Wine Closure Performance of Three Common Closure Types: Chemical and Sensory Impact on a Sauvignon Blanc Wine
Abstract
:1. Introduction
2. Results
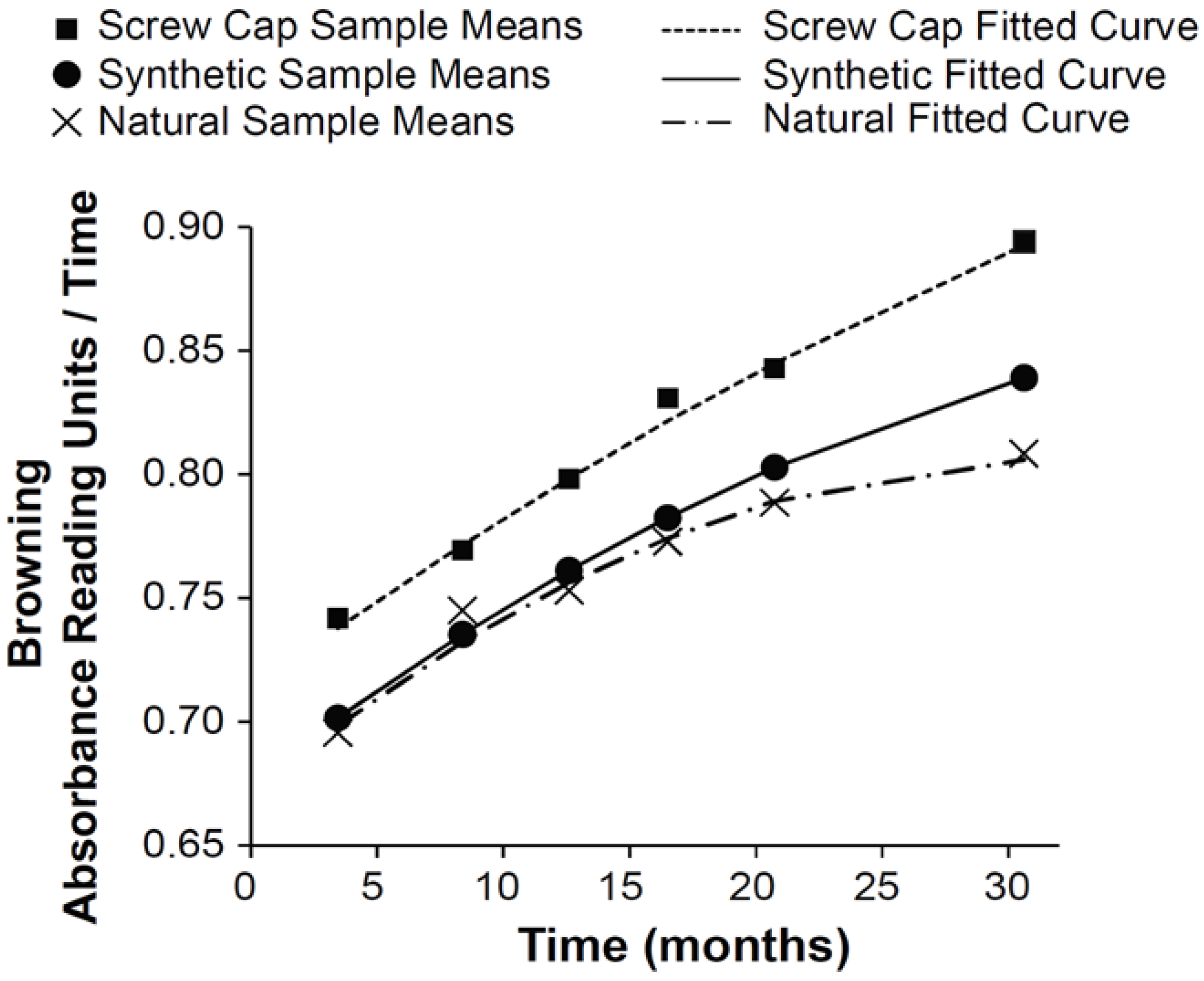
2.1. Browning Trajectories of a Sauvignon Blanc Wine under Three Different Closures
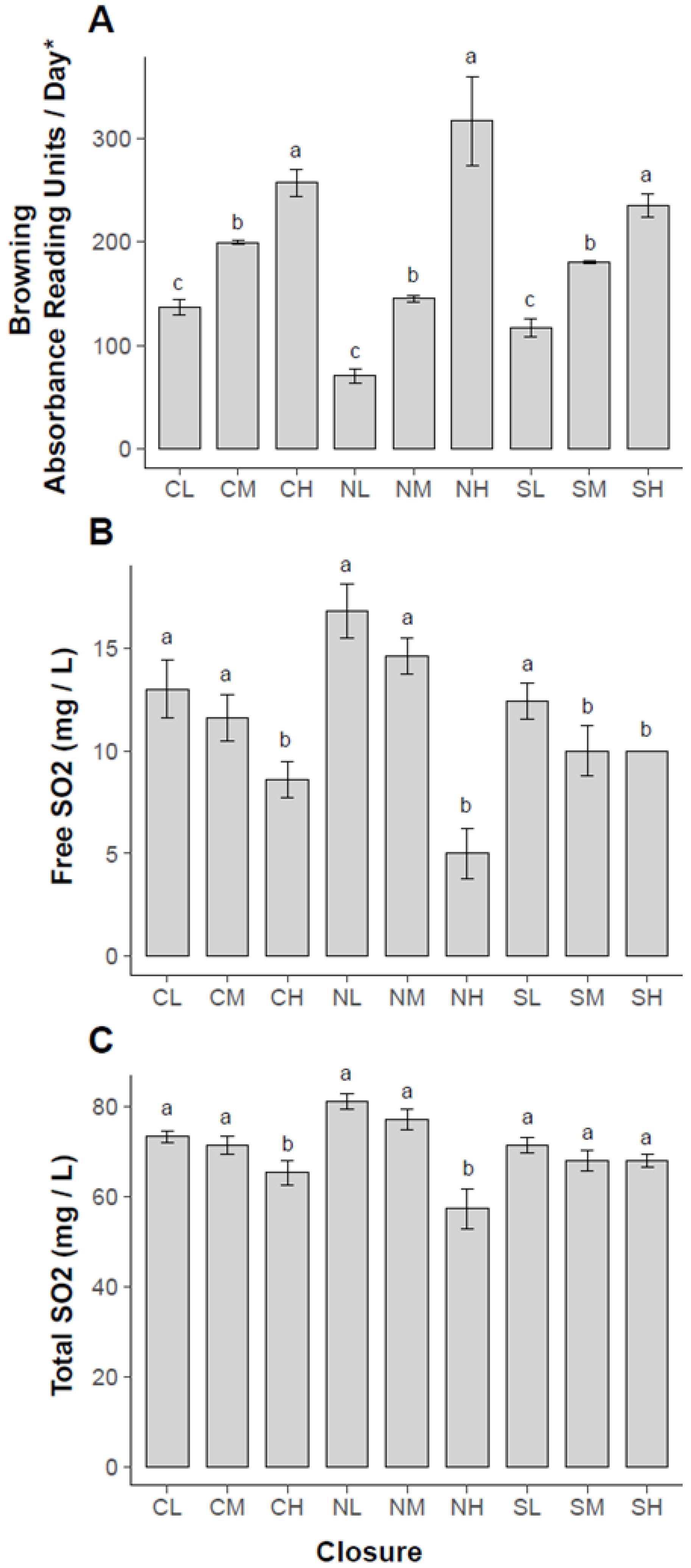
2.2. Results for Degree of Browning, Free and Total Sulfur Dioxide Concentrations and QDA Sensory Analysis
3. Discussion
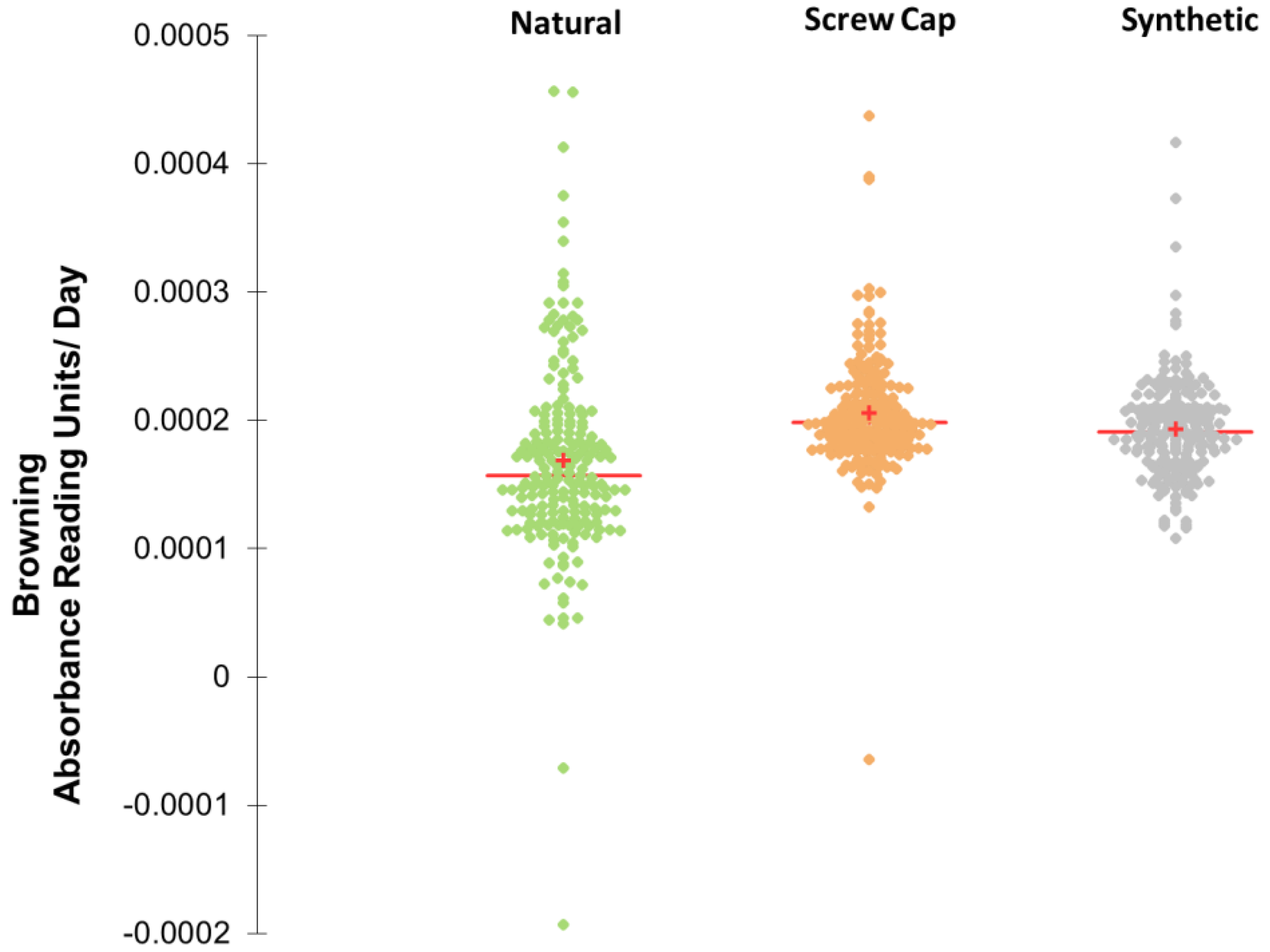
3.1. Browning Trajectories of a Sauvignon Blanc Wine under Three Different Closures and Withing Bottle Variation at 30 Months
3.2. Sensory Evaluation
4. Materials and Methods
4.1. Wine and Winemaking
4.2. Bottles and Closures
4.3. Bottling
4.4. Determination of Browning at 420 nm (a Proxy for Oxidation)
4.5. Sulfur Dioxide Measurements
4.6. Quantitative Descriptive Analysis (QDA)
4.7. Sensory Data Analysis
4.8. Data Analyses of Browning Levels and Free and Total SO2 at 30 Months for Sensory Panel
4.9. Data Analysis of Browning to Estimate Variation within Closure Group
4.10. Data Analysis of Browning Trajectories within Closure Groups
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A. Description for Browning Functions
Appendix B. Panel QDA Definitions of Sensory Terms for the Sauvignon Blanc Wines at 30 Months of Aging
| GOLDEN COLOR * (light-dark)+ | The intensity of a golden color from light to dark. A wine that is light golden may appear pale yellow. A wine that is dark golden may appear similar in color to diluted apple juice or white grape juice. |
| GREEN (light–dark) | The intensity of a green color, like that of lime juice, from light to dark. |
| THICKNESS (thin–thick) | The measure of how thick the wine appears on the side of the glass from thin to thick. A wine that is thin may appear watery. A wine that is thick may appear like syrup or oil. |
| BUBBLES (a little–a lot) | The amount of visible bubbles in the wine from a little to a lot. |
| OAKY | The intensity of an oaky aroma, like that of an oak barrel |
| ALCOHOL | The intensity of an alcohol aroma, like that of rubbing alcohol |
| SOUR | The intensity of a sour aroma, like that of a sour apple, from weak to strong. |
| FRUITY | The intensity of a fruity aroma, like that of peaches, pears, and grapes, from weak to strong. |
| SWEET | The intensity of a sweet aroma, like that of the sweetness from a fruit such as a pear or apple, from weak to strong. |
| SPICE | The intensity of a spice aroma, like that of a spice mixture that may contain cinnamon, nutmeg, and cloves, from weak to strong. |
| GREEN APPLE | The intensity of a green apple flavor from weak to strong. |
| FRUITY | The intensity of a fruity flavor, like that of pears and grapes, from weak to strong. |
| ALCOHOL | The intensity of an alcohol flavor, like that of unflavored liquor, from weak to strong. |
| BITTER | The intensity of a bitter flavor, like that of the pith of a citrus fruit, from weak to strong. |
| SOUR | The intensity of a sour flavor, like that of sour candy, from weak to strong. |
| CITRUS | The intensity of a citrus flavor, like that of a lemon or lime, from weak to strong. |
| SWEET | The intensity of a sweet flavor, like that of the sweetness of an unripe fruit such as a pear or apple, from weak to strong. |
| OAKY | The intensity of an oaky flavor, like that of an oak barrel, from weak to strong. |
| BUTTERY | The intensity of a buttery flavor, like that of warm melted butter, from weak to strong. |
| PUCKERING | The measure of an initial puckering or cringing sensation felt in the mouth, like that from a sour candy or lemon, from weak to strong. |
| TINGLING | The measure of a tingling or numbing sensation felt in the mouth from weak to strong. A weak tingling sensation may feel like water in the mouth. A strong tingling sensation may feel like a carbonated beverage in the mouth. |
| BURNING | The measure of a burning sensation on the tongue, like that from a strong breath mint, from weak to strong. |
| VAPORS IN BACK OF THROAT | The measure of the sensation of vapors in the back of the throat, like those produced from a throat lozenge, from weak to strong. |
| GREEN APPLE | The intensity of a lingering green apple flavor from weak to strong. |
| FRUITY | The intensity of a lingering fruity flavor, like that of pears and grapes, from weak to strong. |
| ALCOHOL | The intensity of a lingering alcohol flavor from weak to strong. |
| BITTER | The intensity of a lingering bitter flavor from weak to strong. |
| SOUR | The intensity of a lingering sour flavor, like that of sour candy, from weak to strong. |
| CITRUS | The intensity of a lingering lemon flavor from weak to strong. |
| OAKY | The intensity of a lingering oaky flavor from weak to strong. |
| DRY | The intensity of a lingering dry or dehydrating effect in the mouth from weak to strong. |
| BURNING * | The intensity of a lingering burning sensation in the mouth from weak to strong. |
| MOUTH COATING | The intensity of a lingering coating or film in the mouth from weak to strong. |
References
- Phillips, C. Wine Business Monthly; Wine Communications Group: Sonoma, CA, USA, 20 June 2011; pp. 28–39. [Google Scholar]
- Phillips, C. Wine Business Monthly; Wine Communications Group: Sonoma, CA, USA, 20 June 2015; pp. 20–27. [Google Scholar]
- Penn, C. Wine Business Monthly; Wine Communications Group: Sonoma, CA, USA, 20 November 2020; pp. 36–41. [Google Scholar]
- Waters, E.J.; Peng, Z.; Pocock, K.F.; Williams, P.J. The Role of Corks in Oxidative Spoilage of White Wines. Aust. J. Grape Wine Res. 1996, 2, 191–197. [Google Scholar] [CrossRef]
- Godden, P.; Francis, L.; Field, J.; Gishen, M.; Coulter, A.; Valente, P.; Høj, P.; Robinson, E. Wine Bottle Closures: Sensory Properties of a Semillon Wine-Performance up to 20 Month Post-Bottling. Wine Ind. J. 2001, 16, 93–112. [Google Scholar]
- Mas, A.; Puig, J.; Llado, N.; Zamora, F. Sealing and Storage Position Effects on Wine Evolution. Food Chem. Toxicol. 2002, 67, 1374–1378. [Google Scholar] [CrossRef]
- Brajkovich, M.; Tibbits, N.; Peron, G.; Lund, C.M.; Dykes, S.I.; Kilmartin, P.A.; Nicolau, L. Effect of Screwcap and Cork Closures on SO2 Levels and Aromas in a Sauvignon Blanc Wine. J. Agric. Food Chem. 2005, 53, 10006–10011. [Google Scholar] [CrossRef]
- Skouroumounis, G.K.; Kwiatkowski, M.J.; Francis, I.L.; Oakey, H.; Capone, D.L.; Duncan, B.; Sefton, M.A.; Waters, E.J. The Impact of Closure Type and Storge Conditions on the Composition, Colour and Flavour Properties of a Riesling and a Wooded Chardonnay Wine during Five Years’ Storage. Aust. J. Grape Wine Res. 2005, 11, 369–377. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Glories, Y. Nondestructive Colorimetric Method to Determine the Oxygen Diffusion Rate through Closures Used in Winemaking. J. Agric. Food Chem. 2005, 53, 6967–6973. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Teissedre, P.L.; Glories, Y. Main Routes of Oxygen Ingress through Different Closures into Wine Bottles. J. Agric. Food Chem. 2007, 55, 5167–5170. [Google Scholar] [CrossRef]
- Kwiatkowski, M.J.; Skouroumounis, G.K.; Lattey, K.A.; Waters, E.J. The Impact of Closures, Including Screw Cap with Three Different Headspace Volumes, on the Composition, Colour and Sensory Properties of a Cabernet Sauvignon Wine during Two Years’ Storage. Aust. J. Grape Wine Res. 2007, 13, 81–94. [Google Scholar] [CrossRef]
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.; Saucier, C.; Darriet, P.; Teissedre, P.-L.; Dubourdieu, D. Impact of Oxygen Dissolved at Bottling and Transmitted through Closures on the Composition and Sensory Properties of a Sauvignon Blanc Wine during Bottle Storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef]
- Brotto, L.; Battistutta, F.; Tat, L.; Comuzzo, P.; Zironi, R. Modified Nondestructive Colorimetric Method to Evaluate the Variability of Oxygen Diffusion Rate through Wine Bottle Closures. J. Agric. Food Chem. 2010, 58, 3567–3572. [Google Scholar] [CrossRef]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.B.; Vidal, S.; Cheynier, V. Sensory Characteristics Changes of Red Grenache Wines Submitted to Different Oxygen Exposures Pre and Post Bottling. Anal. Chim. Acta 2010, 660, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, M.L. Etudes Sur Le Vin, 2nd ed.; Librairie, F., Ed.; Savy: Paris, France, 1875. [Google Scholar]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of Oxygenation on Polyphenol Changes Occurring in the Course of Wine-Making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Wirth, J.; Morel-Salmi, C.; Souquet, J.M.; Dieval, J.B.; Aagaard, O.; Vidal, S.; Fulcrand, H.; Cheynier, V. The Impact of Oxygen Exposure before and after Bottling on the Polyphenolic Composition of Red Wines. Food Chem. 2010, 123, 107–116. [Google Scholar] [CrossRef]
- Singleton, V.L. Oxygen with Phenols and Related Reactions in Musts, Wines, and Model Systems: Observations and Practical Implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar]
- Godden, P.; Francis, I.L.; Field, J.; Gishen, M.; Coulter, A.; Valente, P.; Hoj, P.; Robinson, E. Wine Bottle Closures: Physical Characteristics and Effect on Composition and Sensory Properties of a Semillon Wine. I. Performance up to 20 Months Post-Bottling. Aust. J. Grape Wine Res. 2001, 7, 64–105. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.B.; Aagaard, O.; Waters, E.J. Evolution of 3-Mercaptohexanol, Hydrogen Sulfide, and Methyl Mercaptan during Bottle Storage of Sauvignon Blanc Wines. Effect of Glutathione, Copper, Oxygen Exposure, and Closure-Derived Oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef]
- Dieval, J.B.; Vidal, S.; Aagaard, O. Measuremnet of the Oxygen Transmission Rate of Co-Extruded Wine Bottle Closures Using a Luminescene-Based Technique. Packag. Technol. Sci. 2011, 24, 375–385. [Google Scholar] [CrossRef]
- Lequin, S.; Chassagne, D.; Karbowiak, T.; Simon, J.M.; Paulin, C.; Bellat, J.P. Diffusion of Oxygen in Cork. J. Agric. Food Chem. 2012, 60, 3348–3356. [Google Scholar] [CrossRef]
- He, J.; Zhou, Q.; Peck, J.; Soles, R.; Qian, M.C. The Effect of Wine Closures on Volatile Sulfur and Other Compounds during Post-Bottle Ageing. Flavour. Fragr. J. 2013, 28, 118–128. [Google Scholar] [CrossRef]
- Crochiere, B.Y.G.K. Oxygen Ingress During Bottling/Storage. Prat. Winery Vineyard 2007, 74–84. [Google Scholar]
- Oliveira, V.; Lopes, P.; Cabral, M.; Pereira, H. Kinetics of Oxygen Ingress into Wine Bottles Closed with Natural Cork Stoppers of Different Qualities. Am. J. Enol. Vitic. 2013, 64, 395–399. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Teissedre, P.L.; Glories, Y. Impact of Storage Position on Oxygen Ingress through Different Closures into Wine Bottles. J. Agric. Food Chem. 2006, 54, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Dimkou, E.; Ugliano, M.; Diéval, J.B.; Vidal, S.; Jung, R. Impact of Dissolved Oxygen at Bottling on Sulfur Dioxide and Sensory Properties of a Riesling Wine. Am. J. Enol. Vitic. 2013, 64, 325–332. [Google Scholar] [CrossRef]
- Dimkou, E.; Ugliano, M.; Dieval, J.B.; Vidal, S.; Aagaard, O.; Rauhut, D.; Jung, R. Impact of Headspace Oxygen and Closure on Sulfur Dioxide, Color, and Hydrogen Sulfide Levels in a Riesling Wine. Am. J. Enol. Vitic. 2011, 62, 261–269. [Google Scholar] [CrossRef]
- Cook, R.D.; Weisberg, S. Diagnostics for Heteroscedasticity in Regression. Biometrika 1983, 70, 1–10. [Google Scholar] [CrossRef]
- AWRI Applying Screw Cap Closures. Available online: https://www.awri.com.au/industry_support/winemaking_resources/storage-and-packaging/packaging-operations/applying-screw-cap-closures/#title6 (accessed on 10 January 2022).
- Ugliano, M. Oxygen Contribution to Wine Aroma Evolution during Bottle Aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.L.; Brazinha, C.; Pereira, H.; Crespo, J.G.; Teodoro, O.M.N.D. Permeability of Cork for Water and Ethanol. J. Agric. Food Chem. 2013, 61, 9672–9679. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Lopes, P.; Cabral, M.; Pereira, H. Influence of Cork Defects in the Oxygen Ingress through Wine Stoppers: Insights with X-Ray Tomography. J. Food Eng. 2015, 165, 66–73. [Google Scholar] [CrossRef]
- Granger, J.; Gobinaud, C.; Resende, D.; McGrath, N.; Nicolini, B.; Armbruster, H.; Anquetil, N.; Bonnel, B.; Moret, F.; Zordan, G.; et al. Metallic Screw Capping for Still Wines on BVS Glass Finishes. In Bottling Guide 9; Cetie: Paris, France, 2016; pp. 1–53. [Google Scholar]
- Heymann, H. A Comparison Of Descriptive Analysis Of Vanilla By Two Independently Trained Panels. J. Sens Stud. 1994, 9, 21–32. [Google Scholar] [CrossRef]
- Zamora, M.C.; Guirao, M. Performance Comparison Between Trained Assessors And Wine Experts Using Specific Sensory Attributes. J. Sens Stud. 2004, 19, 530–545. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Thibon, C.; Redon, P.; Loisel, C.; Dubourdieu, D.; Darriet, P. Impact of Closure OTR on the Volatile Compound Composition and Oxidation Aroma Intensity of Sauvignon Blanc Wines during and after 10 Years of Bottle Storage. J. Agric. Food Chem. 2021, 69, 9883–9894. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, C.; van Wyngaard, E.; Šuklje, K.; Silva Ferreira, A.C.; du Toit, W.J. Chemical and Sensory Study on the Evolution of Aromatic and Nonaromatic Compounds during the Progressive Oxidative Storage of a Sauvignon Blanc Wine. J. Agric. Food Chem. 2016, 64, 7979–7993. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.L.; Howe, P.A.; Standing, M.; Danilewicz, J.C. Free, Bound, and Total Sulfur Dioxide (SO2 ) during Oxidation of Wines. Am. J. Enol. Vitic. 2020, 71, 266–277. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Magiatis, P.; Waterhouse, A.L. Measuring Protection of Aromatic Wine Thiols from Oxidation by Competitive Reactions vs Wine Preservatives with Ortho-Quinones. Food Chem. 2014, 163, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.R.; Jofre, V.P.; Fanzone, M.L.; Assof, M.V.; Catania, A.A.; Diaz-Sambueza, A.M.; Heredia, F.J.; Mercado, L.A. Effect of Different Closure Types and Storage Temperatures on the Color and Sensory Characteristics Development of Argentinian Torrontes Riojano White Wines Aged in Bottles. Food Control 2021, 130, 108343. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Thorngate, J.H. Wine Chemistry and Flavor: Looking into the Crystal Glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef] [PubMed]
- Skouroumounis, G.K.; Kwiatkowski, M.; Sefton, M.A.; Gawel, R.; Waters, E.J. In Situ Measurement of White Wine Absorbance in Clear and in Coloured Bottles Using a Modified Laboratory Spectrophotometer. Aust. J. Grape Wine Res. 2003, 9, 138–148. [Google Scholar] [CrossRef]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Sensory Evaluation Practices, 5th ed.; Elsevier/Academic Press Ltd.: San Diego, CA, USA, 2020. [Google Scholar]
- Davidian, M.; Giltinan, D.M. Nonlinear Models for Repeated Measurement Data: An Overview and Update. J. Agric. Biol. Environ. Stat. 2003, 8, 387–419. [Google Scholar] [CrossRef]

| Closure | Fit Index | No Growth | Linear Growth | Quadratic Growth |
|---|---|---|---|---|
| Synthetic (n = 200) | −2lnL | −3101.6 | −5031.9 | −5152.0 |
| AIC | −3095.6 | −5023.9 | −5142.0 | |
| BIC | −3085.7 | −5010.7 | −5125.5 | |
| Natural (n = 199) | −2lnL | −3262.3 | −4099.0 | −4214.7 |
| AIC | −3256.3 | −4091.0 | −4204.7 | |
| BIC | −3246.4 | −4077.8 | −4188.2 | |
| Screw cap (n = 200) | −2lnL | −3011.7 | −5583.4 | −5646.5 |
| AIC | −3005.7 | −5575.4 | −5636.4 | |
| BIC | −2995.8 | −5562.3 | −5620.0 |
| Closure | Parameter | MLE (SE) | 95% CI |
|---|---|---|---|
| Synthetic | β0, browning * at 18 months | 0.79(0.006) | (0.78, 0.80) |
| β0, browning at 30 months | 0.84(0.006) | (0.83, 0.85) | |
| β1, linear change at 18 months | 0.0049 (0.00006) | (0.0048, 0.0050) | |
| β1, linear change at 30 months | 0.0030 (0.00019) | (0.0027, 0.0034) | |
| β2, acceleration rate | −0.00008(6.9 × 106) | (−0.00009, −0.00006) | |
| φb0, between-bottle variance | 0.0073 (0.0007) | ||
| σ2j, within-bottle variance | 0.0003 (0.00002) | ||
| Natural | β0, browning at 18 months | 0.78 (0.005) | (0.77, 0.79) |
| β0, browning at 30 months | 0.81(0.005) | (0.80, 0.82) | |
| β1, linear change at 18 months | 0.0037(0.00010) | (0.0035, 0.0039) | |
| β1, linear change at 30 months | 0.0006(0.00031) | (0.00001, 0.00124) | |
| β2, acceleration rate | −0.00013 (0.00001) | (−0.00015, −0.00010) | |
| φb0, between-bottle variance | 0.0052 (0.0005) | ||
| σ2, within-bottle variance | 0.0009 (0.00004) | ||
| Screw cap | β0, browning at 18 months | 0.83 (0.005) | (0.82, 0.84) |
| β0, browning at 30 months | 0.89 (0.005) | (0.88, 0.90) | |
| β1, linear change at 18 months | 0.0056 (0.00005) | (0.0055, 0.0057) | |
| β1, linear change at 30 months | 0.0046 (0.00015) | (0.0043, 0.0049) | |
| β2, acceleration rate | −0.00005 (5.6 × 106) | (−0.00006, −0.00003) | |
| φb0, between-bottle variance | 0.0049 (0.0005) | ||
| σ2, within-bottle variance | 0.0002 (1.0 × 105) |
| Closure Type and Oxidation/Browning Level |
|---|
| Screw cap Low O2 (CL) |
| Screw cap Middle O2 (CM) |
| Screw cap High O2 (CH) |
| Natural cork Low O2 (NL) Natural cork Middle O2 (NM) Natural cork High O2 (NH) |
| Synthetic cork Low O2 (SL) |
| Synthetic cork Middle O2 (SM) |
| Synthetic cork High O2 (SH) |
| Screw Cap | Synthetic Cork | Natural Cork | |
|---|---|---|---|
| HS (cm) | 4.8 | 1.5 | 1.3 |
| S.D. | 0.1 | 0.2 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantu, A.; Guernsey, J.; Anderson, M.; Blozis, S.; Bleibaum, R.; Cyrot, D.; Waterhouse, A.L. Wine Closure Performance of Three Common Closure Types: Chemical and Sensory Impact on a Sauvignon Blanc Wine. Molecules 2022, 27, 5881. https://doi.org/10.3390/molecules27185881
Cantu A, Guernsey J, Anderson M, Blozis S, Bleibaum R, Cyrot D, Waterhouse AL. Wine Closure Performance of Three Common Closure Types: Chemical and Sensory Impact on a Sauvignon Blanc Wine. Molecules. 2022; 27(18):5881. https://doi.org/10.3390/molecules27185881
Chicago/Turabian StyleCantu, Annegret, Jillian Guernsey, Mauri Anderson, Shelley Blozis, Rebecca Bleibaum, Danielle Cyrot, and Andrew L. Waterhouse. 2022. "Wine Closure Performance of Three Common Closure Types: Chemical and Sensory Impact on a Sauvignon Blanc Wine" Molecules 27, no. 18: 5881. https://doi.org/10.3390/molecules27185881
APA StyleCantu, A., Guernsey, J., Anderson, M., Blozis, S., Bleibaum, R., Cyrot, D., & Waterhouse, A. L. (2022). Wine Closure Performance of Three Common Closure Types: Chemical and Sensory Impact on a Sauvignon Blanc Wine. Molecules, 27(18), 5881. https://doi.org/10.3390/molecules27185881






