of the Central Properties of Several Hypericum Species from the Canary Islands. Phyther. Res. 2002, 16, 740–744. [Google Scholar] [CrossRef]
- Sánchez-Mateo, C.C.; Bonkanka, C.X.; Prado, B.; Rabanal, R.M. Antidepressant Properties of Some Hypericum Canariense L. and Hypericum Glandulosum Ait. Extracts in the Forced Swimming Test in Mice. J. Ethnopharmacol. 2005, 97, 541–547. [Google Scholar] [CrossRef]
- Rabanal, R.M.; Bonkanka, C.X.; Hernández-Pérez, M.; Sánchez-Mateo, C.C. Analgesic and Topical Anti-Inflammatory Activity of Hypericum Canariense L. and Hypericum Glandulosum Ait. J. Ethnopharmacol. 2005, 96, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mateo, C.C.; Bonkanka, C.X.; Hernández-Pérez, M.; Rabanal, R.M. Evaluation of the Analgesic and Topical Anti-Inflammatory Effects of Hypericum Reflexum L. Fil. J. Ethnopharmacol. 2006, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mateo, C.C.; Bonkanka, C.X.; Prado, B.; Rabanal, R.M. Antidepressant Activity of Some Hypericum Reflexum L. Fil. Extracts in the Forced Swimming Test in Mice. J. Ethnopharmacol. 2007, 112, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bonkanka, C.X.; Smelcerovic, A.; Zuehlke, S.; Rabanal, R.M.; Spiteller, M.; Sánchez-Mateo, C.D.C. HPLC-MS Analysis and Anti-Oedematogenic Activity of Hypericum Grandifolium Choisy (Hypericaceae). Planta Med. 2008, 74, 719–725. [Google Scholar] [CrossRef]
- Sánchez-Mateo, C.C.; Bonkanka, C.X.; Rabanal, R.M. Hypericum Grandifolium Choisy: A Species Native to Macaronesian Region with Antidepressant Effect. J. Ethnopharmacol. 2009, 121, 297–303. [Google Scholar] [CrossRef]
- Bonkanka, C.X.; Sánchez-Mateo, C.D.C.; Rabanal, R.M. Antinociceptive Activity of Hypericum Grandifolium Choisy in Mice. J. Nat. Med. 2011, 65, 122–128. [Google Scholar] [CrossRef]
- Macías, F.A.; Lacret, R.; Varela, R.M.; Nogueiras, C.; Molinillo, J.M.G. Bioactive Apocarotenoids from Tectona Grandis. Phytochemistry 2008, 69, 2708–2715. [Google Scholar] [CrossRef]
- Ion, V.; Ielciu, I.; Cârje, A.G.; Muntean, D.L.; Crişan, G.; Păltinean, R. Hypericum Spp.—An Overview of the Extraction Methods and Analysis of Compounds. Separations 2022, 9, 17. [Google Scholar] [CrossRef]
- Dresler, S.; Kováčik, J.; Strzemski, M.; Sowa, I.; Wójciak-Kosior, M. Methodological Aspects of Biologically Active Compounds Quantification in the Genus Hypericum. J. Pharm. Biomed. Anal. 2018, 155, 82–90. [Google Scholar] [CrossRef]
- Wilson, B.A.P.; Thornburg, C.C.; Henrich, C.J.; Grkovic, T.; O’Keefe, B.R. Creating and Screening Natural Product Libraries. Nat. Prod. Rep. 2020, 37, 893–918. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Britt, J.R.; Evans, J.R.; Akee, R.K.; Whitt, J.A.; Trinh, S.K.; Harris, M.J.; Thompson, J.R.; Ewing, T.L.; Shipley, S.M.; et al. NCI Program for Natural Product Discovery: A Publicly-Accessible Library of Natural Product Fractions for High-Throughput Screening. ACS Chem. Biol. 2018, 13, 2484–2497. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, T.; Akee, R.K.; Thornburg, C.C.; Trinh, S.K.; Britt, J.R.; Harris, M.J.; Evans, J.R.; Kang, U.; Ensel, S.; Henrich, C.J.; et al. National Cancer Institute (NCI) Program for Natural Products Discovery: Rapid Isolation and Identification of Biologically Active Natural Products from the NCI Prefractionated Library. ACS Chem. Biol. 2020, 15, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M. Protocols for Screening Chemical Agents and Natural Products against Animal Tumors and Other Biological Systems. Cancer Chemother. Rep. 1972, 3, 17–19. [Google Scholar]
- Friedl, P.; Wolf, K. Tumour-Cell Invasion and Migration: Diversity and Escape Mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Hu, Y.; Reggiori, F. Molecular Regulation of Autophagosome Formation. Biochem. Soc. Trans. 2022, 50, 55–69. [Google Scholar] [CrossRef]
- Alvarez-Meythaler, J.G.; Garcia-Mayea, Y.; Mir, C.; Kondoh, H.; LLeonart, M.E. Autophagy Takes Center Stage as a Possible Cancer Hallmark. Front. Oncol. 2020, 10, 6069. [Google Scholar] [CrossRef]
- Shindo-Okada, N.; Takeuchi, K.; Han, B.S.; Nagamachi, Y. Establishment of Cell Lines with High and Low Metastatic Potential from A549 Human Lung Adenocarcinoma. Japanese J. Cancer Res. 2002, 93, 50–60. [Google Scholar] [CrossRef]
- Vaid, B.; Chopra, B.S.; Raut, S.; Sagar, A.; Badmalia, M.D.; Ashish; Khatri, N. Antioxidant and Wound Healing Property of Gelsolin in 3T3-L1 Cells. Oxid. Med. Cell. Longev. 2020, 2020, 4045365. [Google Scholar] [CrossRef]
- Karna, P.; Zughaier, S.; Pannu, V.; Simmons, R.; Narayan, S.; Aneja, R. Induction of Reactive Oxygen Species-Mediated Autophagy by a Novel Microtubule-Modulating Agent. J. Biol. Chem. 2010, 285, 18737–18748. [Google Scholar] [CrossRef]
- Majumdar, S.K.; Valdellon, J.A.; Brown, K.A. In Vitro Investigations on the Toxicity and Cell Death Induced by Tamoxifen on Two Non-Breast Cancer Cell Types. J. Biomed. Biotechnol. 2001, 2001, 99–107. [Google Scholar] [CrossRef]
- Thomé, M.P.; Filippi-Chiela, E.C.; Villodre, E.S.; Migliavaca, C.B.; Onzi, G.R.; Felipe, K.B.; Lenz, G. Ratiometric Analysis of Acridine Orange Staining in the Study of Acidic Organelles and Autophagy. J. Cell Sci. 2016, 129, 4622–4632. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.G.; Scheper, T. Identification of Major Constituents of Hypericum Perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the Major Constituents of Hypericum Perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Yin, F.; Hu, L.H.; Lu, P. Sulfonated Xanthones from Hypericum Sampsonii. Phytochemistry 2004, 65, 2595–2598. [Google Scholar] [CrossRef]
- Ploss, O.; Petereit, F.; Nahrstedt, A. Procyanidins from the Herb of Hypericum Perforatum. Pharmazie 2001, 56, 509–511. [Google Scholar]
- Ersoy, E.; Eroglu Ozkan, E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-Aging Potential and Anti-Tyrosinase Activity of Three Hypericum Species with Focus on Phytochemical Composition by LC–MS/MS. Ind. Crops Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Camas, N.; Radusiene, J.; Ivanauskas, L.; Jakstas, V.; Kayikci, S.; Cirak, C. Chemical Composition of Hypericum Species from the Taeniocarpium and Drosanthe Sections. Plant Syst. Evol. 2014, 300, 953–960. [Google Scholar] [CrossRef]
- Macías, F.A.; Lacret, R.; Varela, R.M.; Nogueiras, C.; Molinillo, J.M.G. Isolation and Phytotoxicity of Terpenes from Tectona Grandis. J. Chem. Ecol. 2010, 36, 396–404. [Google Scholar] [CrossRef]
- González-Coloma, A.; Reina, M.; Sáenz, C.; Lacret, R.; Ruiz-Mesia, L.; Arán, V.J.; Sanz, J.; Martínez-Díaz, R.A. Antileishmanial, Antitrypanosomal, and Cytotoxic Screening of Ethnopharmacologically Selected Peruvian Plants. Parasitol. Res. 2012, 110, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, K.A.; Hałasa, R.; Dudek, M.K.; Majdan, M.; Jankowska, K.; Granica, S. Antibacterial and Anti-Inflammatory Activity of Bistort (Bistorta Officinalis) Aqueous Extract and Its Major Components. Justification of the Usage of the Medicinal Plant Material as a Traditional Topical Agent. J. Ethnopharmacol. 2020, 260, 113077. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio-protocol 2016, 6, e1984. [Google Scholar] [CrossRef] [PubMed]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity Screening of Thymus Vulgaris L. Essential Oil in Brine Shrimp Nauplii and Cancer Cell Lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration in Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Gebäck, T.; Schulz, M.M.P.; Koumoutsakos, P.; Detmar, M. TScratch: A Novel and Simple Software Tool for Automated Analysis of Monolayer Wound Healing Assays. Biotechniques 2009, 46, 265–274. [Google Scholar] [CrossRef]
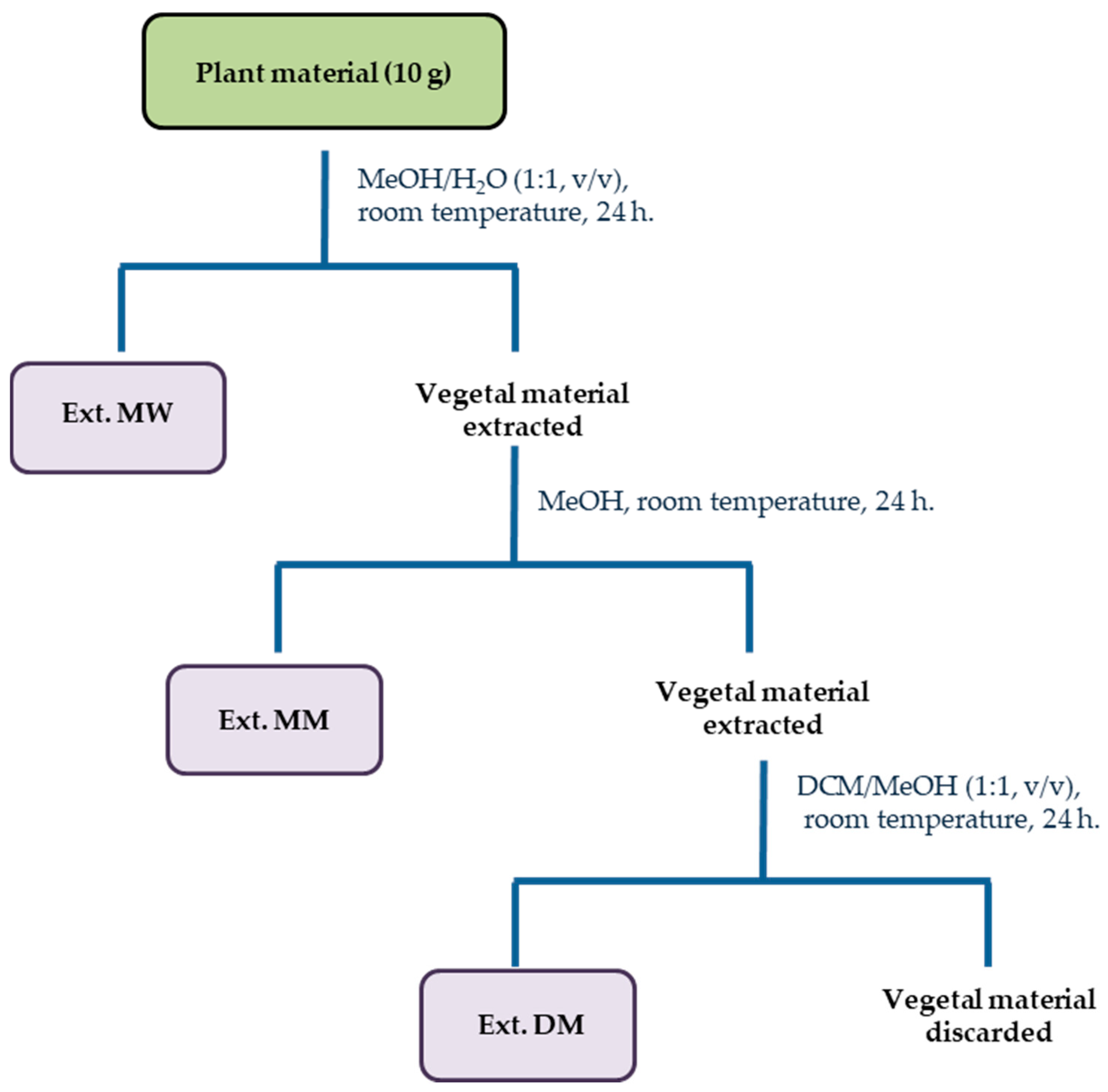

| Yield (%) | ||||
|---|---|---|---|---|
| Species | MW | MM | DM | Total |
| C | 8.5 | 9.5 | 2.4 | 20.4 |
| G | 7.5 | 8.0 | 1.9 | 17.4 |
| L | 13.9 | 7.6 | 9.1 | 30.6 |
| R | 14.3 | 6.9 | 1.2 | 22.1 |
| Peak | RT (min) | (max) | MS [M+H]+ | MS [M-H]− | CMW | GMW | LMW | Putative Compound |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.3 | 269 | 171.03 | 169.03 | - | ✔ | - | gallic acid [59] |
| 2 | 6.6 | 259, 293 | 154.99 | 153.07 | ✔ | ✔ | - | 3,4-dihydroxybenzoic acid [60] |
| 3 | 7.6 | 214, 256 | 205.30 | 203.18 | - | - | ✔ | unidentified compound |
| 4 | 7.8 | 202, 279 | - | 315.22 | ✔ | - | - | unidentified compound |
| 5 | 8.1 | 205, 262, 294 | 343.19 | 341.18 | - | ✔ | - | unidentified compound |
| 6 | 11.3 | 214, 325 | 355.2 | 353.25 | ✔ | ✔ | ✔ | neochlorogenic acid [61] |
| 7 | 12.9 | 287, 316 | 343.21 | 341.28 | - | - | ✔ | unidentified compound |
| 8 | 14.4 | 209, 311 | 339.27 | 337.27 | - | ✔ | ✔ | p-coumaroylquinic acid isomer [62,63] |
| 9 | 15.4 | 217, 310 | 339.20 | 337.21 | ✔ | ✔ | ✔ | p-coumaroylquinic acid isomer [62,63] |
| 10 | 16.5 | 279 | 579.19 | 577.19 | ✔ | - | - | dimeric procyanidin type B [64] |
| 11 | 17.9 | 216, 325 | 355.20 | 353.25 | ✔ | - | ✔ | chlorogenic acid [42] |
| 12 | 20.0 | 214, 325 | 355.19 | 353.24 | - | - | ✔ | cryptochlorogenic acid [63] |
| 13 | 21.7 | 280 | 579.20 | 577.19 | ✔ | - | - | dimeric procyanidin type B [64] |
| 14 | 23.3 | 279 | 579.17 | 577.19 | ✔ | ✔ | ✔ | dimeric procyanidin type B |
| 15 | 24.6 | 279 | 291.13 | 289.17 | ✔ | ✔ | ✔ | catechin [64] |
| 16 | 25.9 | 203, 257, 318, 369 | 423.19 | 421.16 | ✔ | - | - | mangiferin/isomangiferin [63] |
| 17 | 27.2 | 203 | 407.18 | 405.28 | - | ✔ | - | neolancerin/xanthohypericosider [60] |
| 18 | 29.2 | 279 | 865.23 | 863.23 | - | ✔ | - | unidentified procyanidin |
| 19 | 30.2 | 279 | 867.24 | 865.26 | ✔ | ✔ | ✔ | trimeric procyanidin type C [64] |
| 20 | 33.2 | 279 | - | 576.17 a | ✔ | ✔ | ✔ | tetrameric procyanidin B type [64] |
| 21 | 35.9 | 279 | - | 720.22 b | - | ✔ | ✔ | pentameric procyanidn B type [64] |
| 22 | 37.6 | 209, 255, 354 | 611.21 | 609.20 | - | - | ✔ | rutin [19,42] |
| 23 | 38.5 | 256, 353 | 465.18 | 463.14 | ✔ | - | ✔ | quercetin 3-O-glucoside [63] |
| 24 | 39.8 | 203, 255, 353 | 479.16 | 477.18 | ✔ | - | - | quercetin 3-O-glucuronide [63] |
| 25 | 41.4 | 286 | - | 361.17 | ✔ | - | - | unidentified compound |
| 26 | 42.4 | 203, 265, 344 | 595.20 | 593.19 | - | - | ✔ | kaempferol 3-O-rhamnoglucoside |
| 27 | 43.7 | 203, 255, 350 | 449.19 | 447.17 | - | ✔ | - | quercetin 3-O-rhamnoside [19,42] |
| 28 | 55.5 | 203, 254, 368 | 303.11 | 301.1 | ✔ | ✔ | ✔ | quercetin [19,42] |
| 29 | 60.0 | 217, 310, 353 | 611.17 | 609.20 | ✔ | ✔ | - | quercetin O-p-coumaroylhexoside [63] |
| 30 | 65.1 | 218, 368 | 287.10 | 285.08 | - | - | ✔ | kaempferol [19] |
| 31 | 67.7 | 219, 342 | 749.16 | 747.18 | - | ✔ | - | unidentified compound |
| 32 | 68.4 | 265, 340 | 749.15 | 747.17 | - | ✔ | ✔ | unidentified compound |
| 33 | 71.1 | 268, 335 | 539.16 | 537.30 | ✔ | ✔ | ✔ | amentoflavone [19] |
| 34 | 78.8 | 284, 369, 483, 565 | 367.34 | 365.30 | - | - | ✔ | unidentified compound |
| 35 | 79.2 | 287, 369, 483 | 381.32 | 379.36 | - | - | ✔ | unidentified compound |
| 36 | 86.7 | 221, 291 | 363.32 | 361.32 | - | - | ✔ | unidentified compound |
| 37 | 87.4 | 287, 369 | 349.31 | 347.33 | - | - | ✔ | unidentified compound |
| 38 | 91.9 | 221, 291 | 363.35 | 361.35 | - | - | ✔ | unidentified compound |
| 39 | 97.3 | 221, 290 | 333.31 | 331.27 | - | - | ✔ | unidentified compound |
| 40 | 100.0 | 221, 291 | 347.33 | 345.33 | - | - | ✔ | unidentified compound |
| 41 | 101.0 | 221, 287 | 333.34 | 331.34 | - | - | ✔ | unidentified compound |
| Chemical Novelty | Anticancer Potential (mL/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microextracts | N | Ctotal | Cknown | Ctotal/Cknown | A549 | HBL-100 | HeLa | SW1573 | T-47D | WiDr |
| CMW | 0 | 18 | 16 | 1.125 | 1074 | 1087 | 1427 | 1242 | 1347 | 846 |
| GMW | 2 | 19 | 15 | 1.266 | 3475 | 2689 | 7699 | 3559 | 5180 | 2966 |
| LMW | 0 | 27 | 16 | 1.688 | 2897 | 3503 | 6175 | 3977 | 3207 | 2637 |
| Plant Species | Common Name | Collection Site | Voucher |
|---|---|---|---|
| Hypericum canariense L. | granadillo | Breña Baja. Las Ledas (La Palma island) | 53,351 |
| Hypericum glandulosum Aiton | malfurada del monte | In between Mirador de Gallegos and Roque Faro (La Palma island) | 53,775 |
| Hypericum grandifolium Choisy | malfurada | Villa de Mazo. La Tablada (La Palma island) | 53,350 |
| Hypericum reflexum L.f | cruzadilla | Arico-Granadilla (Tenerife island) | 53,346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacret, R.; Puerta, A.; Granica, S.; González-Bakker, A.; Hevia, D.; Teng, Y.; Sánchez-Mateo, C.C.; Pérez de Paz, P.L.; Padrón, J.M. Bioactive Potential: A Pharmacognostic Definition through the Screening of Four Hypericum Species from the Canary Islands. Molecules 2022, 27, 6101. https://doi.org/10.3390/molecules27186101
Lacret R, Puerta A, Granica S, González-Bakker A, Hevia D, Teng Y, Sánchez-Mateo CC, Pérez de Paz PL, Padrón JM. Bioactive Potential: A Pharmacognostic Definition through the Screening of Four Hypericum Species from the Canary Islands. Molecules. 2022; 27(18):6101. https://doi.org/10.3390/molecules27186101
Chicago/Turabian StyleLacret, Rodney, Adrián Puerta, Sebastian Granica, Aday González-Bakker, Danela Hevia, Yiling Teng, Candelaria C. Sánchez-Mateo, Pedro Luis Pérez de Paz, and José M. Padrón. 2022. "Bioactive Potential: A Pharmacognostic Definition through the Screening of Four Hypericum Species from the Canary Islands" Molecules 27, no. 18: 6101. https://doi.org/10.3390/molecules27186101
APA StyleLacret, R., Puerta, A., Granica, S., González-Bakker, A., Hevia, D., Teng, Y., Sánchez-Mateo, C. C., Pérez de Paz, P. L., & Padrón, J. M. (2022). Bioactive Potential: A Pharmacognostic Definition through the Screening of Four Hypericum Species from the Canary Islands. Molecules, 27(18), 6101. https://doi.org/10.3390/molecules27186101