Photolyase Production and Current Applications: A Review
Abstract
:1. Introduction
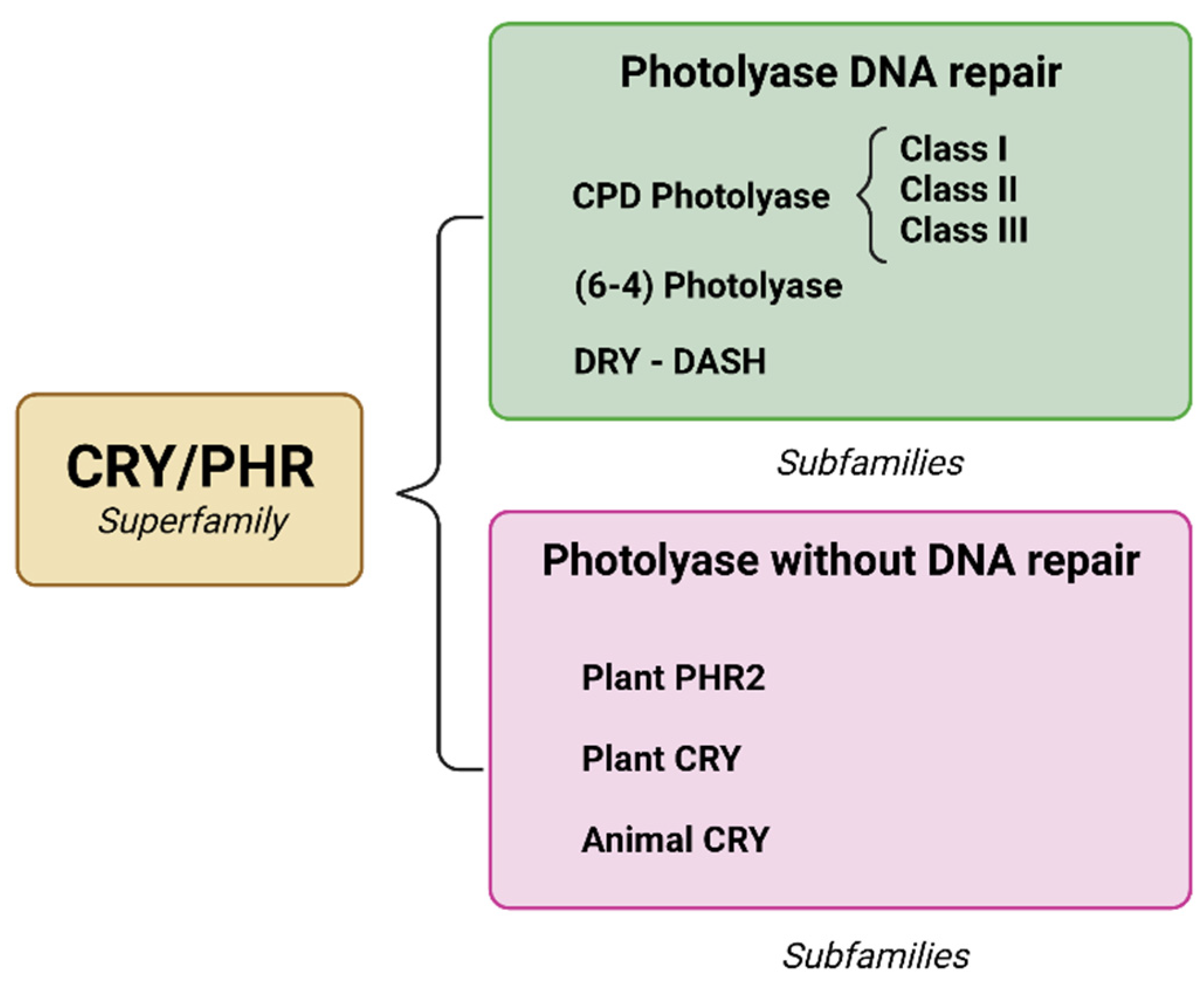
2. Photolyase
2.1. Type of Enzyme
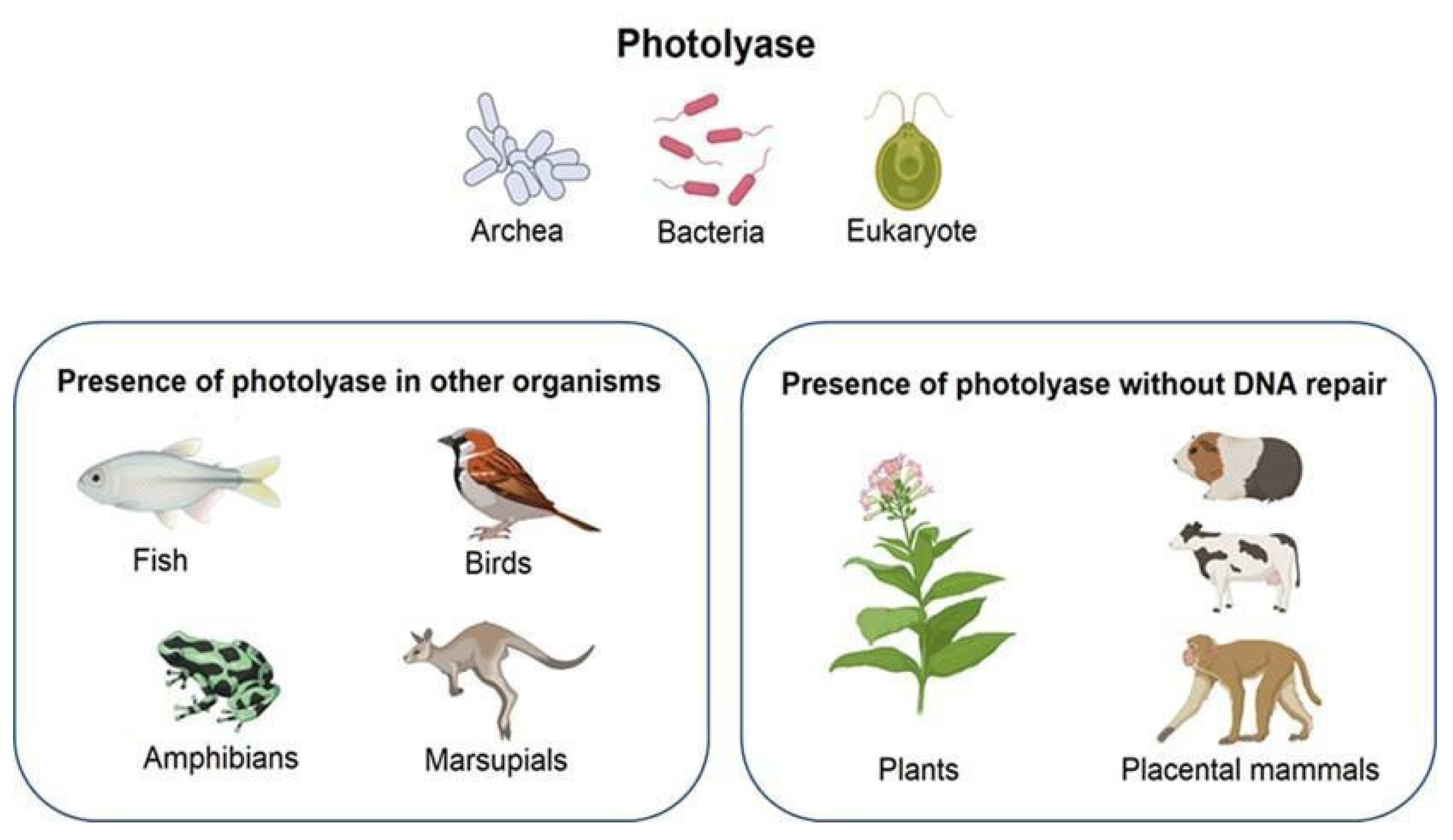
2.2. Photolyase and Microorganisms
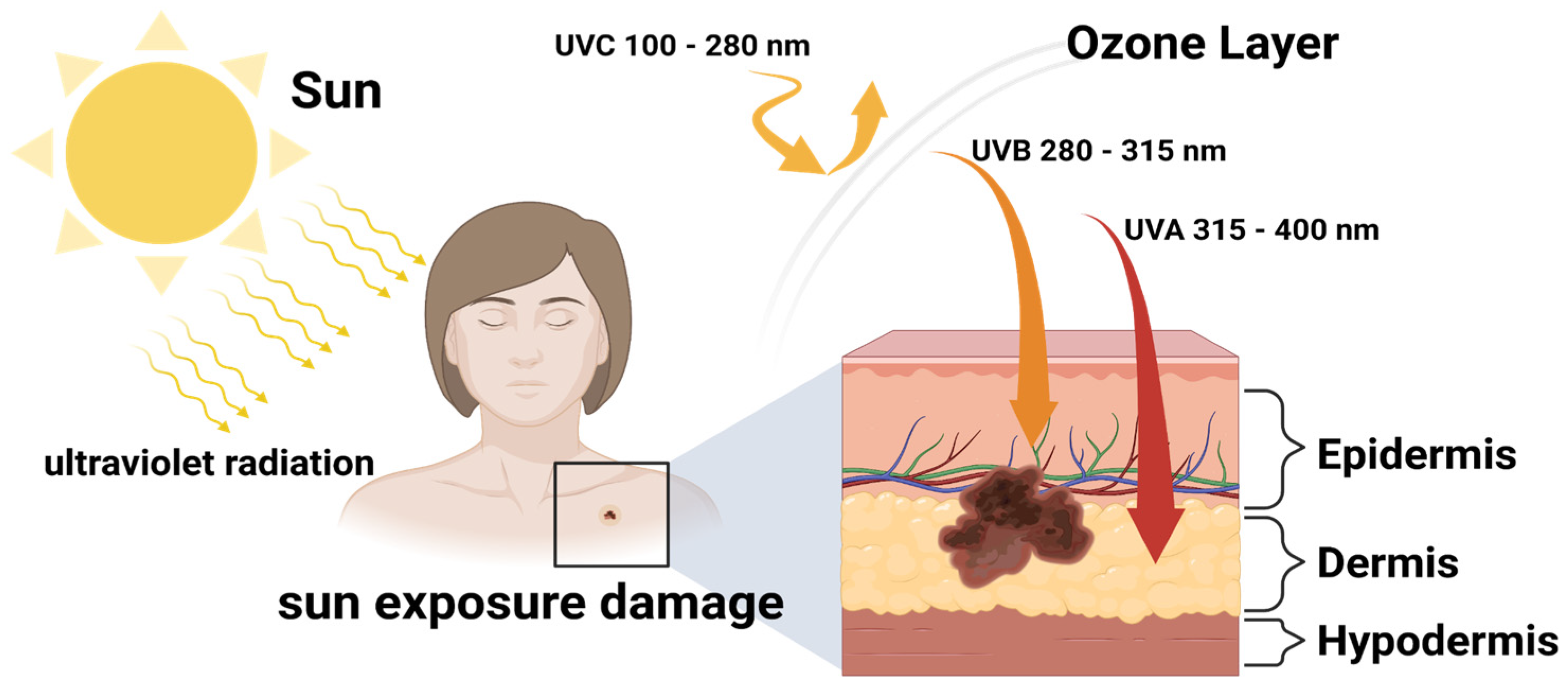
2.3. DNA Damage by UV Irradiation
2.4. Photolyase Mechanism of Action
2.5. Immobilization and Biocarriers
3. Photolyase Applications
3.1. Current Photolyase Production
3.2. Sunscreen with Photolyase as an Ingredient
3.3. Photoaging
| Study | Skin Condition | Therapy Evaluation | Duration | Assessment | Results | Reference |
|---|---|---|---|---|---|---|
| Clinical series | AK | Eryfotona | 3 months | Clinical photography | Great improvement in AK lesions count. | [97] |
| Retrospective case study | Xeroderma Pigmentosum | Eryfotona | 12 months | Histological records | Reduction of 65% for AK, 56% for BCC, and 100% for SCC lesions. | [98] |
| Longitudinal, observational clinical | AK | Eryfotona | 3 months | Clinical, dermoscopy, and confocal microscopy analysis | Grade I AK clinical and dermoscopy improvement. Reduction in desquamation. Improvement in the epidermal architectural pattern. Grade II AK, no improvements. | [99] |
| Pilot study | AK | Eryfotona | 1 month | Clinical, dermoscopy, and reflectance confocal microscopy assessments | Erythema and scaling improvement. | [100] |
| Clinical | AK | Eryfotona | 9 months | Telethermografy | Hyperthermic halos area reduced from 3.46 to 0.64 cm2. | [101] |
| Randomized, assessor-blinded parallel-group | AK | Eryfotona | 9 months | Lesion count | Significant reduction in new AK lesions. No additional photodynamic therapy required. | [102] |
| Prospective observational study | AK | Eryfotona Cryotherapy | 6 months | Epidemiologic, clinical, and therapeutic variables | No adverse cutaneous effects and 84% improvement in AK lesion count. | [103] |
| Prospective, single-arm, case-series | AK | Eryfotona | 3 months | Clinical photography | Partial response in 100% of patients. 50% reduction in lesion count. | [104] |
| Randomized, double-blind parallel-group Pilot study | AK | Eryfotona | 6 months | Clinical, dermoscopy, and reflectance confocal Microscopy evaluation | Significant reduction in mean AK lesion number up to 31%. | [105] |
| -- | Photoaging | Tinted facial sunscreen with high sun protection, peptide complex, and encapsulated photolyase | 1 month | Periocular wrinkles, skin firmness and elasticity, UV spots, and patient subjective questionnaire | Wrinkle count −6.9%. Wrinkle volume −10.4%. UV spots area −9%. Firmness +8.2. Elasticity +11.3%. | [94] |
| -- | UV exposure | Sunscreen amended with photolyase | 4 days | Skin biopsies after experimental irradiations | 93% prevention of CPD formation. 82% apoptosis prevention. | [95] |
| Head-to-head comparison studies | UV exposure | Triple-protection factor broad-spectrum sunscreen (TPF50) | -- | Skin biopsies after experimental irradiations | Reduction in CPD and protein carboxylation. | [96] |
| Randomized, double-blind, factorial clinical trial | AK | Sunscreen amended with photolyase | 2 months | Clinical and demographic variables | No significant differences with common sunscreen. | [106] |
| -- | AK | Eryfotona | 1 month | Histopathological and molecular assessment | Improvement in the field of cancerization. Restoration of normal phenotype through CPI-17 up-regulation. | [107] |
| Randomized, clinical study | AK | Sunscreen amended with photolyase | 6 months | Fluorescence diagnostics using methylaminolevulinate Skin biopsies | Superior to sunscreen in reduction in field cancerization and UVR-associated molecular signatures. | [108] |
3.4. Actinic Keratosis
3.5. Skin Cancer
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelner, A. Photoreactivation of ultraviolet-irradiated Escherichia coli, with special reference to the dose-reduction principle and to ultraviolet-induced mutation. J. Bacteriol. 1949, 58, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.P.; Sancar, A. A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity. Proc. Natl. Acad. Sci. USA 2006, 103, 17696–17700. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Lledó, J.I.; Lynch, M. Evolution of mutation rates: Phylogenomic analysis of the photolyase/cryptochrome family. Mol. Biol. Evol. 2009, 26, 1143–1153. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Skin Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/skin-cancer-statistics/ (accessed on 16 August 2022).
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients. JAMA 2019, 321, 2082. [Google Scholar] [CrossRef]
- Matta, M.K.; Florian, J.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Yang, Y.; Oh, L.; Bashaw, E.; Zineh, I.; et al. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients. JAMA 2020, 323, 256. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, I.H.; Ozturk, N.; Gul, S. DNA repair by photolyases. Adv. Protein Chem. Struct. Biol. 2019, 115, 1–19. [Google Scholar]
- Menck, C.F.M. Shining a light on photolyases. Nat. Genet. 2002, 32, 338–339. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.O.; Van Der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef]
- Malhotra, K.; Kim, S.T.; Batschauer, A.; Dawut, L.; Sancar, A. Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity. Biochemistry 1995, 34, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Kelner, A. Effect of Visible Light on the Recovery of Streptomyces Griseus Conidia from Ultra-violet Irradiation Injury. Proc. Natl. Acad. Sci. USA 1949, 35, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulbecco, R. Reactivation of ultra-violet-inactivated bacteriophage by visible light. Nature 1949, 163, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Mathis, P.; Eker, A.P.M.; Brettel, K. Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans. Proc. Natl. Acad. Sci. USA 1999, 96, 5423–5427. [Google Scholar] [CrossRef]
- Karentz, D.; Cleaver, J.E.; Mitchell, D.L. Cell survival characteristics and molecular responses of Antarctic phytoplankton to ultraviolet-B radiation. J. Phycol. 1991, 27, 326–341. [Google Scholar] [CrossRef]
- Li, C.; Ma, L.; Mou, S.; Wang, Y.; Zheng, Z.; Liu, F.; Qi, X.; An, M.; Chen, H.; Miao, J. Cyclobutane pyrimidine dimers photolyase from extremophilic microalga: Remarkable UVB resistance and efficient DNA damage repair. Mutat. Res. 2015, 773, 37–42. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Elsevier: London, UK, 1965; pp. 97–166. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Sancar, A. Structure and Function of DNA Photolyase and Cryptochrome Blue-Light Photoreceptors. Chem. Rev. 2003, 103, 2203–2238. [Google Scholar] [CrossRef]
- Sancar, G.B. DNA photolyases: Physical properties, action mechanism, and roles in dark repair. Mutat. Res. 1990, 236, 147–160. [Google Scholar] [CrossRef]
- Müller, M.; Carell, T. Structural biology of DNA photolyases and cryptochromes. Curr. Opin. Struct. Biol. 2009, 19, 277–285. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zhong, D. Photolyase: Dynamics and Mechanisms of Repair of Sun-Induced DNA Damage. Photochem. Photobiol. 2017, 93, 78–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Q.; Dvornyk, V. Evolutionary History of the Photolyase/Cryptochrome Superfamily in Eukaryotes. PLoS ONE 2015, 10, e0135940. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Song, S.H.; Özgür, S.; Selby, C.P.; Morrison, L.; Partch, C.; Zhong, D.; Sancar, A. Structure and function of animal cryptochromes. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Essen, L.O.; Klar, T. Light-driven DNA repair by photolyases. Cell. Mol. Life Sci. 2006, 63, 1266–1277. [Google Scholar] [CrossRef]
- Graf, D.; Wesslowski, J.; Ma, H.; Scheerer, P.; Krauß, N.; Oberpichler, I.; Zhang, F.; Lamparter, T. Key Amino Acids in the Bacterial (6-4) Photolyase PhrB from Agrobacterium fabrum. PLoS ONE 2015, 10, e0140955. [Google Scholar] [CrossRef]
- He, Y.; Qu, C.; Zhang, L.; Miao, J. DNA photolyase from Antarctic marine bacterium Rhodococcus sp. NJ-530 can repair DNA damage caused by ultraviolet. 3 Biotech 2021, 11, 102. [Google Scholar] [CrossRef]
- An, M.; Zheng, Z.; Qu, C.; Wang, X.; Chen, H.; Shi, C.; Miao, J. The first (6-4) photolyase with DNA damage repair activity from the Antarctic microalga Chlamydomonas sp. ICE-L. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2018, 809, 13–19. [Google Scholar] [CrossRef]
- Marizcurrena, J.J.; Martínez-López, W.; Ma, H.; Lamparter, T.; Castro-Sowinski, S. A highly efficient and cost-effective recombinant production of a bacterial photolyase from the Antarctic isolate Hymenobacter sp. UV11. Extremophiles 2019, 23, 49–57. [Google Scholar] [CrossRef]
- Kiontke, S.; Geisselbrecht, Y.; Pokorny, R.; Carell, T.; Batschauer, A.; Essen, L.O. Crystal structures of an archaeal class II DNA photolyase and its complex with UV-damaged duplex DNA. EMBO J. 2011, 30, 4437–4449. [Google Scholar] [CrossRef]
- An, M.; Qu, C.; Miao, J.; Sha, Z. A Class II CPD Photolyase and a 6-4 Photolyase with Photorepair Activity from the Antarctic Moss Pohlia nutans M211. Photochem. Photobiol. 2021, 97, 1527–1533. [Google Scholar] [CrossRef]
- An, M.; Qu, C.; Miao, J.; Sha, Z. Two class II CPD photolyases, PiPhr1 and PiPhr2, with CPD repair activity from the Antarctic diatom Phaeodactylum tricornutum ICE-H. 3 Biotech 2021, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Kao, Y.T.; Selby, C.P.; Kavakli, I.H.; Partch, C.L.; Zhong, D.; Sancar, A. Purification and Characterization of a Type III Photolyase from Caulobacter crescentus. Biochemistry 2008, 47, 10255. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Niemann, N.; Kock, D.; Dadaeva, T.; Gutiérrez, G.; Engelsdorf, T.; Kiontke, S.; Corrochano, L.M.; Batschauer, A.; Garre, V. The DASH-type Cryptochrome from the Fungus Mucor circinelloides Is a Canonical CPD-Photolyase. Curr. Biol. 2020, 30, 4483–4490.e4. [Google Scholar] [CrossRef] [PubMed]
- Tagua, V.G.; Pausch, M.; Eckel, M.; Gutiérrez, G.; Miralles-Durán, A.; Sanz, C.; Eslava, A.P.; Pokorny, R.; Corrochano, L.M.; Batschauer, A. Fungal cryptochrome with DNA repair activity reveals an early stage in cryptochrome evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 15130–15135. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; Heßling, B.; Illarionova, V.; Bacher, A.; Weber, S.; Richter, G.; Gerwert, K. Light-induced reactions of Escherichia coli DNA photolyase monitored by Fourier transform infrared spectroscopy. FEBS J. 2005, 272, 1855–1866. [Google Scholar] [CrossRef]
- Brash, D.E.; Franklin, W.A.; Sancar, G.B.; Haseltine, W.A. Escherichia coli DNA photolyase reverses cyclobutane pyrimidine dimers but not pyrimidine-pyrimidone (6-4) photoproducts. J. Biol. Chem. 1985, 260, 11438–11441. [Google Scholar] [CrossRef]
- Sancar, G.B.; Sancar, A. Purification and characterization of DNA photolyases. Methods Enzymol. 2006, 408, 121–156. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Marrot, L.; Meunier, J.R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zhong, D. Photolyase: Dynamics and electron-transfer mechanisms of DNA repair. Arch. Biochem. Biophys. 2017, 632, 158–174. [Google Scholar] [CrossRef]
- Ahmad, A.; Nay, S.L.; O’Connor, T.R. Direct Reversal Repair in Mammalian Cells. In Advances in DNA Repair; Chen, C., Ed.; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Petruseva, I.O.; Evdokimov, A.N.; Lavrik, O.I. Molecular mechanism of global genome nucleotide excision repair. Acta Nat. 2014, 6, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Fukui, K. DNA Mismatch Repair in Eukaryotes and Bacteria. J. Nucleic Acids 2010, 2010, 260512. [Google Scholar] [CrossRef] [PubMed]
- Vítor, A.C.; Huertas, P.; Legube, G.; de Almeida, S.F. Studying DNA Double-Strand Break Repair: An Ever-Growing Toolbox. Front. Mol. Biosci. 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.K. DNA Damage Repair: Historical Perspectives, Mechanistic Pathways and Clinical Translation for Targeted Cancer Therapy; Springer: Greer, SA, USA, 2021; Volume 6, ISBN 4139202100648. [Google Scholar]
- Pathak, J.; Rajneesh; Singh, P.R.; Häder, D.P.; Sinha, R.P. UV-induced DNA damage and repair: A cyanobacterial perspective. Plant Gene 2019, 19, 100194. [Google Scholar] [CrossRef]
- Ramírez, N.; Serey, M.; Illanes, A.; Piumetti, M.; Ottone, C. Immobilization strategies of photolyases: Challenges and perspectives for DNA repairing application. J. Photochem. Photobiol. B Biol. 2021, 215, 112113. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, X.; Pan, W.; Wang, X.; Wu, W. Photoactivation of the cryptochrome/photolyase superfamily. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 84–102. [Google Scholar] [CrossRef]
- Brettel, K.; Müller, P.; Yamamoto, J. Kinetics of Electron Returns in Successive Two-Photon DNA Repair by (6-4) Photolyase. ACS Catal. 2022, 12, 3041–3045. [Google Scholar] [CrossRef]
- Terai, Y.; Sato, R.; Matsumura, R.; Iwai, S.; Yamamoto, J. Enhanced DNA repair by DNA photolyase bearing an artificial light-harvesting chromophore. Nucleic Acids Res. 2020, 48, 10076–10086. [Google Scholar] [CrossRef]
- Kavakli, I.H.; Ozturk, N.; Gul, S. DNA repair by photolyases. In Advances in Protein Chemistry and Structural Biology; Elsevier Inc.: London, UK, 2019; Volume 115, pp. 1–19. [Google Scholar]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444. [Google Scholar] [CrossRef]
- Pavlou, P.; Siamidi, A.; Varvaresou, A.; Vlachou, M. Skin Care Formulations and Lipid Carriers as Skin Moisturizing Agents. Cosmetics 2021, 8, 89. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Eskens, O.; Amin, S. Challenges and effective routes for formulating and delivery of epidermal growth factors in skin care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Montenegro, L. Nanocarriers for skin delivery of cosmetic antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 73–92. [Google Scholar]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Travis, B.; Darren, S.; Zimei, W. Pharmaceutical Strategies for the Topical Dermal Delivery of Peptides/Proteins for Cosmetic and Therapeutic Applications. Austin J. Pharmacol. Ther. 2014, 2, 1–10. [Google Scholar]
- Nair, R.; Maseeh, A. Vitamin D: The sunshine vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Hoel, D.G.; Berwick, M.; de Gruijl, F.R.; Holick, M.F. The risks and benefits of sun exposure 2016. Dermatoendocrinology 2016, 8, e1248325. [Google Scholar] [CrossRef]
- Mead, M.N. Benefits of Sunlight: A Bright Spot for Human Health. Environ. Health Perspect. 2008, 116, A160–A167. [Google Scholar] [CrossRef]
- Mmbando, G.S.; Teranishi, M.; Hidema, J. Transgenic rice Oryza glaberrima with higher CPD photolyase activity alleviates UVB-caused growth inhibition. GM Crop. Food 2021, 12, 435–448. [Google Scholar] [CrossRef]
- Tong, S.; Feng, M. Molecular basis and regulatory mechanisms underlying fungal insecticides’ resistance to solar ultraviolet irradiation. Pest Manag. Sci. 2022, 78, 30–42. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef]
- Lyons, A.B.; Trullas, C.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Photoprotection beyond ultraviolet radiation: A review of tinted sunscreens. J. Am. Acad. Dermatol. 2021, 84, 1393–1397. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef]
- Singer, S.; Karrer, S.; Berneburg, M. Modern sun protection. Curr. Opin. Pharmacol. 2019, 46, 24–28. [Google Scholar] [CrossRef]
- Seité, S.; Fourtanier, A.M.A. The benefit of daily photoprotection. J. Am. Acad. Dermatol. 2008, 58, S160–S166. [Google Scholar] [CrossRef]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.L.; Green, A.C.; van der Pols, J.C.; Bernard, B.A.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–931. [Google Scholar] [CrossRef]
- Neale, R.E.; Khan, S.R.; Lucas, R.M.; Waterhouse, M.; Whiteman, D.C.; Olsen, C.M. The effect of sunscreen on vitamin D: A review. Br. J. Dermatol. 2019, 181, 907–915. [Google Scholar] [CrossRef]
- Marks, R.; Foley, P.A.; Jolley, D.; Knight, K.R.; Harrison, J.; Thompson, S.C. The Effect of Regular Sunscreen Use on Vitamin D Levels in an Australian Population: Results of a Randomized Controlled Trial. Arch. Dermatol. 1995, 131, 415–421. [Google Scholar] [CrossRef]
- Siller, A.; Blaszak, S.C.; Lazar, M.; Olasz Harken, E. Update About the Effects of the Sunscreen Ingredients Oxybenzone and Octinoxate on Humans and the Environment. Plast. Surg. Nurs. 2018, 38, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef]
- Rigel, D.S.; Lim, H.W.; Draelos, Z.D.; Weber, T.M.; Taylor, S.C. Photoprotection for all: Current gaps and opportunities. J. Am. Acad. Dermatol. 2022, 86, S18–S26. [Google Scholar] [CrossRef] [PubMed]
- Mezei, M.; Gulasekharam, V.; Straubinger, R.; Hong, K.; Friend, D. Purification and administration of dna repair enzymes. Int. J. Pharm. 1988, 173, 3415–3422. [Google Scholar]
- Puig, S.; Granger, C.; Garre, A.; Trullàs, C.; Sanmartin, O.; Argenziano, G. Review of Clinical Evidence over 10 Years on Prevention and Treatment of a Film-Forming Medical Device Containing Photolyase in the Management of Field Cancerization in Actinic Keratosis. Dermatol. Ther. 2019, 9, 259–270. [Google Scholar] [CrossRef]
- Garinis, G.A.; Jans, J.; van der Horst, G.T.J. Photolyases: Capturing the light to battle skin cancer. Futur. Oncol. 2006, 2, 191–199. [Google Scholar] [CrossRef]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Müllegger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact on photoageing—A systematic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Yarosh, D.B.; Rosenthal, A.; Moy, R. Six critical questions for DNA repair enzymes in skincare products: A review in dialog. Clin. Cosmet. Investig. Dermatol. 2019, 12, 617–624. [Google Scholar] [CrossRef]
- Addor, F.A.S. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef]
- Huang, A.H.; Chien, A.L. Photoaging: A Review of Current Literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- The Skin Cancer Foundation. Photoaging: What You Need to Know about the Other Kind of Aging. Available online: https://www.skincancer.org/blog/photoaging-what-you-need-to-know/ (accessed on 25 January 2022).
- Kligman, L.H. Photoaging. Manifestations, prevention, and treatment. Dermatol. Clin. 1986, 4, 517–528. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Photoaging. J. Invest. Dermatol. 2013, 133, E2–E6. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “Active” photoprotection: Sunscreens with DNA repair enzymes. Ital. J. Dermatol. Venereol. 2017, 152, 302–307. [Google Scholar] [CrossRef]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef]
- Han, A.; Chien, A.L.; Kang, S. Photoaging. Dermatol. Clin. 2014, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily photoprotection to prevent photoaging. Photodermatol. Photoimmunol. Photomed. 2021, 37, 482–489. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Granger, C.; Trullàs, C.; Bauza, G.; Garre, A. 17895 Anti-photoaging effect of a novel tinted facial sunscreen with high sun protection, peptide complex, and encapsulated photolyase after 1 month of use. J. Am. Acad. Dermatol. 2020, 83, AB202. [Google Scholar] [CrossRef]
- Emanuele, E. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: Clues to skin cancer prevention. Mol. Med. Rep. 2011, 5, 570–574. [Google Scholar] [CrossRef]
- Emanuele, E.; Spencer, J.M.; Braun, M. An experimental double-blind irradiation study of a novel topical product (TPF 50) compared to other topical products with DNA repair enzymes, antioxidants, and growth factors with sunscreens: Implications for preventing skin aging and cancer. J. Drugs Dermatol. 2014, 13, 309–314. [Google Scholar]
- Puviani, M.; Barcella, A.; Milani, M. Efficacy of a photolyase-based device in the treatment of cancerization field in patients with actinic keratosis and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. 2013, 148, 693–698. [Google Scholar] [PubMed]
- Giustini, S.; Miraglia, E.; Berardesca, E.; Milani, M.; Calvieri, S. Preventive long-term effects of a topical film-forming medical device with ultra-high uv protection filters and dna repair enzyme in xeroderma pigmentosum: A retrospective study of eight cases. Case Rep. Dermatol. 2014, 6, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Rstom, S.A.; Abdalla, B.M.Z.; Rezze, G.G.; Paschoal, F.M. Evaluation of the effects of a cream containing liposomeencapsulated photolyase and SPF 100 sunscreen on facial actinic keratosis: Clinical, dermoscopic, and confocal microscopy based analysis. Surg. Cosmet. Dermatol. 2014, 6, 226–231. [Google Scholar]
- Malvehy, J.S.P. Field Cancerisation Improvement with Topical Application of a Film-Forming Medical Device Containing Photolyase and UV Filters in Patients with Actinic Keratosis, a Pilot Study. J. Clin. Exp. Dermatol. Res. 2014, 5, 220. [Google Scholar] [CrossRef]
- Laino, L.; Elia, F.; Desiderio, F.; Scarabello, A.; Sperduti, I.; Cota, C.; DiCarlo, A. The efficacy of a photolyase-based device on the cancerization field: A clinical and thermographic study. J. Exp. Clin. Cancer Res. 2015, 34, 84. [Google Scholar] [CrossRef]
- Eibenschutz, L.; Silipo, V.; De Simone, P.; Buccini, P.L.; Ferrari, A.; Carbone, A.; Catricalà, C. A 9-month, randomized, assessor-blinded, parallel-group study to evaluate clinical effects of film-forming medical devices containing photolyase and sun filters in the treatment of field cancerization compared with sunscreen in patients after successful p. Br. J. Dermatol. 2016, 175, 1391–1393. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Jiménez, N.; Grillo, E.; Ballester, A. Estudio observacional sobre la efectividad y tolerabilidad de un producto tópico con fotoliasa y filtros solares en pacientes con queratosis actínicas tratados con crioterapia en condiciones de uso habitual. Piel 2016, 31, 532–536. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Molgó, M. The use of a sunscreen containing DNA-photolyase in the treatment of patients with field cancerization and multiple actinic keratoses: A case-series. Dermatol. Online J. 2016, 23, 4–7. [Google Scholar] [CrossRef]
- Moscarella, E.; Argenziano, G.; Longo, C.; Aladren, S. Management of cancerization field with a medical device containing photolyase: A randomized, double-blind, parallel-group pilot study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e401–e403. [Google Scholar] [CrossRef]
- Alvares, B.A.; Miola, A.C.; Schimitt, J.V.; Miot, H.A.; Abbade, L.P.F. Efficacy of sunscreen with photolyase or regular sunscreen associated with topical antioxidants in treating advanced photodamage and cutaneous field cancerization: A randomized clinical trial. An. Bras. Dermatol. 2022, 97, 157–165. [Google Scholar] [CrossRef]
- Puig-Butillé, J.A.; Malvehy, J.; Potrony, M.; Trullas, C.; Garcia-García, F.; Dopazo, J.; Puig, S. Role of CPI-17 in restoring skin homoeostasis in cutaneous field of cancerization: Effects of topical application of a film-forming medical device containing photolyase and UV filters. Exp. Dermatol. 2013, 22, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Carducci, M.; Pavone, P.S.; De Marco, G.; Lovati, S.; Altabas, V.; Altabas, K.; Emanuele, E.; De Marco, G.; Lovati, S.; Altabas, V.; et al. Comparative Effects of Sunscreens Alone vs. Sunscreens Plus DNA Repair Enzymes in Patients With Actinic Keratosis: Clinical and Molecular Findings from a 6-Month, Randomized, Clinical Study. J. Drugs Dermatol. 2015, 14, 986–990. [Google Scholar] [PubMed]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of care for the management of actinic keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Salasche, S.J. Epidemiology of actinic keratoses and squamous cell carcinoma. J. Am. Acad. Dermatol. 2000, 42, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.R.; Weinstock, M.A. Keratinocyte Carcinoma. CA. Cancer J. Clin. 2003, 53, 292–302. [Google Scholar] [CrossRef]
- Krutmann, J.; Berking, C.; Berneburg, M.; Diepgen, T.L.; Dirschka, T.; Szeimies, M. New Strategies in the Prevention of Actinic Keratosis: A Critical Review. Skin Pharmacol. Physiol. 2015, 28, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, A.; Stoddard, M.; Chipps, L.; Herrmann, J. Skin cancer prevention: A review of current topical options complementary to sunscreens. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1261–1267. [Google Scholar] [CrossRef]
- Parker, E.R. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Women’s Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef]
- American Cancer Society; The American Cancer Society. Skin Cancer|Skin Cancer Facts|Common Skin Cancer Types. Available online: https://www.cancer.org/cancer/skin-cancer.html (accessed on 27 January 2022).
- Chabaane, M.; Ayadi, K.; Rkhami, M.; Drissi, C.; Houimli, S.; Bahri, K.; Zammel, I.; Badri, M. Management of a recurrence of a squamous cell carcinoma of the scalp with extension to the brain: A case report and literature review. Surg. Neurol. Int. 2020, 11, 347. [Google Scholar] [CrossRef]
- Cobanoglu, H.B.; Constantinides, M.; Ural, A. Nonmelanoma Skin Cancer of the Head and Neck: Molecular Mechanisms. Facial Plast. Surg. Clin. N. Am. 2012, 20, 437–443. [Google Scholar] [CrossRef]
- The American Cancer Society. What Is Melanoma Skin Cancer? Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/what-is-melanoma.html (accessed on 27 January 2022).
- Agamohammadi, D.; Reihan, M.D.; Mirzaei, F.; Payandeh, Z.; Farzin, H.; Marahem, M. New Therapies for Melanoma Cancer Strategies. Crescent J. Med. Biol. Sci. 2021, 8, 3–9. [Google Scholar]


| Microorganisms | Genus | Type Photolyase | Extraction and Purification | References |
|---|---|---|---|---|
| Agrobacterium fabrum | Prokaryote | (6-4) Photolyase | Heated and cleared by centrifugation HPLC column from Macherey and Nagel | [30] |
| Rhodococcus sp. NJ-530 | Marine bacterium | CPD Class I | Disrupted with ultrasonication, Ni-NTA resin | [31] |
| Chlamydomonas sp. ICE-L | Psychrophilic microalga | (6-4) Photolyase | Disrupted with ultrasonication, Ni-NTA resin | [32] |
| Hymenobacter sp. | Antarctic bacterium | CPD Class I | Lysed with sonication, Ni-NTA resin | [33] |
| Methanosarcina mazei Mm0852 | Archaea | CPD Class II | Cell disruption with lysozyme, EDTA and PMSF with an emulsifier, Ni-NTA resin | [34] |
| Pohlia nutans M211 | Antarctic Moss | CPD Class II and (6-4) Photolyase | Ultrasonic cell disruptor, Ni-NTA resin | [35] |
| Phaeodactylum tricornutum ICE-H | Antarctic diatom | CPD Class II | Ultrasonic cell disruptor, Ni-NTA resin | [36] |
| Caulobacter crescentus | Oligotrophic bacterium | CPD Class III | Heated and cleared with centrifugation, purified by affinity chromatography on amylose resin | [37] |
| Mucor circinelloides | Fungus | CRY-DASH | Disrupted with a French press, affinity chromatography-HisTrap HP column | [38] |
| Phycomyces blakesleeanus (NRRL1555) | Fungus | CRY-DASH | Disrupted with a French press, affinity chromatography-His Trap HP column | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Meléndez-Sánchez, E.R.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; López-Arellanes, M.E.; Sosa-Hernández, J.E.; Coronado-Apodaca, K.G.; Gámez-Méndez, A.; Afewerki, S.; et al. Photolyase Production and Current Applications: A Review. Molecules 2022, 27, 5998. https://doi.org/10.3390/molecules27185998
Ramírez-Gamboa D, Díaz-Zamorano AL, Meléndez-Sánchez ER, Reyes-Pardo H, Villaseñor-Zepeda KR, López-Arellanes ME, Sosa-Hernández JE, Coronado-Apodaca KG, Gámez-Méndez A, Afewerki S, et al. Photolyase Production and Current Applications: A Review. Molecules. 2022; 27(18):5998. https://doi.org/10.3390/molecules27185998
Chicago/Turabian StyleRamírez-Gamboa, Diana, Ana Laura Díaz-Zamorano, Edgar Ricardo Meléndez-Sánchez, Humberto Reyes-Pardo, Karen Rocio Villaseñor-Zepeda, Miguel E. López-Arellanes, Juan Eduardo Sosa-Hernández, Karina G. Coronado-Apodaca, Ana Gámez-Méndez, Samson Afewerki, and et al. 2022. "Photolyase Production and Current Applications: A Review" Molecules 27, no. 18: 5998. https://doi.org/10.3390/molecules27185998
APA StyleRamírez-Gamboa, D., Díaz-Zamorano, A. L., Meléndez-Sánchez, E. R., Reyes-Pardo, H., Villaseñor-Zepeda, K. R., López-Arellanes, M. E., Sosa-Hernández, J. E., Coronado-Apodaca, K. G., Gámez-Méndez, A., Afewerki, S., Iqbal, H. M. N., Parra-Saldivar, R., & Martínez-Ruiz, M. (2022). Photolyase Production and Current Applications: A Review. Molecules, 27(18), 5998. https://doi.org/10.3390/molecules27185998








