Investigating Localized Electrochemical of Ferrocenyl-Imidazolium in Ionic Liquid Using Scanning Electrochemical Microscopy Configuration
Abstract
:1. Introduction
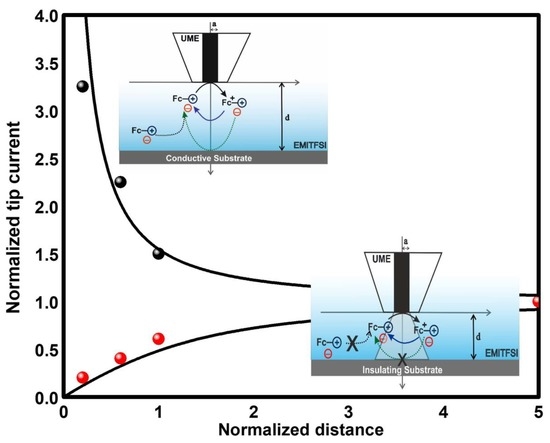
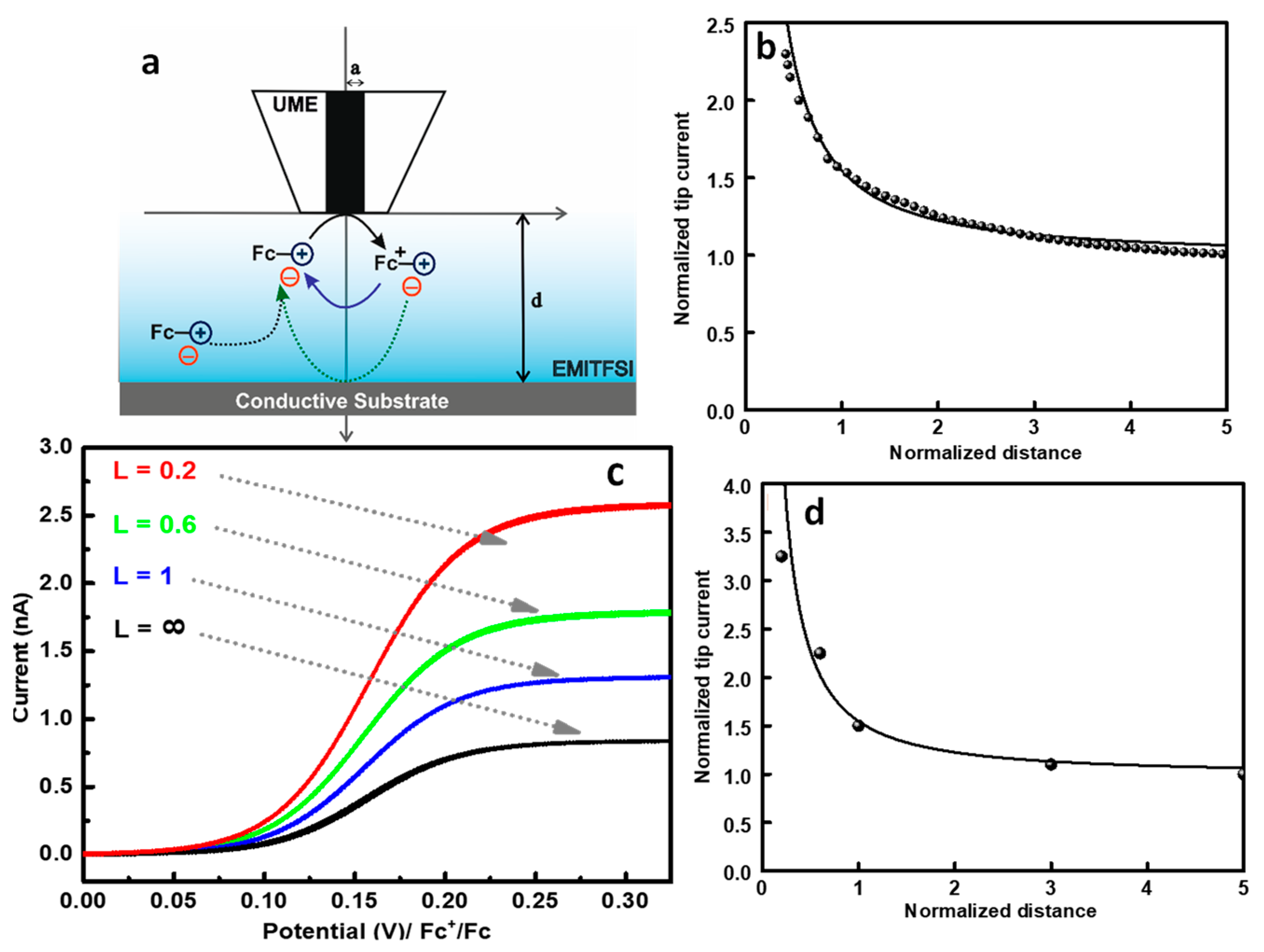
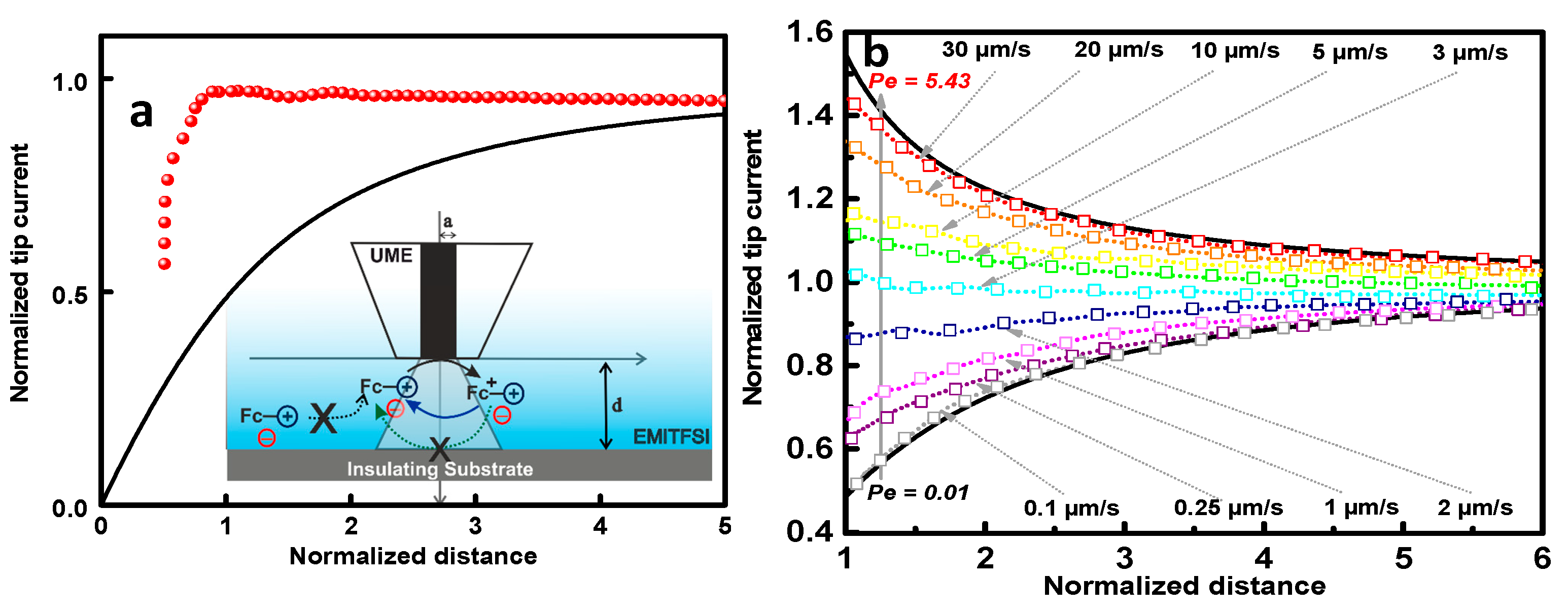
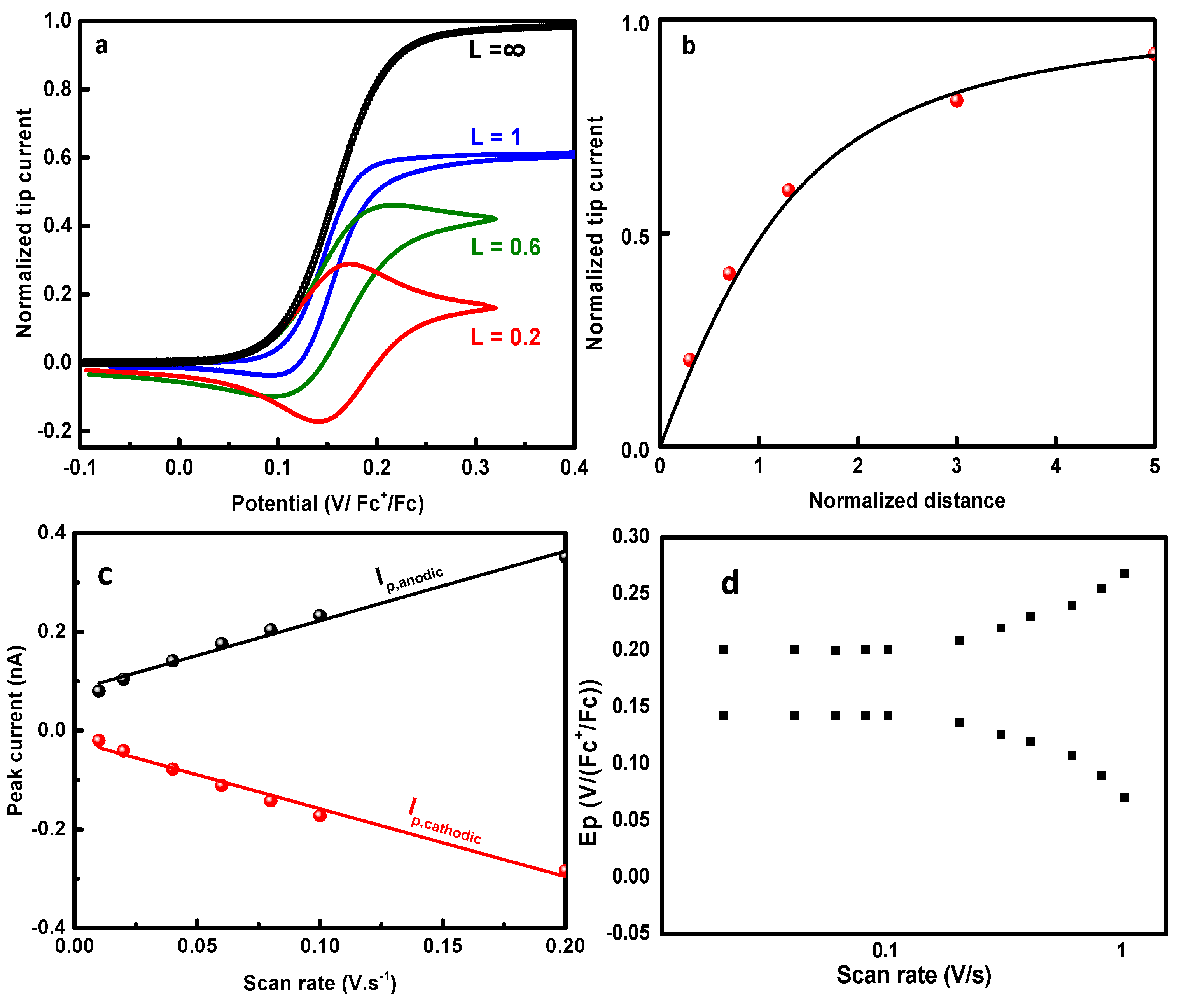
2. Results and Discussions
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yin, K.; Zhang, Z.; Li, X.; Yang, L.; Tachibana, K.; Hirano, S. Polymer electrolytes based on dicationic polymeric ionic liquids: Application in lithium metal batteries. J. Mater. Chem. A 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Sato, T.; Morinaga, T.; Marukane, S.; Narutomi, T.; Igarashi, T.; Kawano, Y.; Ohno, K.; Fukuda, T.; Tsujii, Y. Novel solid-state polymer electrolyte of colloidal crystal decorated with ionic-liquid polymer brush. Adv. Mater. 2011, 23, 4868–4872. [Google Scholar] [CrossRef]
- Kawano, R.; Katakabe, T.; Shimosawa, H.; Nazeeruddin, M.K.; Graetzel, M.; Matsui, H.; Kitamura, T.; Tanabe, N.; Watanabe, M. Solid-state dye-sensitized solar cells using polymerized ionic liquid electrolyte with platinum-free counter electrode. Phys. Chem. Chem. Phys. 2010, 12, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Feng, T.; Chu, F.; Zhang, S.; Yuan, N. Poly(ionic liquid)/ionic liquid/graphene oxide composite quasi solid-state electrolytes for dye sensitized solar cells. RSC Adv. 2015, 5, 57216–57222. [Google Scholar] [CrossRef]
- Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Electrodeposition of Nanocrystalline Metals and Alloys from Ionic Liquids. Angew. Chem.-Int. Ed. 2003, 42, 3428–3430. [Google Scholar] [CrossRef] [PubMed]
- Pham-Truong, T.N.; Randriamahazaka, H.; Ghilane, J. Polymer Brushes Ionic Liquid as a Catalyst for Oxygen Reduction and Oxygen Evolution Reactions. ACS Catal. 2018, 8, 869–875. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Zullo, V.; Górecki, M.; Guazzelli, L.; Mezzetta, A.; Pescitelli, G.; Iuliano, A. Exploiting isohexide scaffolds for the preparation of chiral ionic liquids tweezers. J. Mol. Liq. 2021, 322, 114528. [Google Scholar] [CrossRef]
- Piatti, E.; Guglielmero, L.; Tofani, G.; Mezzetta, A.; Guazzelli, L.; D’Andrea, F.; Roddaro, S.; Pomelli, C.S. Ionic liquids for electrochemical applications: Correlation between molecular structure and electrochemical stability window. J. Mol. Liq. 2022, 364, 120001. [Google Scholar] [CrossRef]
- Taylor, A.W.; Qiu, F.; Hu, J.; Licence, P.; Walsh, D.A. Heterogeneous Electron Transfer Kinetics at the Ionic Liquid/Metal Interface Studied Using Cyclic Voltammetry and Scanning Electrochemical Microscopy. J. Phys. Chem. B 2008, 112, 13292–13299. [Google Scholar] [CrossRef]
- Ghilane, J.; Lacroix, J.-C. Formation of a bifunctional redox system using electrochemical reduction of platinum in ferrocene based ionic liquid and its reactivity with aryldiazonium. J. Am. Chem. Soc. 2013, 135, 4722–4728. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.L.; Bond, A.M.; Hollenkamp, A.F.; Mahon, P.J.; Zhang, J. Electrode Reaction and Mass-Transport Mechanisms Associated with the Iodide/Triiodide Couple in the Ionic Liquid 1—Ethyl-3- methylimidazolium Bis(tri-fluoromethanesulfonyl)imide. J. Phys. Chem. C 2014, 118, 22439–22449. [Google Scholar] [CrossRef]
- Hapiot, P.; Lagrost, C. Electrochemical Reactivity in Room-Temperature Ionic Liquids. Chem. Rev. 2008, 108, 2238–2264. [Google Scholar] [CrossRef]
- Guglielmero, L.; Meskinfam Langroudi, M.; Al Khatib, M.; Aysla Costade Oliveira, M.; Mecheri, B.; De Leo, M.; Mezzetta, A.; Guazzelli, L.; Giglioli, R.; D’Epifanio, A.; et al. Electrochemical and spectroscopic study of vanadyl acetylacetonate–ionic liquids interactions. Electrochim. Acta 2021, 373, 137865. [Google Scholar] [CrossRef]
- Bentley, C.L.; Li, J.; Bond, A.M.; Zhang, J. Mass-Transport and Heterogeneous Electron-Transfer Kinetics Associated with the Ferrocene/Ferrocenium Process in Ionic Liquids. J. Phys. Chem. C 2019, 120, 16516–16525. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Klymenko, O.V.; Wadhawan, J.D.; Hardacre, C.; Seddon, K.R.; Compton, R.G. Voltammetry of Oxygen in the Room-Temperature Ionic Liquids 1-Ethyl-3-methylimidazolium Bis((trifluoromethyl)sulfonyl)imide and Hexyltriethylammonium Bis((trifluoromethyl)sulfonyl)imide: One-Electron Reduction To Form Superoxide. Steady-State and Transient Behavior in the Same Cyclic Voltammogram Resulting from Widely Different Diffusion Coefficients of Oxygen and Superoxide. J. Phys. Chem. A 2003, 107, 8872–8878. [Google Scholar]
- Ghilane, J.; Lagost, C.; Hapiot, P. Scanning electrochemical microscopy in nonusual solvents: Inequality of diffusion coefficients problem. Anal. Chem. 2007, 79, 7383–7391. [Google Scholar] [CrossRef]
- Zigah, D.; Ghilane, J.; Lagrost, C.; Hapiot, P. Variations of diffusion coefficients of redox active molecules in room temperature ionic liquids upon electron transfer. J. Phys. Chem. B 2008, 112, 14952–14958. [Google Scholar] [CrossRef]
- Walsh, D.A.; Lovelock, K.R.J.; Licence, P.; Walsh, D.A. Ultramicroelectrode voltammetry and scanning electrochemical microscopy in room-temperature ionic liquid electrolytes. Chem. Soc. Rev. 2010, 39, 4185–4194. [Google Scholar] [CrossRef]
- Nkuku, C.A.; Lesuer, R.J. Electrochemistry in Deep Eutectic Solvents. J. Phys. Chem. B 2007, 111, 13271–13277. [Google Scholar] [CrossRef]
- Bertoncello, P. Advances on scanning electrochemical microscopy (SECM) for energy. Energy Environ. Sci. 2010, 3, 1620–1633. [Google Scholar] [CrossRef]
- Amemiya, S.; Guo, J.; Xiong, H.; Gross, D.A. Biological applications of scanning electrochemical microscopy: Chemical imaging of single living cells and beyond. Anal. Bioanal. Chem. 2006, 386, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Bard, A.J. Scanning electrochemical microscopy. Theory of the feedback mode. Anal. Chem. 1989, 61, 1221–1227. [Google Scholar] [CrossRef]
- Barhdadi, R.; Laurent, M.; Troupel, M.; Marne, P. Determination of Viscosity, Ionic Conductivity, and Diffusion Coefficients in Some Binary Systems: Ionic Liquids + Molecular Solvents. J. Chem. Eng. Data 2006, 51, 680–685. [Google Scholar]
- Shin, S.J.; Kim, J.Y.; An, S.; Kim, M.; Seo, M.; Go, S.Y.; Chung, H.; Lee, M.; Kim, M.-G.; Lee, H.G.; et al. Revisiting thin-layer electrochemistry in a chip-type cell for the study of electro-organic reactions. Anal. Chem. 2022, 94, 1248–1255. [Google Scholar] [CrossRef]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Pham-Truong, T.N.; Randriamahazaka, H.; Ghilane, J. Electrochemistry of bi-redox ionic liquid from solution to bi-functional carbon surface. Electrochim. Acta 2020, 354, 136689. [Google Scholar] [CrossRef]
- Pham-Truong, T.N.; Lafolet, F.; Ghilane, J.; Randriamahazaka, H. Surface functionalization with redox active molecule-based imidazolium via click chemistry. Electrochem. Commun. 2016, 70, 13–17. [Google Scholar] [CrossRef]
- Hanna, K.M.; Sanborn, C.D.; Ardo, S.; Yang, J.Y. Interfacial Electron Transfer of Ferrocene Immobilized onto Indium Tin Oxide through Covalent and Noncovalent Interactions. ACS Appl. Mater. Interfaces 2018, 10, 13211–13217. [Google Scholar] [CrossRef]
- Schrage, B.R.; Zhang, B.; Petrochko, S.C.; Zhao, Z.; Frkonja-Kuczin, A.; Boika, A.; Ziegler, C.J. Highly soluble imidazolium ferrocene bis(sulfonate) salts for redox flow battery applications. Inorg. Chem. 2021, 60, 10764–10771. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham-Truong, T.-N.; Ghilane, J. Investigating Localized Electrochemical of Ferrocenyl-Imidazolium in Ionic Liquid Using Scanning Electrochemical Microscopy Configuration. Molecules 2022, 27, 6004. https://doi.org/10.3390/molecules27186004
Pham-Truong T-N, Ghilane J. Investigating Localized Electrochemical of Ferrocenyl-Imidazolium in Ionic Liquid Using Scanning Electrochemical Microscopy Configuration. Molecules. 2022; 27(18):6004. https://doi.org/10.3390/molecules27186004
Chicago/Turabian StylePham-Truong, Thuan-Nguyen, and Jalal Ghilane. 2022. "Investigating Localized Electrochemical of Ferrocenyl-Imidazolium in Ionic Liquid Using Scanning Electrochemical Microscopy Configuration" Molecules 27, no. 18: 6004. https://doi.org/10.3390/molecules27186004
APA StylePham-Truong, T.-N., & Ghilane, J. (2022). Investigating Localized Electrochemical of Ferrocenyl-Imidazolium in Ionic Liquid Using Scanning Electrochemical Microscopy Configuration. Molecules, 27(18), 6004. https://doi.org/10.3390/molecules27186004