High-Pressure-Based Strategies for the Inactivation of Bacillus subtilis Endospores in Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Culture Media, and Solutions
2.2. Botanical Origin of Honey
2.3. Sample Preparation
2.4. High-Pressure Processing at Room Temperature
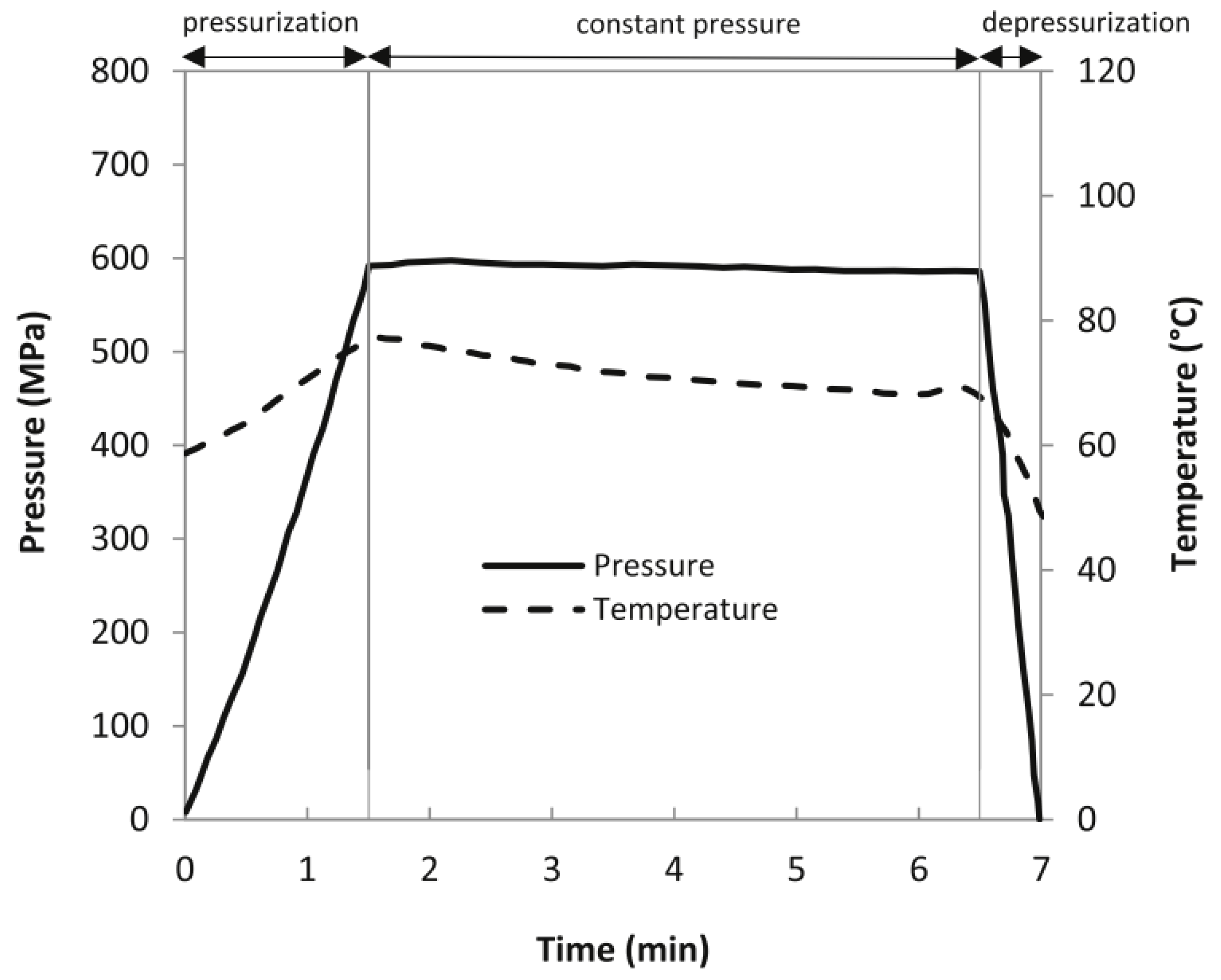
2.5. High Pressure Combined with Thermal Processing
2.6. Hyperbaric Storage
2.7. Thermal Processing
2.8. Microbiology
2.8.1. Endospore Inoculations
2.8.2. Endospore Preparation
2.8.3. Determination of Endospore Germination and Inactivation
3. Results
3.1. Botanical Origin
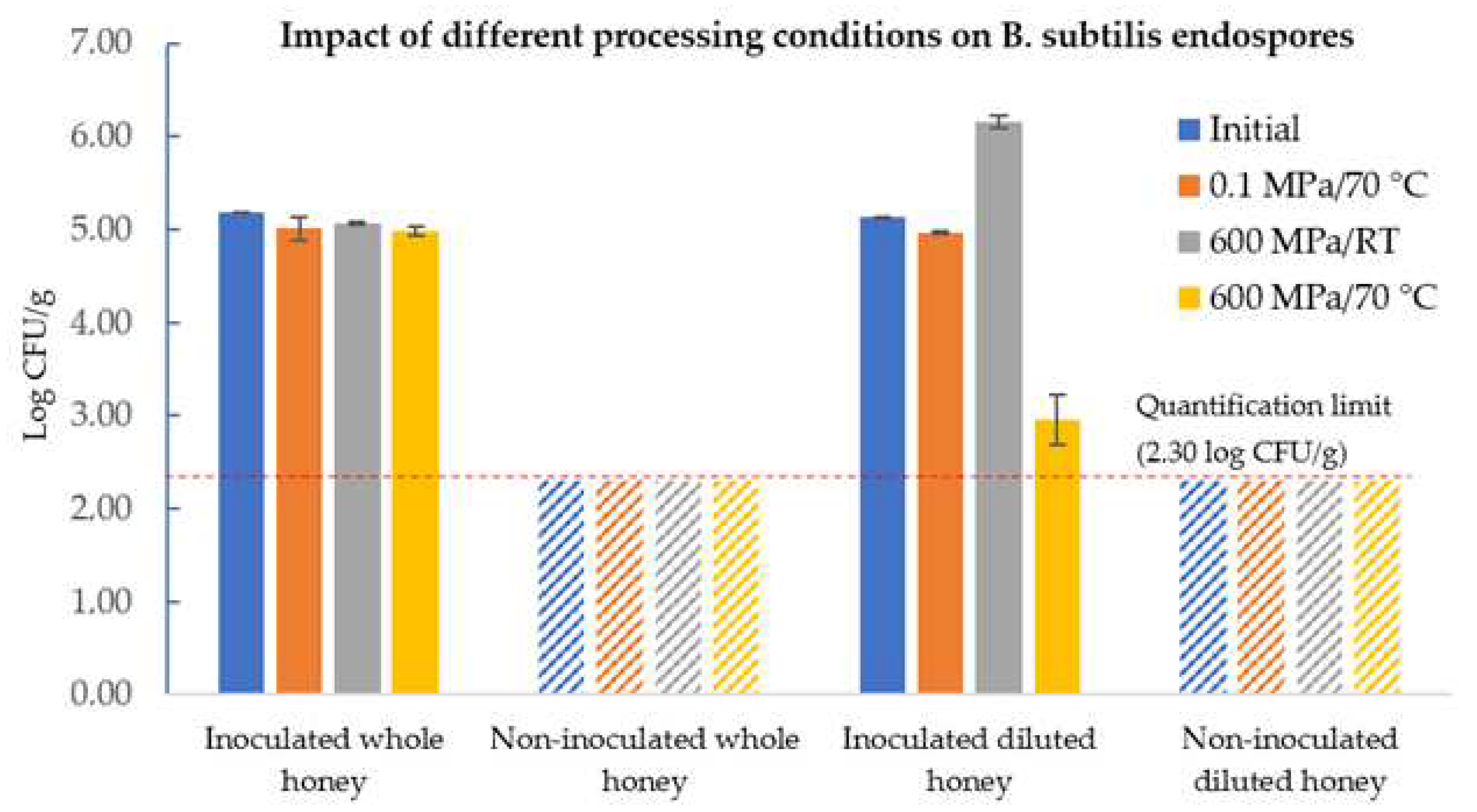
3.2. Bacillus Subtilis Endospores’ Response in Honey towards Different HPP Techniques
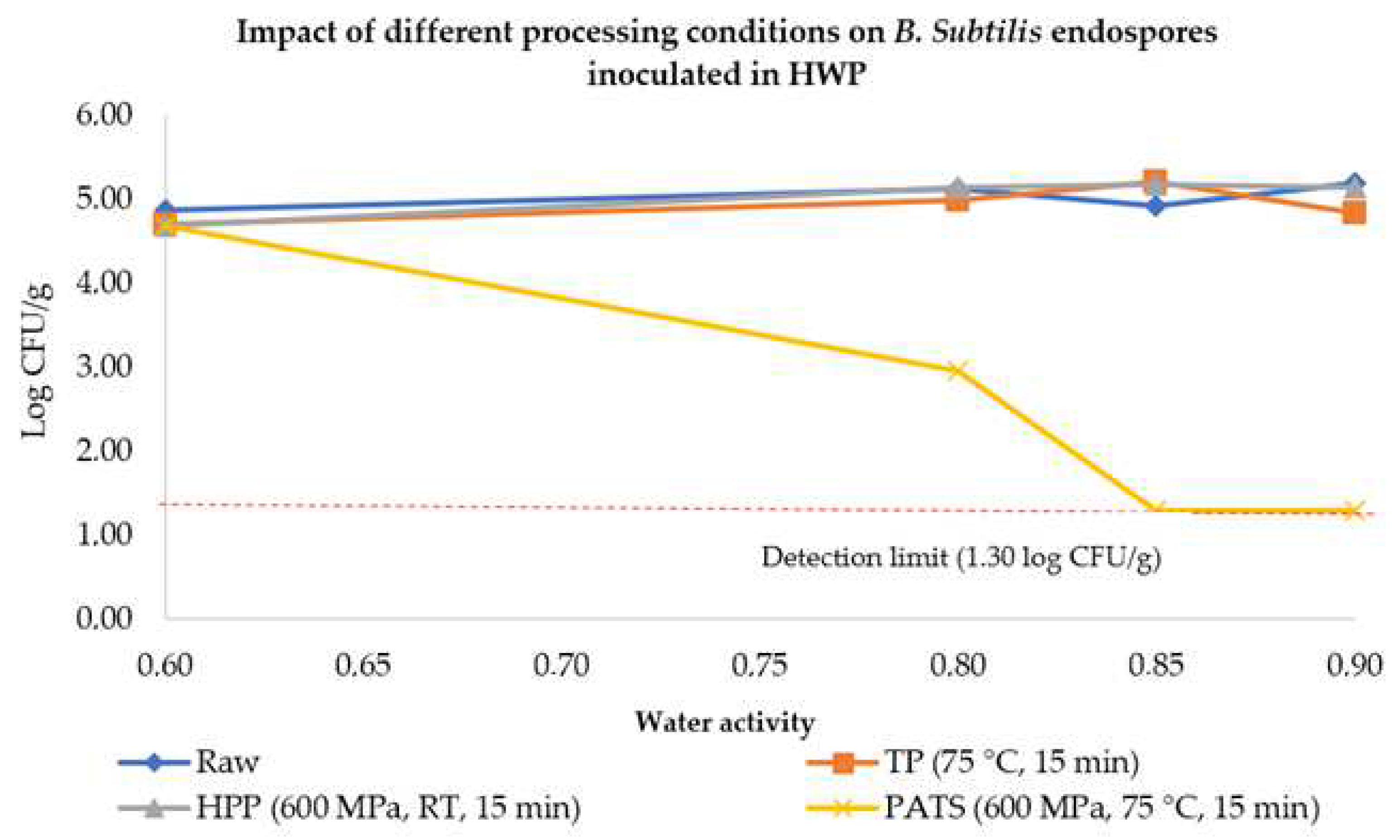
3.2.1. Pressure-Assisted Thermal Processing
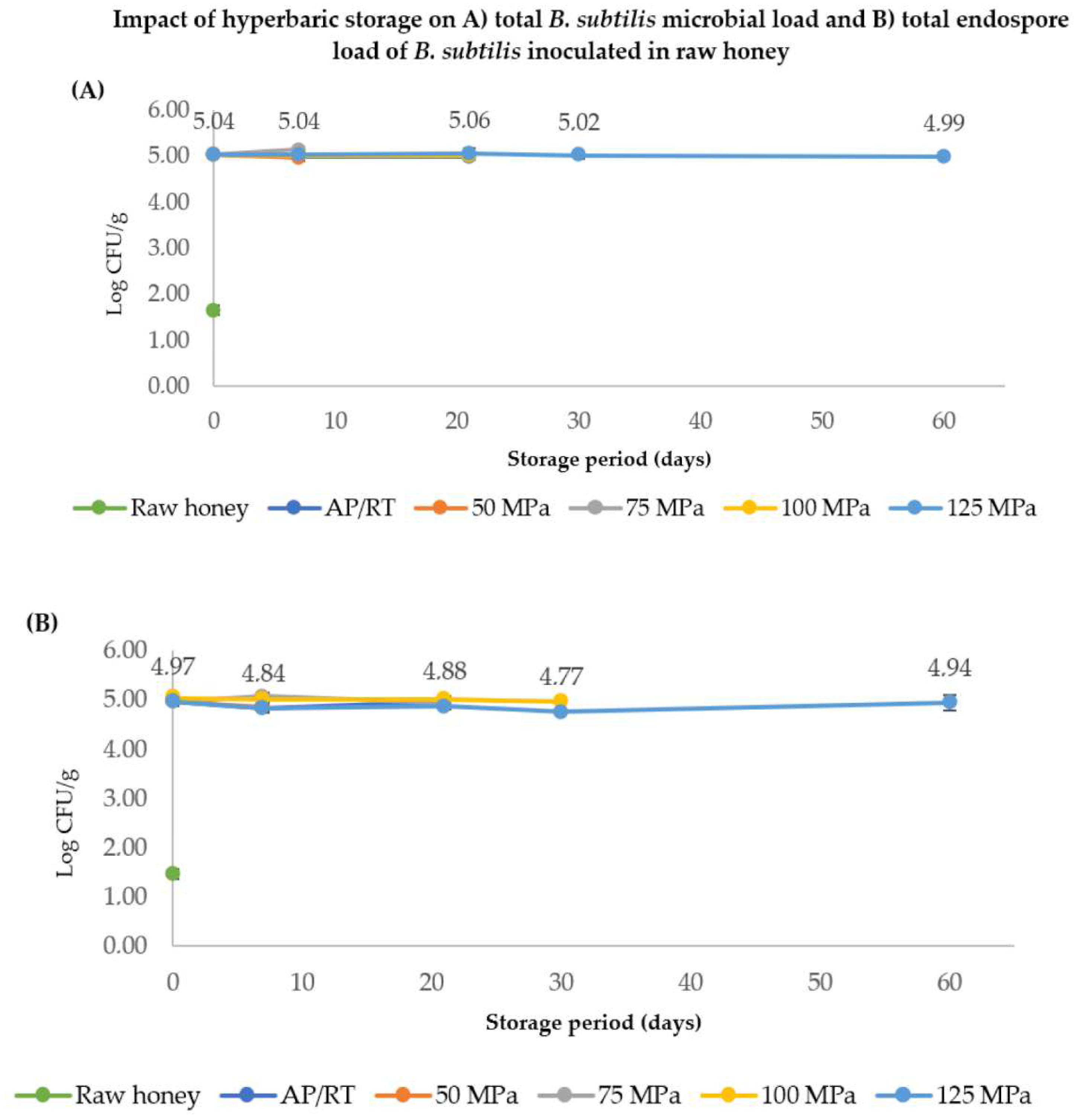
3.2.2. Hyperbaric Storage
3.3. B. subtilis Endospores’ Response in Diluted Honey with Adjusted Water Activity after Different HPP Techniques
3.3.1. HPP and PATP of Honey–Water Preparations with Increased aW
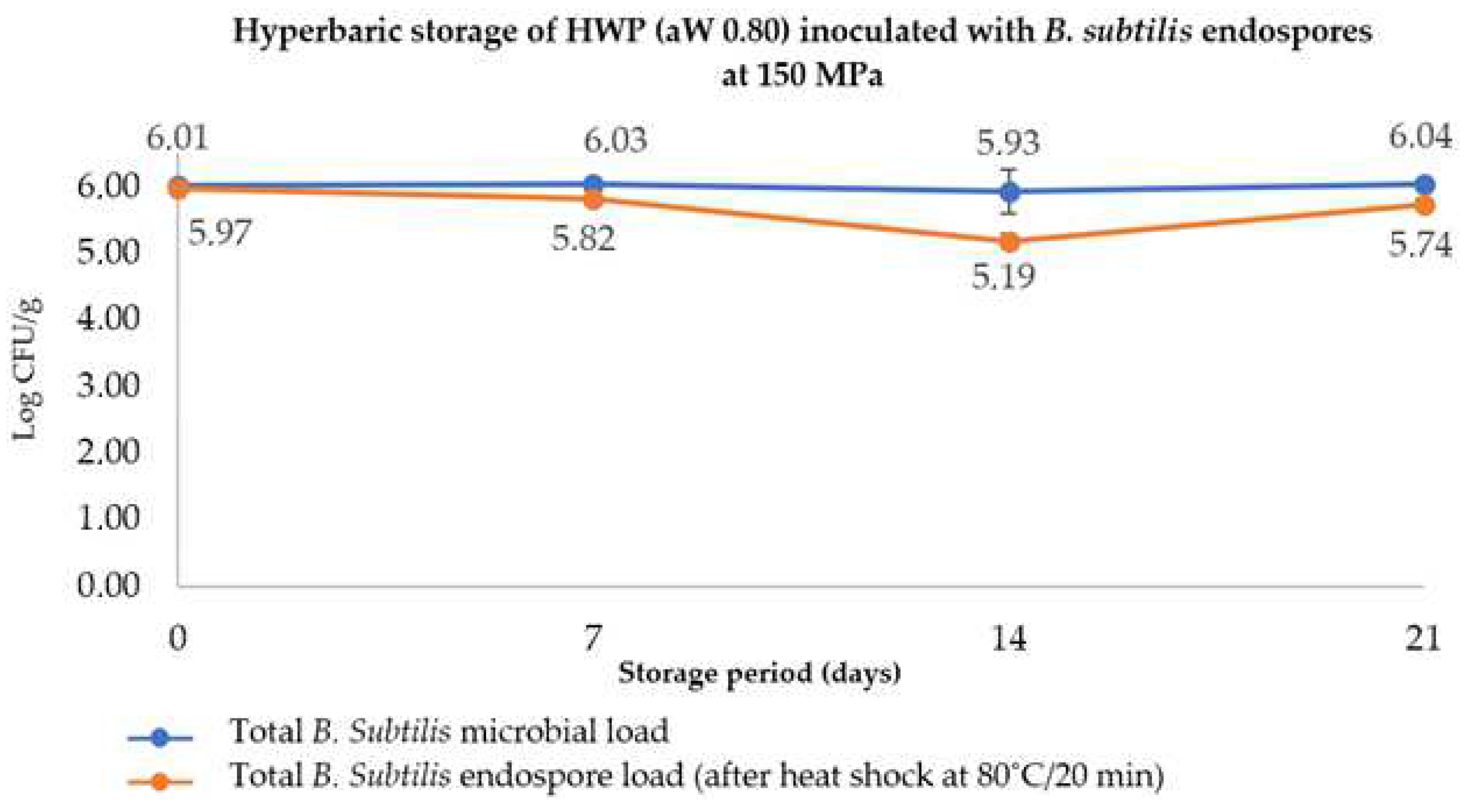
3.3.2. Hyperbaric Storage of Diluted Honey with Adjusted aW
4. Discussion
4.1. Survival of B. subtilis Endospores Inoculated in Honey after HPP, PATP, and HS
4.2. Inactivation of Vegetative Cells and Endospores of B. subtilis in Diluted Honey with Increased aW Using HPP, PATP, and HS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schell, K.R.; Fernandes, K.E.; Shanahan, E.; Wilson, I.; Blair, S.E.; Carter, D.A.; Cokcetin, N.N. The Potential of Honey as a Prebiotic Food to Re-Engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 2022, 9, 957932. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Saraiva, J.A.; Estevinho, L.M. Honey Health Benefits and Uses in Medicine. In Bee Products-Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 83–96. ISBN 978-3-319-59689-1. [Google Scholar]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef] [PubMed]
- Hossain, L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-Based Medicinal Formulations: A Critical Review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- Hakim, D.A.; Tjahjaningsih, W. Sudarno Antibacterial Activity of Honey in Preserving High- Pressure Cooked Milkfish Stored at Room Temperature. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012079. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Karczmarczyk, R.; Wiszniewska-Łaszczych, A.; Gomółka-Pawlicka, M.; Szteyn, J.; Liedtke, K. Honey Sold Directly by Producers in the Silesian Region of Poland as a Source of Clostridium Botulinum Types A, B, E and F. Czech J. Food Sci. 2017, 35, 194–199. [Google Scholar] [CrossRef]
- Grenda, T.; Grabczak, M.; Sieradzki, Z.; Kwiatek, K.; Pohorecka, K.; Skubida, M.; Bober, A. Clostridium Botulinum Spores in Polish Honey Samples. J. Vet. Sci. 2018, 19, 635–642. [Google Scholar] [CrossRef]
- World Health Organization. Botulism. Available online: https://www.who.int/news-room/fact-sheets/detail/botulism (accessed on 15 August 2022).
- Tamrin, M.; Islahuddin, M. The Dilemma of Diagnosing Wound Botulism in an Infant: A Rare Case of Paralysis with Topical Application of Honey. Int. J. Infect. Dis. 2020, 95, 157–159. [Google Scholar] [CrossRef]
- Chen, C. Relationship between Water Activity and Moisture Content in Floral Honey. Foods 2019, 8, 30. [Google Scholar] [CrossRef]
- FAO. Standard for Honey 12-1981. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 15 August 2022).
- Scepankova, H.; Pinto, C.A.; Paula, V.; Estevinho, L.M.; Saraiva, J.A. Conventional and Emergent Technologies for Honey Processing: A Perspective on Microbiological Safety, Bioactivity, and Quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5393–5420. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.M.; Gibbs, P.A. Non-Proteolytic Clostridium Botulinum Spores in Distributed Foods and Design of Pasteurization Processes. Food Sci. Technol. 2010, 21, 95–105. [Google Scholar] [CrossRef]
- Majkut, M.; Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T. Antimicrobial Activity of Heat-Treated Polish Honeys. Food Chem. 2021, 343, 128561. [Google Scholar] [CrossRef]
- Molan, P.C.; Allen, K.L. The Effect of Gamma-Irradiation on the Antibacterial Activity of Honey. J. Pharm. Pharmacol. 1996, 48, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Castell-Perez, M.E.; Moreira, G.R. Irradiation and Consumers Acceptance. In Innovative Food Procesing Technologies; Commonwealth Scientific and Industrial Research Organisation (CSIRO), Agriculture and Food: Werribee, VIC, Australia, 2021; pp. 122–135. ISBN 9780128157824. [Google Scholar]
- Roobab, U.; Abida, A.; Afzal, R.; Madni, G.M.; Zeng, X.-A.; Rahaman, A.; Aadil, R.M. Impact of High-Pressure Treatments on Enzyme Activity of Fruit-Based Beverages: An Overview. Int. J. Food Sci. Technol. 2022, 57, 801–815. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Zhang, W.; Zhang, J.; Pérez-Santaescolástica, C.; Lorenzo, J.M. High-Pressure Processing in Inactivation of Salmonella Spp. in Food Products. Trends Food Sci. Technol. 2021, 107, 31–37. [Google Scholar] [CrossRef]
- Bolton, D.; Bover-cid, S.; Chemaly, M.; Davies, R.; Cesare, A.D.; Herman, L.; Hilbert, F.; Lindqvist, R.; Nauta, M.; Peixe, L.; et al. The Efficacy and Safety of High-Pressure Processing of Food. EFSA J. 2022, 20, 7128. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy Expectations of High Hydrostatic Pressure Treatment in Food Processing Industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of High Hydrostatic Pressure Food Processing: Perspectives on Technology and Food Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef]
- Santos, M.D.; Fidalgo, L.G.; Pinto, C.A.; Duarte, R.V.; Lemos, Á.T.; Delgadillo, I.; Saraiva, J.A. Hyperbaric Storage at Room like Temperatures as a Possible Alternative to Refrigeration: Evolution and Recent Advances. Crit. Rev. Food Sci. Nutr. 2020, 61, 2078–2089. [Google Scholar] [CrossRef]
- Setikaite, I.; Koutchma, T.; Patazca, E.; Parisi, B. Effects of Water Activity in Model Systems on High-Pressure Inactivation of Escherichia Coli. Food Bioprocess Technol. 2009, 2, 213–221. [Google Scholar] [CrossRef]
- Erdtman, G. The Acetolysis Method-a Revised Description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Evelyn; Silva, F.V.M. Heat Assisted HPP for the Inactivation of Bacteria, Moulds and Yeasts Spores in Foods: Log Reductions and Mathematical Models. Trends Food Sci. Technol. 2019, 88, 143–156. [Google Scholar] [CrossRef]
- Patazca, E.; Koutchma, T.; Balasubramaniam, V.M. Quasi-Adiabatic Temperature Increase during High Pressure Processing of Selected Foods. J. Food Eng. 2007, 80, 199–205. [Google Scholar] [CrossRef]
- Vercammen, A.; Vivijs, B.; Lurquin, I.; Michiels, C.W. Germination and Inactivation of Bacillus Coagulans and Alicyclobacillus Acidoterrestris Spores by High Hydrostatic Pressure Treatment in Buffer and Tomato Sauce. Int. J. Food Microbiol. 2012, 152, 162–167. [Google Scholar] [CrossRef]
- Reineke, K.; Schlumbach, K.; Baier, D.; Mathys, A.; Knorr, D. The Release of Dipicolinic Acid—The Rate-Limiting Step of Bacillus Endospore Inactivation during the High Pressure Thermal Sterilization Process. Int. J. Food Microbiol. 2013, 162, 55–63. [Google Scholar] [CrossRef]
- Reineke, K.; Ellinger, N.; Berger, D.; Baier, D.; Mathys, A.; Setlow, P.; Knorr, D. Structural Analysis of High Pressure Treated Bacillus Subtilis Spores. Innov. Food Sci. Emerg. Technol. 2013, 17, 43–53. [Google Scholar] [CrossRef]
- Wuytack, E.Y.; Boven, S.; Michiels, C.W. Comparative Study of Pressure-Induced Germination of Bacillus Subtilis Spores at Low and High Pressures. Appl. Environ. Microbiol. 1998, 64, 3220–3224. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of Honey Criteria and Standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Bermejo-Prada, A.; Colmant, A.; Otero, L.; Guignon, B. Industrial Viability of the Hyperbaric Method to Store Perishable Foods at Room Temperature. J. Food Eng. 2017, 193, 76–85. [Google Scholar] [CrossRef]
- Black, E.P.; Setlow, P.; Hocking, A.D.; Stewart, C.M.; Kelly, A.L.; Hoover, D.G. Response of Spores to High-Pressure Processing. Compr. Rev. Food Sci. Food Saf. 2007, 6, 103–119. [Google Scholar] [CrossRef]
- Reineke, K.; Doehner, I.; Baier, D.; Mathys, A.; Knorr, D. The Different Pathways of Spore Germination and Inactivation of Bacillus Subtilis under High Pressure and Elevated Temperatures. Procedia Food Sci. 2011, 1, 792–799. [Google Scholar] [CrossRef][Green Version]
- Fauzi, N.A. An Insight on the Relationship between Food Compressibility and Microbial Inactivation during High Pressure Processing. J. Food Sci. Technol. 2017, 8, 802–809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leyva-Daniel, D.E.; Escobedo-Avellaneda, Z.; Villalobos-Castillejos, F.; Alamilla-Beltrán, L.; Welti-Chanes, J. Effect of High Hydrostatic Pressure Applied to a Mexican Honey to Increase Its Microbiological and Functional Quality. Food Bioprod. Processing 2017, 102, 299–306. [Google Scholar] [CrossRef]
- Pinto, C.A.; Moreira, S.A.; Fidalgo, L.G.; Inácio, R.S.; Barba, F.J.; Saraiva, J.A. Effects of High-Pressure Processing on Fungi Spores: Factors Affecting Spore Germination and Inactivation and Impact on Ultrastructure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 553–573. [Google Scholar] [CrossRef]
- Reineke, K.; Mathys, A. Endospore Inactivation by Emerging Technologies: A Review of Target Structures and Inactivation Mechanisms. Annu. Rev. Food Sci. Technol. 2020, 11, 255–274. [Google Scholar] [CrossRef]
- Aldrete-Tapia, J.A.; Torres, J.A. Enhancing the Inactivation of Bacterial Spores during Pressure-Assisted Thermal Processing. Food Eng. Rev. 2021, 13, 431–441. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; Wang, X.; Wang, P.; Liao, X.; Wu, X.; Chen, F.; Hu, X. Building of Pressure-Assisted Ultra-High Temperature System and Its Inactivation of Bacterial Spores. Front. Microbiol. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Safe Practices for Food Processes—Kinetics of Microbial Inactivation for Alternative Food Processing Technologies—High Pressure Processing; Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2014. [Google Scholar]
- Podolak, R.; Whitman, D.; Black, D.G. Factors Affecting Microbial Inactivation during High Pressure Processing in Juices and Beverages: A Review. J. Food Prot. 2020, 83, 1561–1575. [Google Scholar] [CrossRef]
- Sevenich, R.; Reineke, K.; Hecht, P.; Fröhling, A.; Rauh, C.; Schlüter, O.; Knorr, D. Impact of Different Water Activities (Aw) Adjusted by Solutes on High Pressure High Temperature Inactivation of Bacillus Amyloliquefaciens Spores. Front. Microbiol. 2015, 6, 689. [Google Scholar] [CrossRef]
- Akhmazillah, M.F.N.; Farid, M.M.; Silva, F.V.M. High Pressure Processing of Honey: Preliminary Study of Total Microorganism Inactivation and Identification of Bacteria. J. Sci. Technol. 2012, 4, 1–12. [Google Scholar]
- Grant, K.A.; McLauchlin, J.; Amar, C. Infant botulism: Advice on Avoiding Feeding Honey to Babies and Other Possible Risk Factors. Community Pract. 2013, 86, 44–46. [Google Scholar]
- Pinto, C.A.; Santos, M.D.; Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. Enhanced Control of Bacillus Subtilis Endospores Development by Hyperbaric Storage at Variable/Uncontrolled Room Temperature Compared to Refrigeration. Food Microbiol. 2018, 74, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Reineke, K.; Mathys, A.; Heinz, V.; Knorr, D. Mechanisms of Endospore Inactivation under High Pressure. Trends Microbiol. 2013, 21, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, M.; Corbo, M.R.; Altieri, C.; Campaniello, D.; D’Amato, D.; Bevilacqua, A. Combined Effects of Temperature, Water Activity, and PH on Alicyclobacillus Acidoterrestris Spores. J. Food Prot. 2003, 66, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, R.; Kleinstueck, E.; Crews, C.; Anderson, W.; Pye, C.; Riddellova, K.; Hradecky, J.; Moravcova, E.; Reineke, K.; Knorr, D. High-Pressure Thermal Sterilization: Food Safety and Food Quality of Baby Food Puree. J. Food Sci. 2014, 79, 230–237. [Google Scholar] [CrossRef]
- Reddy, N.R.; Patazca, E.; Morrissey, T.R.; Skinner, G.E.; Loeza, V.; Schill, K.M.; Larkin, J.W. Thermal and Pressure-Assisted Thermal Destruction Kinetics for Spores of Type A Clostridium Botulinum and Clostridium Sporogenes PA3679. J. Food Prot. 2016, 79, 253–262. [Google Scholar] [CrossRef]
- Daryaei, H.; Balasubramaniam, V.M.; Yousef, A.E.; Legan, J.D.; Tay, A. Lethality Enhancement of Pressure-Assisted Thermal Processing against Bacillus Amyloliquefaciens Spores in Low-Acid Media Using Antimicrobial Compounds. Food Control 2016, 59, 234–242. [Google Scholar] [CrossRef]
- Stewart, C.M.; Dunne, C.P.; Keener, L. Pressure-Assisted Thermal Sterilization Validation. In High Pressure Processing of Food: Principles, Technology and Applications; Balasubramaniam, V.M., Barbosa-Cánovas, G.V., Lelieveld, H.L.M., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2016; pp. 687–716. ISBN 978-1-4939-3234-4. [Google Scholar]
- Daher, D. Effect of High Pressure Processing on the Microbial Inactivation in Fruit Preparations and Other Vegetable Based Beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef]

| Sample | °Brix | aW |
|---|---|---|
| Honey (raw, non-diluted) | 84.6 | 0.57 |
| Diluted honey | ||
| HWP 0.80 | 63.3 | 0.80 |
| HWP 0.85 | 52.9 | 0.85 |
| HWP 0.90 | 43.4 | 0.90 |
| HWP 0.96 | 33.9 | 0.96 |
| Quantitative Pollen Spectrum (%) | ||||
|---|---|---|---|---|
| D | A | I | R | Type of Honey |
| Castanea sativa | Erica australias | Trifolium repens | Cistus ladanifer | Multifloral |
| (53.3%) | (19.1%) | (12.8%) | (2.9%) | |
| Quercus suber | ||||
| (4.3%) | ||||
| Acasia dealbata | ||||
| (3.8%) | ||||
| Prunus lusitanica | ||||
| (3.8%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scepankova, H.; Pinto, C.A.; Estevinho, L.M.; Saraiva, J.A. High-Pressure-Based Strategies for the Inactivation of Bacillus subtilis Endospores in Honey. Molecules 2022, 27, 5918. https://doi.org/10.3390/molecules27185918
Scepankova H, Pinto CA, Estevinho LM, Saraiva JA. High-Pressure-Based Strategies for the Inactivation of Bacillus subtilis Endospores in Honey. Molecules. 2022; 27(18):5918. https://doi.org/10.3390/molecules27185918
Chicago/Turabian StyleScepankova, Hana, Carlos A. Pinto, Letícia M. Estevinho, and Jorge A. Saraiva. 2022. "High-Pressure-Based Strategies for the Inactivation of Bacillus subtilis Endospores in Honey" Molecules 27, no. 18: 5918. https://doi.org/10.3390/molecules27185918
APA StyleScepankova, H., Pinto, C. A., Estevinho, L. M., & Saraiva, J. A. (2022). High-Pressure-Based Strategies for the Inactivation of Bacillus subtilis Endospores in Honey. Molecules, 27(18), 5918. https://doi.org/10.3390/molecules27185918







