Valorization of Amaranth (Amaranthus cruentus) Grain Extracts for the Development of Alginate-Based Active Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity and Total Phenolic Content of Amaranth Grain Extracts
2.2. Identification of Amaranth Phenolic Compounds by UHPLC
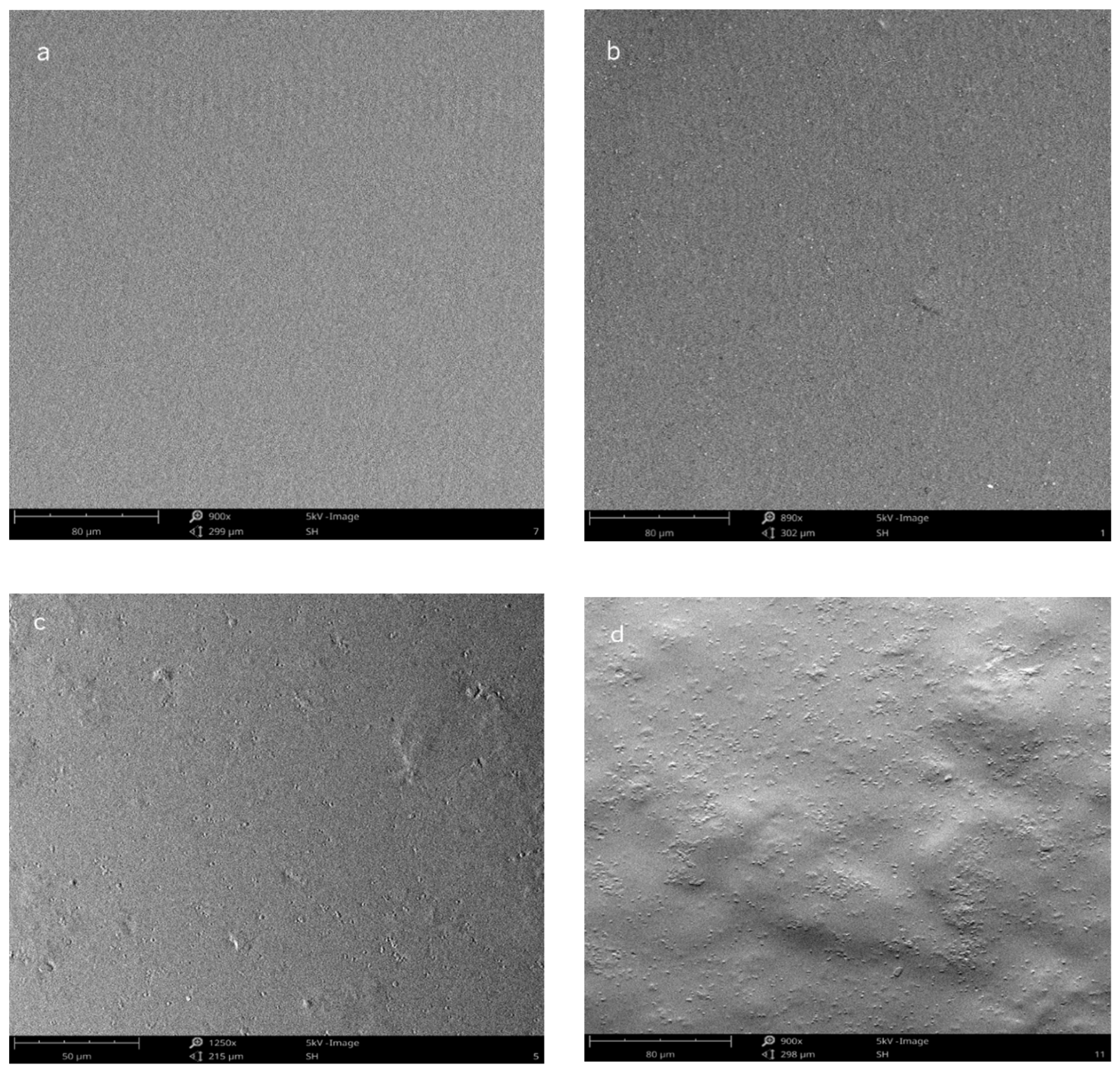
2.3. Film Surface Morphology
2.4. Water Angle Contact, Solubility, Moisture Content (MC), Water Vapor Permeability (WVP) Measurements of Films
2.5. Thermal Stability-Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
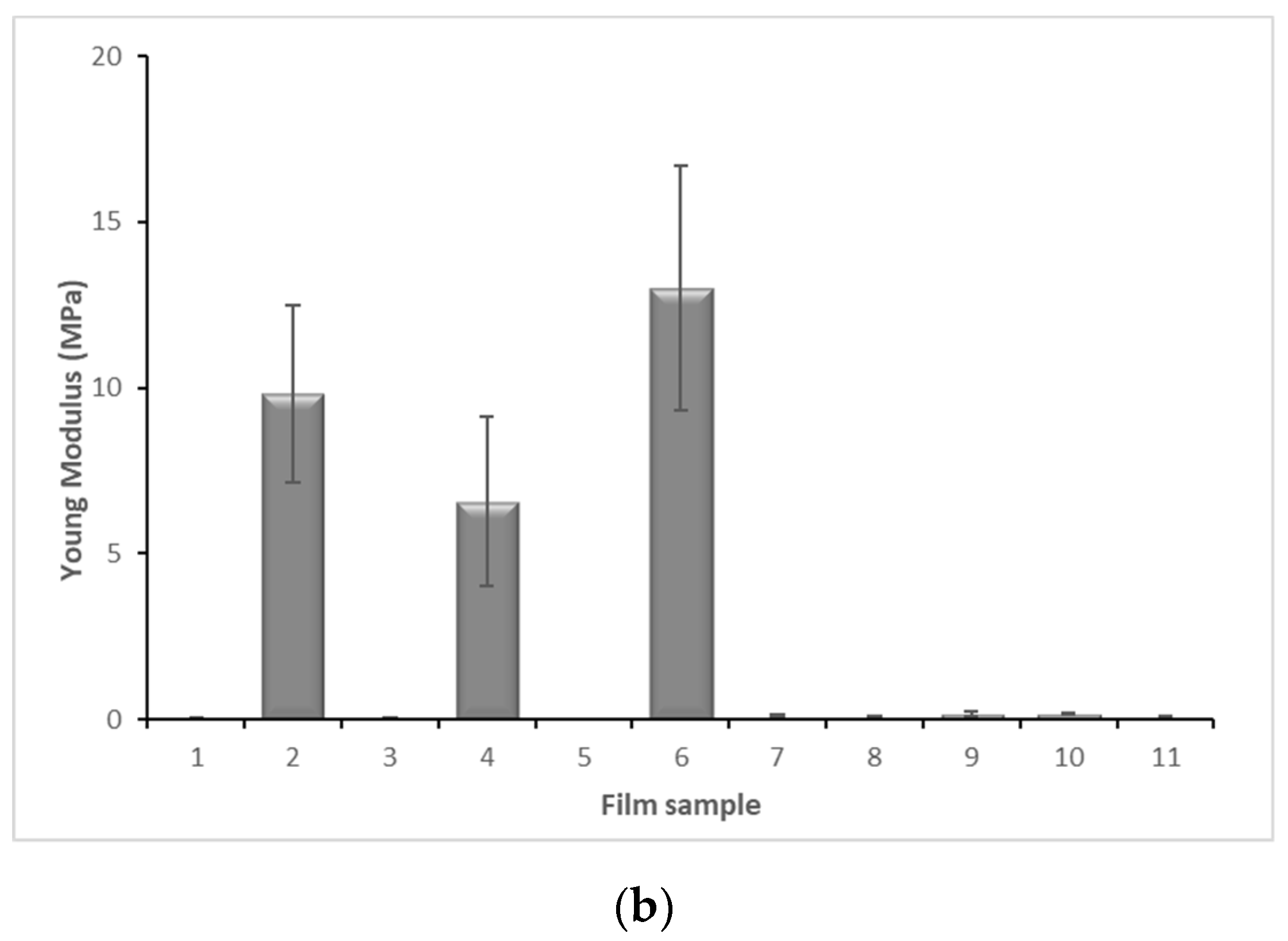
2.6. Mechanical Properties
2.7. Optical Properties-Color and Opacity of the Films
2.8. Optimization of Alginate-Based Films’ Formulation
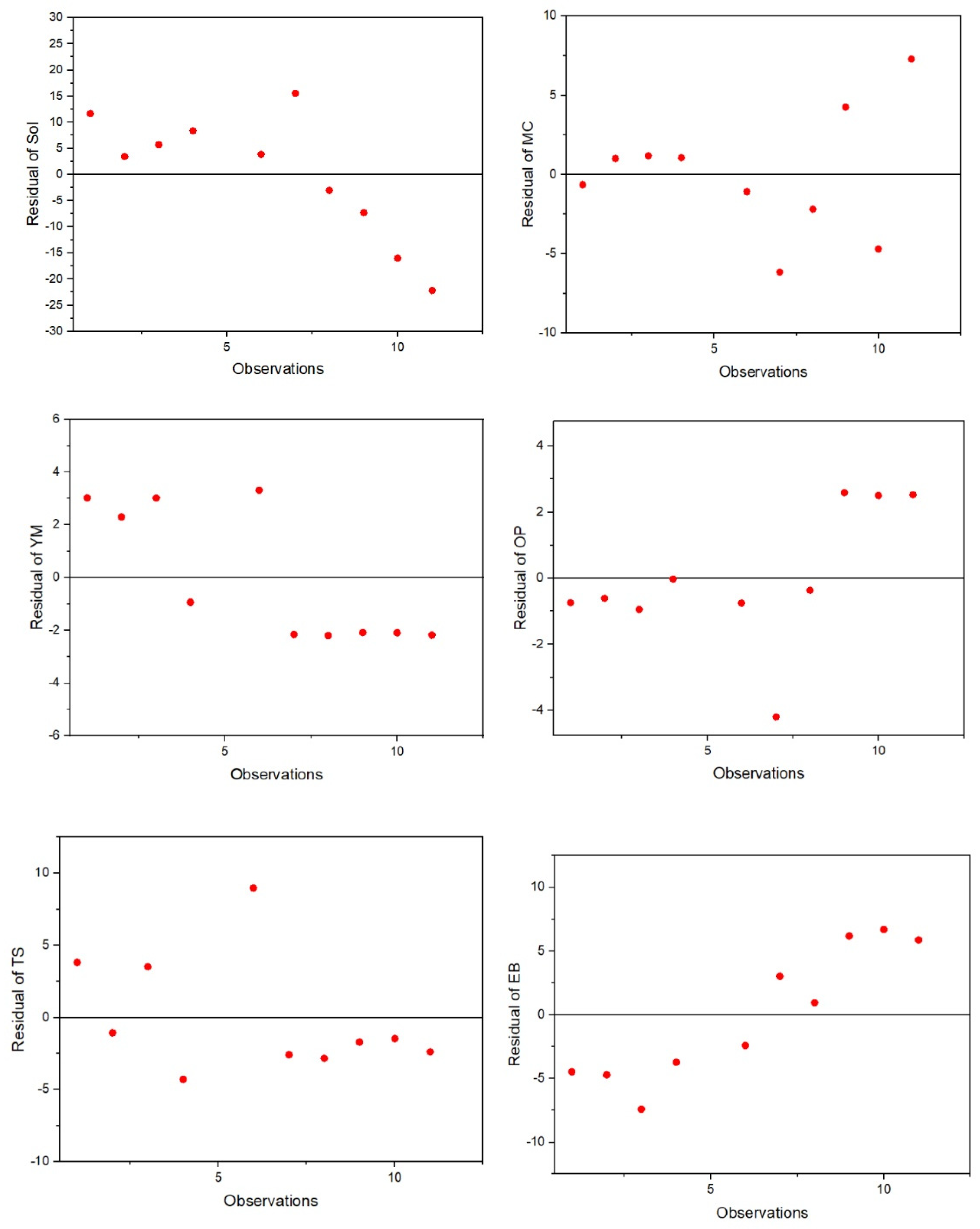
2.9. Multivariate Analysis Using Partial Least Squares (PLS), Principal Component Analysis (PCA), k-Means Cluster Analysis and Pearson’s Correlation Matrices
3. Materials and Methods
3.1. Amaranth Phenolic Extraction
3.2. Identification of Amaranth Phenolic Compounds by Ultra-High-Performance Liquid Chromatography (UHPLC)
3.3. Antioxidant Activity of Freeze-Dried Amaranth Extracts
3.4. Total Phenolic Content of Freeze-Dried Amaranth Extracts
3.5. Experimental Design and Production of Alginate Film with Amaranth Phenolic Compounds
3.6. Characterization of the Alginate-Based Films
3.6.1. Scanning Electron Microscopy (SEM)
3.6.2. Water Contact Angle
3.6.3. Film Solubility (Sol)
3.6.4. Moisture Content (MC)
3.6.5. Water Vapor Permeability (WVP)
3.6.6. Thermal Analysis
3.6.7. Mechanical Properties
3.6.8. Film Thickness
3.6.9. Optical Properties (OP)
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aadil, K.R.; Prajapati, D.; Jha, H. Improvement of physcio-chemical and functional properties of alginate film by Acacia lignin. Food Packag. Shelf Life 2016, 10, 25–33. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Basanta, M.F.; Rojas, A.M.; Martinefski, M.R.; Tripodi, V.P.; De’Nobili, M.D.; Fissore, E.N. Cherry (Prunus avium) phenolic compounds for antioxidant preservation at food interfaces. J. Food Eng. 2018, 239, 15–25. [Google Scholar] [CrossRef]
- Huang, S.; Ma, Y.; Zhang, C.; Cai, S.; Pang, M. Bioaccessibility and antioxidant activity of phenolics in native and fermented Prinsepia utilis Royle seed during a simulated gastrointestinal digestion in vitro. J. Funct. Foods 2017, 37, 354–362. [Google Scholar] [CrossRef]
- Demir, T.; Ağaoğlu, S. Antioxidant, Antimicrobial and Metmyoglobin Reducing Activity of Artichoke (Cynara scolymus) Powder Extract-Added Minced Meat during Frozen Storage. Molecules 2021, 26, 5494. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch–chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Coelho, L.M.; Silva, P.M.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A. Emerging opportunities in exploring the nutritional/functional value of amaranth. Food Funct. 2018, 9, 5499–5512. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Sebola, N.A.; Hassan, Z.M.; Ng’ambi, J.W.; Weeks, W.J.; Mabelebele, M. Chemical Composition and Metabolomic Analysis of Amaranthus cruentus Grains Harvested at Different Stages. Molecules 2022, 27, 623. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.R.; do Amaral Sobral, P.J.; Menegalli, F.C. Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT Food Sci. Technol. 2011, 44, 1731–1738. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, E.O.; BC, E.S.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 014–0048. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef]
- Pazinatto, C.; Malta, L.G.; Pastore, G.M.; Maria Netto, F. Antioxidant capacity of amaranth products: Effects of thermal and enzymatic treatments. Food Sci. Technol. 2013, 33, 485–493. [Google Scholar] [CrossRef]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol. 2007, 225, 321–328. [Google Scholar] [CrossRef]
- Parliament, E. Directive 2009/32/EC of The European Parliament and of the Council On the approximation of the laws of the member states on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 141, 11. [Google Scholar]
- Conforti, F.; Statti, G.; Loizzo, M.R.; Sacchetti, G.; Poli, F.; Menichini, F. In Vitro antioxidant effect and inhibition of alpha-amylase of two varieties of Amaranthus caudatus seeds. Biol. Pharm. Bull. 2005, 28, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.A.; Steffensen, S.K.; Christophersen, C.; Mortensen, A.G.; Jørgensen, L.N.; Niveyro, S.; de Troiani, R.M.; Rodríguez-Enríquez, R.J.; Barba-de la Rosa, A.P.; Fomsgaard, I.S. Synthesis and Quantitation of Six Phenolic Amides in Amaranthus spp. J. Agric. Food. Chem. 2010, 58, 6306–6311. [Google Scholar] [CrossRef]
- Venskutonis, R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.S.; Cerqueira, M.A.; Pereira, R.N.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Effect of moderate electric fields in the properties of starch and chitosan films reinforced with microcrystalline cellulose. Carbohydr. Polym. 2017, 174, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Thivya, P.; Bhosale, Y.K.; Anandakumar, S.; Hema, V.; Sinija, V.R. Study on the characteristics of gluten/alginate-cellulose/onion waste extracts composite film and its food packaging application. Food Chem. 2022, 390, 133221. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Wang, Y.; Liao, Y.; Xiong, H.; Selomulya, C.; Hu, J.; Zhao, Q. Complete waste recycling strategies for improving the accessibility of rice protein films. Green Chem. 2020, 22, 490–503. [Google Scholar] [CrossRef]
- Paixão, L.C.; Lopes, I.A.; Barros Filho, A.K.D.; Santana, A.A. Alginate biofilms plasticized with hydrophilic and hydrophobic plasticizers for application in food packaging. J. Appl. Polym. Sci. 2019, 136, 48263. [Google Scholar] [CrossRef]
- Santos, L.G.; Silva, G.F.A.; Gomes, B.M.; Martins, V.G. A novel sodium alginate active films functionalized with purple onion peel extract (Allium cepa). Biocatal. Agric. Biotechnol. 2021, 35, 102096. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.S.; Vicente, A.A. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; da Silva, M.A.; Kieckbusch, T.G. Natamycin release from alginate/pectin films for food packaging applications. J. Food Eng. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of antioxidant–antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.C.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef]
- Xiao, M.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Properties of film-forming emulsions and films based on corn starch/sodium alginate/gum Arabic as affected by virgin coconut oil content. Food Packag. Shelf Life 2022, 32, 100819. [Google Scholar] [CrossRef]
- Guan, T.; Li, N.; Zhang, G.; Xue, P. Characterization and evaluation of sodium alginate-based edible films by incorporation of star anise ethanol extract/hydroxypropyl-β-cyclodextrin inclusion complex. Food Packag. Shelf Life 2022, 31, 100785. [Google Scholar] [CrossRef]
- Roos, Y.; Karel, M. Plasticizing Effect of Water on Thermal Behavior and Crystallization of Amorphous Food Models. J. Food Sci. 1991, 56, 38–43. [Google Scholar] [CrossRef]
- Mokrejs, P.; Langmaier, F.; Janacova, D.; Mladek, M.; Kolomaznik, K.; Vasek, V. Thermal study and solubility tests of films based on amaranth flour starch–protein hydrolysate. J. Therm. Anal. Calorim. 2009, 98, 299–307. [Google Scholar] [CrossRef]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Menzel, C.; González-Martínez, C.; Chiralt, A.; Vilaplana, F. Antioxidant starch films containing sunflower hull extracts. Carbohydr. Polym. 2019, 214, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Ouwehand, A.C.; Salminen, S.J.; Forssell, P.; Myllärinen, P. Effect of starch- and lipid-based encapsulation on the culturability of two Bifidobacterium longum strains. Lett. Appl. Microbiol. 2007, 44, 500–505. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Fu, Z.-Q.; Wang, L.-J.; Li, D.; Wei, Q.; Adhikari, B. Effects of high-pressure homogenization on the properties of starch-plasticizer dispersions and their films. Carbohydr. Polym. 2011, 86, 202–207. [Google Scholar] [CrossRef]
- Fu, Z.-Q.; Guo, S.-X.; Sun, Y.; Wu, H.-J.; Huang, Z.-G.; Wu, M. Effect of Glycerol Content on the Properties of Potato Flour Films. Starch-Stärke 2021, 73, 2000203. [Google Scholar] [CrossRef]
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-K.; Song, K.B. Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Packag. Shelf Life 2018, 18, 157–163. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Genisheva, Z.; Oliveira, H.; de Freitas, V.; Teixeira, J.A.; Vicente, A.A. Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT 2016, 74, 493–503. [Google Scholar] [CrossRef]
- Omodamiro, O.; Jimoh, M.; Ezurike, P. In vitro Antioxidant Activity and Hypolipidemic Effects of an Ethanol Extract of Amaranthus viridis Leaves on Hyperlipidemic Wistar Albino Rats. Br. J. Pharm. Res. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Perales Sanchez, X.; Reyes Moreno, C.; Gómez-Favela, M.; Milán-Carrillo, J.; Cuevas-Rodriguez, E.-O.; Valdez, A.; Gutiérrez Dorado, R. Increasing the Antioxidant Activity, Total Phenolic and Flavonoid Contents by Optimizing the Germination Conditions of Amaranth Seeds. Plant Foods Hum. Nutr. 2014, 69, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.W.S.; Cerqueira, M.A.; Casariego, A.; Lima, A.M.P.; Teixeira, J.A.; Vicente, A.A. Effect of moderate electric fields in the permeation properties of chitosan coatings. Food Hydrocoll. 2009, 23, 2110–2115. [Google Scholar] [CrossRef][Green Version]
- ASTM. Standard Test Methods for Tensile Properties of Plastics: D 638-99. In ASTM Annual Book of American Standard Testing Methods; ASTM: Philadelphia, PA, USA, 1999. [Google Scholar]
- Maniwara, P.; Nakano, K.; Ohashi, S.; Boonyakiat, D.; Seehanam, P.; Theanjumpol, P.; Poonlarp, P. Evaluation of NIRS as non-destructive test to evaluate quality traits of purple passion fruit. Sci. Hortic. 2019, 257, 108712. [Google Scholar] [CrossRef]
- Danny Matthew, S.; Daniel, S.; Liniyanti, D.O. Effect of Distance Metrics in Determining K-Value in K-Means Clustering Using Elbow and Silhouette Method. In Proceedings of the Sriwijaya International Conference on Information Technology and Its Applications (SICONIAN 2019), Palembang, Indonesia, 16 November 2019; Atlantis Press: Amsterdam, The Netherlands; pp. 341–346. [Google Scholar]


| Solvent | TPC (mg GAE/g) | AA (mg ACE/g) |
|---|---|---|
| Methanol (50%) 1 | 3.9 ± 0.12 a | 0.24 ± 0.01 a |
| Ethanol (80%) 1 | 3.5 ± 0.09 a | 0.21 ± 0.01 b |
| Water | 2.4 ± 0.07 b | 0.12 ± 0.02 b |
| Freeze-dried ethanol extract | 16.78 ± 2.61 c | 7.54 ± 0.29 c |
| Phenolic Compounds | Concentration (mg/L) |
|---|---|
| Vanillic acid | 6.08 ± 0.01 |
| Ferulic acid | 1.96 ± 0.02 |
| p-coumaric acid + epicatechin | 1.66 ± 0.00 |
| o-coumaric acid | 1.32 ± 0.00 |
| Ellagic acid | 1.08 ± 0.13 |
| Cinnamic acid | 2.23 ± 0.01 |
| Resveratrol | 4.07 ± 0.02 |
| Rutin | 2.42 ± 0.00 |
| Total | 20.82 ± 0.10 |
| Films | Water Contact Angle (°) | MC (%) | Sol (%) | WVP × 1010 [g·(Pa·s·m)−1] | ΔHm (J g−1) | Tg (°C) | Tonset (°C) | TGAp (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 35.30 ± 0.75 b,c | 47.76 ± 1.22 b | 85.63 ± 2.54 b | 0.28 ± 0.02 a | 471.15 ± 11.63 a | 148.56 ± 3.66 a | 188.63 ± 0.04 b,c | 214.47 ± 0.80 b |
| 2 | 0.00 ± 0.00 a | 72.06 ± 0.52 a | 93.88 ± 1.21 a | 0.26 ± 0.03 a | 423.94 ± 90.07 a.b | 144.85 ± 2.38 a | 209.55 ± 0.06 a | 218.03 ± 0.16 a,d |
| 3 | 47.10 ± 1.28 b | 49.60 ± 1.18 b | 79.67 ± 1.55 c | 5.43 ± 0.22 c | 213.48 ± 19.62 c | 123.68 ± 1.07 b | 192.51 ± 2.25 b | 213.47 ± 0.57 b |
| 4 | 0.00 ± 0.00 a | 72.13 ± 0.68 a | 98.86 ± 1.98 a | 3.70 ± 0.41 b | 290.51 ± 5.48 c | 125.23 ± 3.83 b | 209.21 ± 3.40 a | 219.07 ± 1.76 a |
| 5 | 21.30 ± 3.94 c | 35.75 ± 0.85 c | 87.68 ± 1.38 b | 5.63 ± 0.28 c | 504.97 ± 38.19 a | 153.94 ± 3.91 a | 184.96 ± 3.27 b,c | 213.78 ± 0.57 b |
| 6 | 27.97 ± 0.67 c | 74.68 ± 0.55 a | 97.74 ± 0.82 a | 5.15 ± 0.32 c | 294.81 ± 51.19 c | 126.00 ± 3.03 b | 181.89 ± 3.48 c | 209.69 ± 0.11 c |
| 7 | 29.33 ± 1.55 b,c | 53.57 ± 0.82 d | 97.78 ± 0.71 a | 3.08 ± 0.15 d | 532.61 ± 19.23 a | 153.31 ± 1.08 a | 193.71 ± 1.94 b | 215.79 ± 0.08 b,d |
| 8 | 21.87 ± 18.94 c | 57.57 ± 0.91 e | 79.20 ± 1.24 c | 2.72 ± 0.32 d | 230.73 ± 30.06 c | 124.54 ± 1.07 b | 193.04 ± 3.13 b | 216.08 ± 1.17 a,b |
| 9 | 0.00 ± 0.00 a | 63.99 ± 1.87 f | 74.91 ± 1.95 d | 2.66 ± 0.18 d | 469.75 ± 9.57 a,b | 148.23 ± 6.65 a | 187.17 ± 0.18 b,c | 215.20 ± 0.24 b,d |
| 10 | 0.00 ± 0.00 a | 55.04 ± 1.00 d | 66.20 ± 1.20 e | 2.44 ± 0.12 d | 425.27 ± 42.19 a,b | 149.59 ± 9.91 a | 193.48 ± 0.69 b | 214.71 ± 0.78 b |
| 11 | 0.00 ± 0.00 a | 67.03 ± 1.52 f | 60.06 ± 1.50 f | 2.57 ± 0.19 d | 413.16 ± 57.00 a,b | 155.64 ± 2.62 a | 191.04 ± 3.92 b | 215.14 ± 0.05 b,d |
| Films | Thickness (mm) | OP (%) | Color Parameters | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| 1 | 5.00 ± 0.26 a | 7.80 ± 0.53 b | 94.26 ± 0.28 b | −0.21 ± 0.08 b | 6.98 ± 0.52 b,d |
| 2 | 5.03 ± 1.01 a | 9.86 ± 0.87 a | 95.26 ± 0.31 a | 0.00 ± 0.02 a,c | 4.08 ± 0.21 a |
| 3 | 6.43 ± 0.65 a | 7.60 ± 1.93 b | 94.13 ± 0.34 b | −0.22 ± 0.12 b | 7.56 ± 0.49 b |
| 4 | 6.57 ± 0.32 a | 10.44 ± 0.48 a | 95.10 ± 0.16 a | −0.06 ± 0.09 a,c | 4.76 ± 0.30 a |
| 5 | 5.00 ± 0.75 a | 3.35 ± 0.29 c | 96.49 ± 0.07 c | −0.09 ± 0.02 a,b | 2.93 ± 0.23 c |
| 6 | 6.10 ± 1.00 a | 10.11 ± 0.73 a | 95.22 ± 0.36 a | 0.03 ± 0.02 c | 4.25 ± 0.23 a |
| 7 | 5.47 ± 0.86 a | 5.31 ± 0.42 d | 93.97 ± 0.29 b | −0.20 ± 0.06 b | 6.62 ± 0.41 d,e |
| 8 | 4.83 ± 0.63 a | 9.14 ± 0.58 a,b | 94.51 ± 0.47 b | −0.07 ± 0.07 a,c | 6.06 ± 0.91 e |
| 9 | 4.73 ± 0.85 a | 12.09 ± 0.51 e | 95.44 ± 0.23 b | −0.20 ± 0.05 b | 7.77 ± 0.27 b |
| 10 | 4.33 ± 0.35 a | 12.00 ± 0.21 e | 94.59 ± 0.18 b | −0.29 ± 0.02 b | 7.35 ± 0.48 b |
| 11 | 4.03 ± 0.35 a | 12.02 ± 0.60 e | 94.02 ± 0.63 b | −0.30 ± 0.07 b | 8.33 ± 0.56 b |
| Response Variables | Model | r2 | p-Value | Fcalc | Ftab |
|---|---|---|---|---|---|
| Sol | 0.6785 | 0.01069 | 8.4 | 4.46 | |
| MC | 0.8763 | 0.00002 | 63.8 | 5.12 | |
| WVP | NA | NA | 0.48726 | 1.0 | 5.05 |
| CA | NA | NA | 0.31137 | 1.6 | 5.05 |
| Tg | NA | NA | 0.76410 | 0.5 | 5.05 |
| YM | 0.9685 | 0.00001 | 122.9 | 4.46 | |
| OP | 0.7789 | 0.01077 | 8.2 | 4.35 | |
| TGAp | NA | NA | 0.76415 | 0.5 | 5.05 |
| TS | 0.8852 | 0.00017 | 30.9 | 4.46 | |
| EB | 0.7859 | 0.00028 | 33.0 | 5.12 |
| Pearson Correlations | ||||||||
| Al | PC | Sol | MC | YM | OP | TS | EB | |
| Al | 1.00 | 0.00 | 0.48 | 0.92 | 0.86 | 0.35 | 0.81 | −0.44 |
| PC | 0.00 | 1.00 | −0.23 | 0.09 | −0.08 | 0.31 | −0.06 | −0.10 |
| Sol | 0.48 | −0.23 | 1.00 | 0.28 | 0.63 | −0.55 | 0.52 | −0.75 |
| MC | 0.92 | 0.09 | 0.28 | 1.00 | 0.79 | 0.54 | 0.72 | −0.40 |
| YM | 0.86 | −0.08 | 0.63 | 0.79 | 1.00 | 0.14 | 0.94 | −0.77 |
| OP | 0.35 | 0.31 | −0.55 | 0.54 | 0.14 | 1.00 | 0.18 | 0.27 |
| TS | 0.81 | −0.06 | 0.52 | 0.72 | 0.94 | 0.18 | 1.00 | −0.60 |
| EB | −0.44 | −0.10 | −0.75 | −0.40 | −0.77 | 0.27 | −0.60 | 1.00 |
| Spearman Correlations | ||||||||
| Al | PC | Sol | MC | YM | OP | TS | EB | |
| Al | 1.00 | 0.00 | 0.44 | 0.93 | 0.93 | 0.35 | 0.93 | −0.45 |
| PC | 0.00 | 1.00 | −0.21 | 0.19 | −0.15 | 0.21 | −0.13 | −0.04 |
| Sol | 0.44 | −0.21 | 1.00 | 0.24 | 0.38 | −0.50 | 0.27 | −0.71 |
| MC | 0.93 | 0.19 | 0.24 | 1.00 | 0.85 | 0.55 | 0.87 | −0.40 |
| YM | 0.93 | −0.15 | 0.38 | 0.85 | 1.00 | 0.45 | 0.97 | −0.33 |
| OP | 0.35 | 0.21 | −0.50 | 0.55 | 0.45 | 1.00 | 0.53 | 0.38 |
| TS | 0.93 | −0.13 | 0.27 | 0.87 | 0.97 | 0.53 | 1.00 | −0.30 |
| EB | −0.45 | −0.04 | −0.71 | −0.40 | −0.33 | 0.38 | −0.30 | 1.00 |
| Formulation | Alginate (% w/v) | Phenolic Compounds (% w/v) |
|---|---|---|
| 1 | 0.86 (0.64) | 0.30 |
| 2 | 1.39 (0.11) | 0.30 |
| 3 | 0.86 (0.64) | 1.70 |
| 4 | 1.39 (0.11) | 1.70 |
| 5 | 0.75 (0.75) | 1.00 |
| 6 | 1.50 (0.00) | 1.00 |
| 7 | 1.13 (0.37) | 0.00 |
| 8 | 1.13 (0.37) | 2.00 |
| 9 | 1.13 (0.37) | 1.00 |
| 10 | 1.13 (0.37) | 1.00 |
| 11 | 1.13 (0.37) | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, L.M.; Faria, C.; Madalena, D.; Genisheva, Z.; Martins, J.T.; Vicente, A.A.; Pinheiro, A.C. Valorization of Amaranth (Amaranthus cruentus) Grain Extracts for the Development of Alginate-Based Active Films. Molecules 2022, 27, 5798. https://doi.org/10.3390/molecules27185798
Coelho LM, Faria C, Madalena D, Genisheva Z, Martins JT, Vicente AA, Pinheiro AC. Valorization of Amaranth (Amaranthus cruentus) Grain Extracts for the Development of Alginate-Based Active Films. Molecules. 2022; 27(18):5798. https://doi.org/10.3390/molecules27185798
Chicago/Turabian StyleCoelho, Laylla Marques, Carla Faria, Daniel Madalena, Zlatina Genisheva, Joana T. Martins, António A. Vicente, and Ana C. Pinheiro. 2022. "Valorization of Amaranth (Amaranthus cruentus) Grain Extracts for the Development of Alginate-Based Active Films" Molecules 27, no. 18: 5798. https://doi.org/10.3390/molecules27185798
APA StyleCoelho, L. M., Faria, C., Madalena, D., Genisheva, Z., Martins, J. T., Vicente, A. A., & Pinheiro, A. C. (2022). Valorization of Amaranth (Amaranthus cruentus) Grain Extracts for the Development of Alginate-Based Active Films. Molecules, 27(18), 5798. https://doi.org/10.3390/molecules27185798






