Recombinant Human Annexin A5 Alleviated Traumatic-Brain-Injury Induced Intestinal Injury by Regulating the Nrf2/HO-1/HMGB1 Pathway
Abstract
:1. Introduction
2. Results
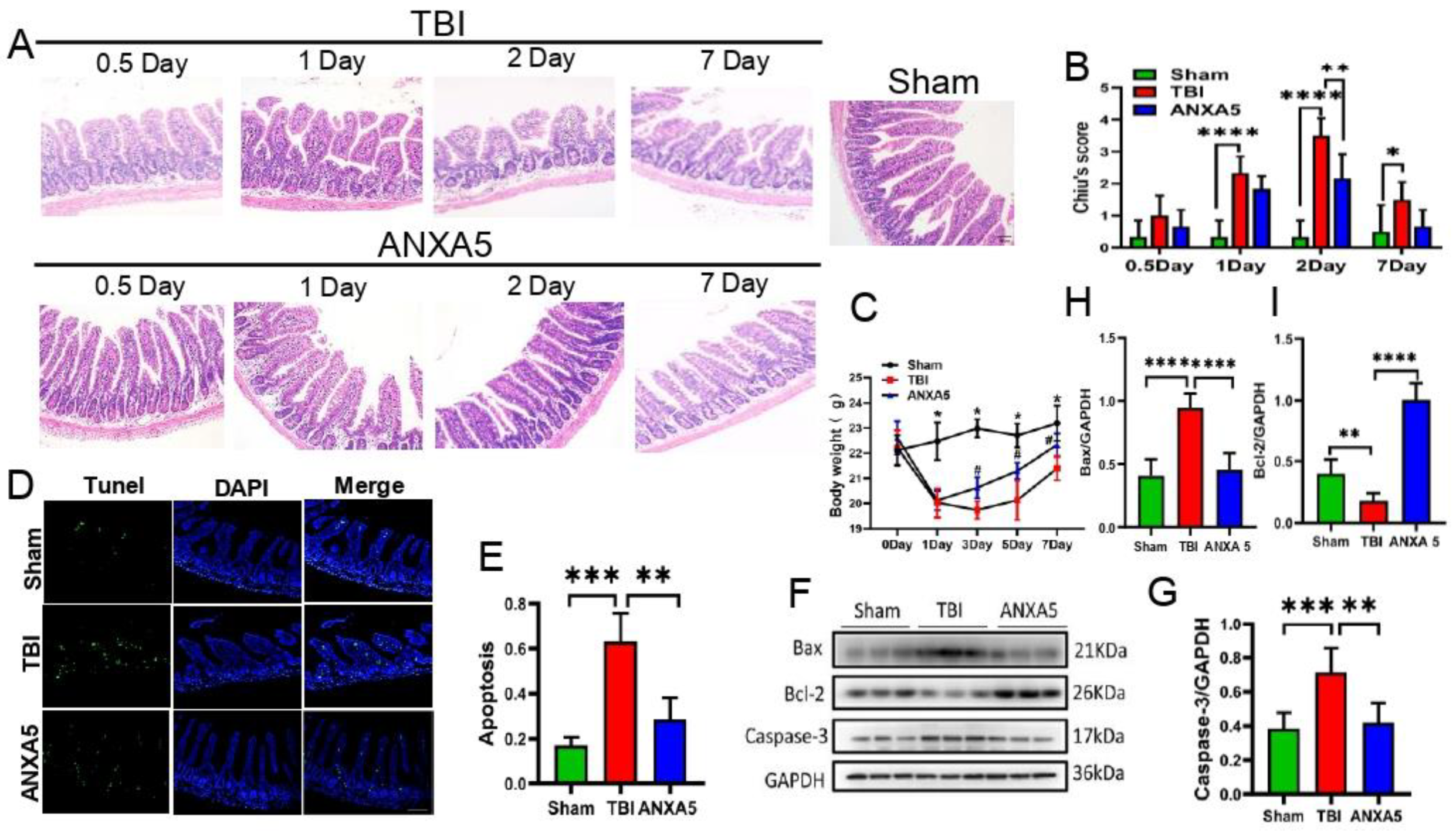
2.1. ANXA5 Reduced Intestinal Lesions Induced by TBI
2.2. ANXA5 Increased Mouse Body Weight after TBI
2.3. ANXA5 Inhibited TBI-Induced Intestinal Apoptosis
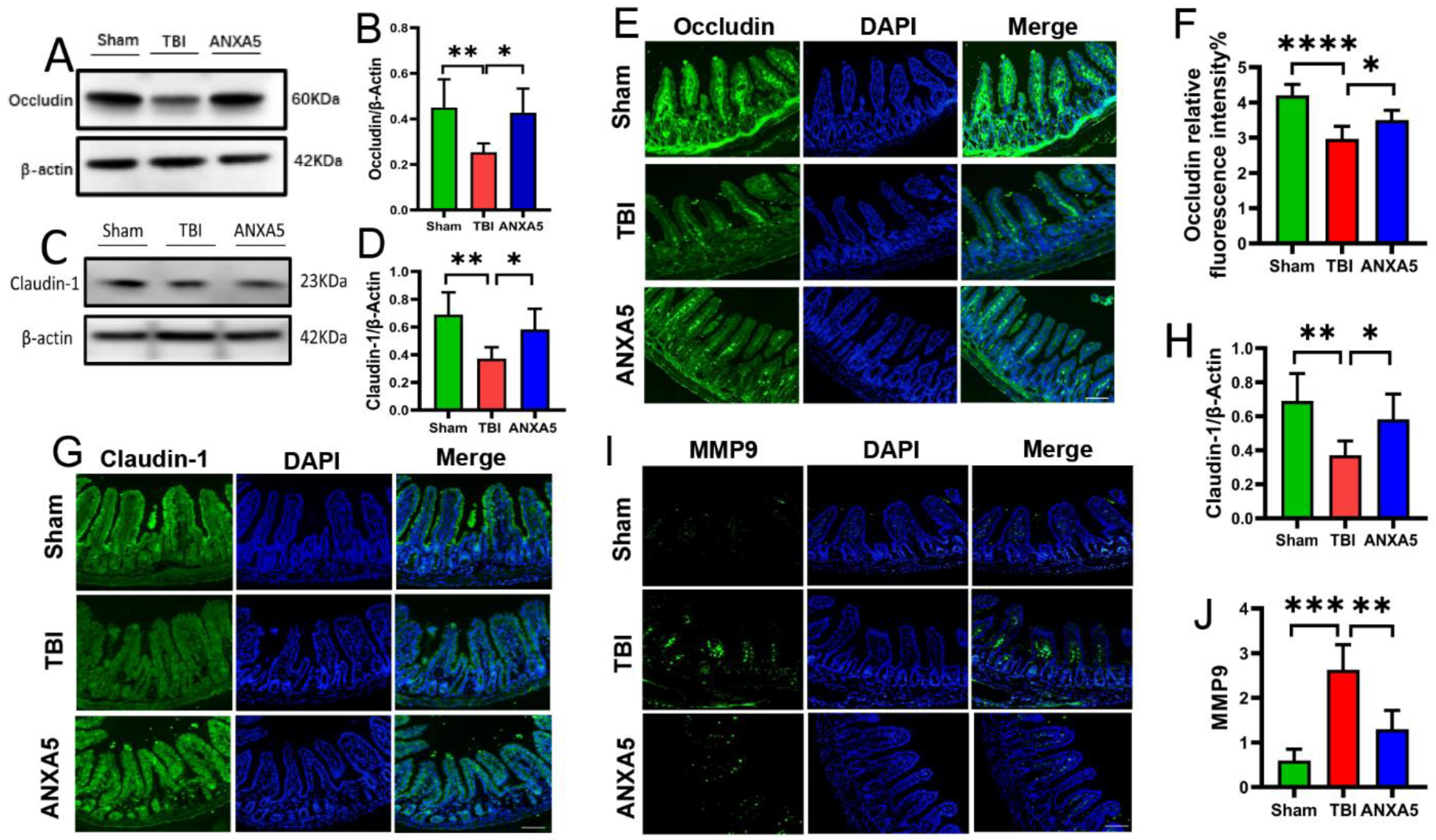
2.4. ANXA5 Alleviated TBI-Induced Intestinal Mucosal Damage
2.5. ANXA5 Mitigated Intestinal MMP-9 after TBI
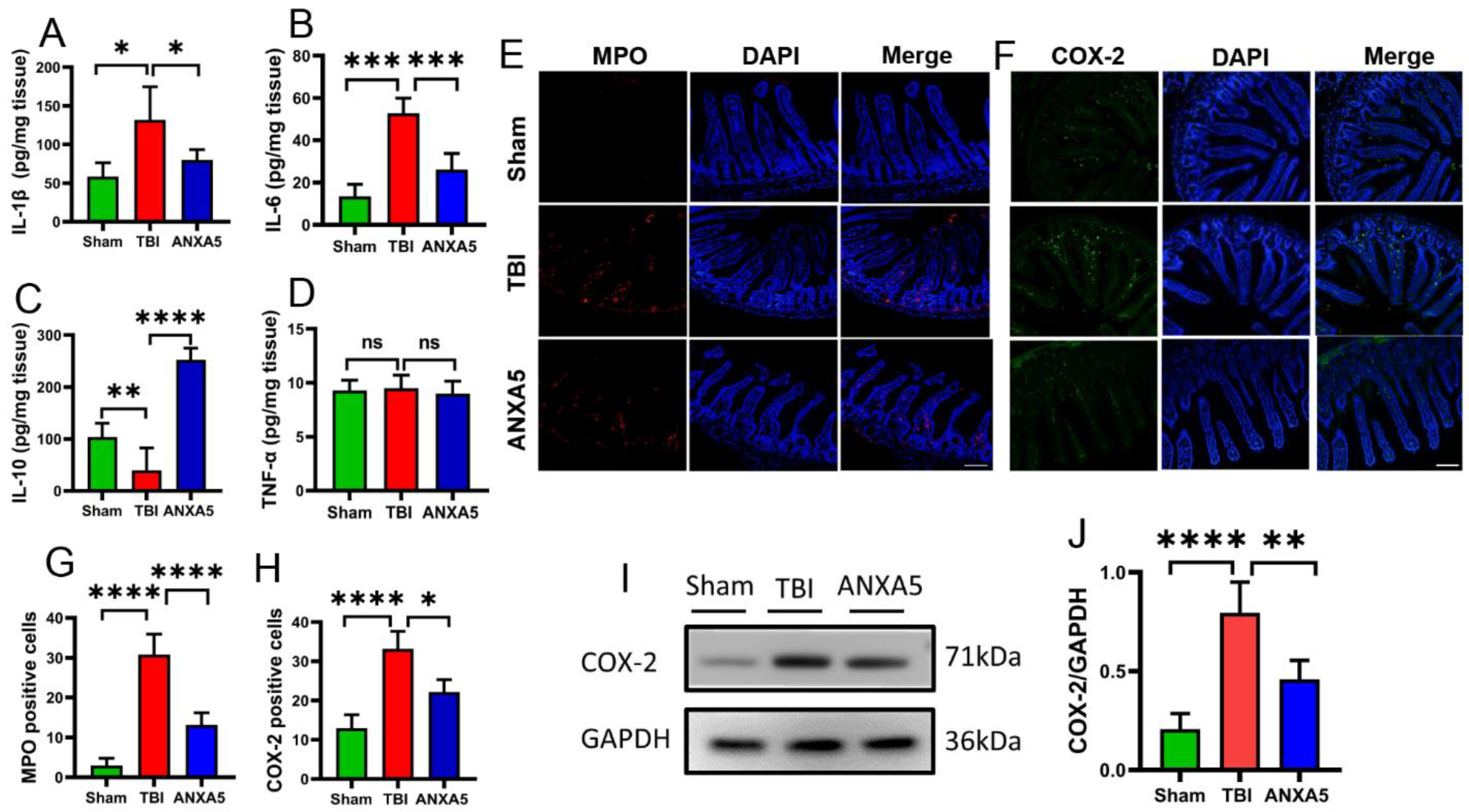
2.6. ANXA5 Increased Anti-Inflammatory Cytokines and Reduced Pro-Inflammatory Cytokines after TBI
2.7. ANXA5 Inhibited Inflammatory Infiltration after TBI
2.8. ANXA5 Attenuated Intestinal Oxidative Stress after TBI
2.9. ANXA5 Reduced HMGB1 and Increased HO-1 and Nrf2 after TBI
3. Materials and Methods
3.1. Mice
3.2. Fluid Percussion Injury Model
3.3. Hematoxylin and Eosin Staining
3.4. Lipid Peroxidation Assay
3.5. Immunofluorescence Staining
3.6. Enzyme-Linked Immunosorbent Assay
3.7. TUNEL Staining
3.8. Western Blotting
3.9. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gao, G.; Wu, X.; Feng, J.; Hui, J.; Mao, Q.; Lecky, F.; Lingsma, H.; Maas, A.I.R.; Jiang, J. Clinical characteristics and outcomes in patients with traumatic brain injury in China: A prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020, 19, 670–677. [Google Scholar] [CrossRef]
- Khan, K.; Saeed, S.; Ramcharan, A.; Gray, S. A case series of closed head trauma with pituitary stalk disruption resulting in hypopituitarism. Int. J. Surg. Case Rep. 2018, 43, 69–71. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Zhu, Y.; Wei, A.; Du, J.; Wang, Y.; Zheng, P.; Tu, M.; Wang, H.; Wen, L.; Yang, X. Traumatic Brain Injury Induces Gastrointestinal Dysfunction and Dysbiosis of Gut Microbiota Accompanied by Alterations of Bile Acid Profile. J. Neurotrauma. 2022, 39, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.L.; Smith, A.D.; Desai, N.; Cheung, L.; Hanscom, M.; Stoica, B.A.; Loane, D.J.; Shea-Donohue, T.; Faden, A.I. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun. 2017, 66, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, Z.; Xu, X.; Chao, H.; Lin, C.; Li, Z.; Liu, Y.; Wang, X.; You, Y.; Liu, N.; et al. Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J. Neurotrauma. 2017, 34, 2119–2131. [Google Scholar] [CrossRef]
- Royes, L.F.F.; Gomez-Pinilla, F. Making sense of gut feelings in the traumatic brain injury pathogenesis. Neurosci. Biobehav. Rev. 2019, 102, 345–361. [Google Scholar] [CrossRef]
- Mazarati, A.; Medel-Matus, J.; Shin, D.; Jacobs, J.P.; Sankar, R. Disruption of intestinal barrier and endotoxemia after traumatic brain injury: Implications for post-traumatic epilepsy. Epilepsia 2021, 62, 1472–1481. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The antioxidant responsive element. Activation by oxidative stress and identifi-cation of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, L.; Jiang, X.; Cheng, S.; Zhang, J.; Qin, X.; Qin, Z.; Chen, C.; Zou, Z. Stabilization of Nrf2 leading to HO-1 activation protects against zinc oxide nanoparticles-induced endothe-lial cell death. Nanotoxicology 2021, 15, 779–797. [Google Scholar] [CrossRef]
- Jimenez, M.T.B.; Frenis, K.; Hahad, O.; Steven, S.; Cohen, G.; Cuadrado, A.; Münzel, T.; Daiber, A. Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressors. Free Radic. Biol. Med. 2022, 187, 72–91. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Ji, Y.; Hu, Q.; Yan, W.; Chen, G.; Yin, H. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine 2008, 44, 135–140. [Google Scholar] [CrossRef]
- van Genderen, H.O.; Kenis, H.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P. Extracellular annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta 2008, 1783, 953–963. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhou, R. Loss of annexin A5 expression attenuates the lipopolysaccharide-induced inflammatory re-sponse of rat alveolar macrophages. Cell Biol. Int. 2020, 44, 391–401. [Google Scholar] [CrossRef]
- Beattie, G.; Cohan, C.; Miraflor, E.; Brigode, W.; Victorino, G.P. Protective effect of phosphatidylserine blockade in sepsis induced organ dysfunction. Surgery 2019, 166, 844–848. [Google Scholar] [CrossRef]
- Danisik, H.; Bogdanova, N.; Markoff, A. Micromolar Zinc in Annexin A5 Anticoagulation as a Potential Remedy for RPRGL3- Associated Recurrent Pregnancy Loss. Reprod. Sci. 2019, 26, 348–356. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, J.H.; Choi, E.J.; Kim, Y.S.; Lee, E.J.; Jung, I.D.; Han, H.D.; Wu, T.C.; Hung, C.F.; Kang, T.H.; et al. Annexin A5 increases survival in murine sepsis model by inhibiting HMGB1-mediated pro-inflammation and coagulation. Mol. Med. 2016, 22, 424–436. [Google Scholar] [CrossRef] [Green Version]
- de Jong, R.C.M.; Pluijmert, N.J.; de Vries, M.R.; Pettersson, K.; Atsma, D.E.; Jukema, J.W.; Quax, P.H.A. Annexin A5 reduces infarct size and improves cardiac function after myocardial ischemia-reperfusion injury by suppression of the cardiac inflammatory response. Sci. Rep. 2018, 8, 6753. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.S.; Park, N.; Kwon, Y.J.; Ye, D.J.; Shin, S.; Chun, Y.J. Annexin A5 suppresses cyclooxygenase-2 expression by downregulating the protein kinase C-zeta-nuclear factor-kappaB signaling pathway in prostate cancer cells. Oncotarget 2017, 8, 74263–74275. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal Mucosal Lesion in Low-Flow States. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, T.; Han, X.; Wan, J.; Jiang, C.; Chen, W.; Lu, H.; Yang, Q.; Wang, J. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. J. Cereb. Blood Flow Metab. 2017, 37, 39–51. [Google Scholar] [CrossRef]
- Gu, J.; Bao, Y.; Chen, J.; Huang, C.; Zhang, X.; Jiang, R.; Liu, Q.; Liu, Y.; Xu, X.; Shi, W. The Expression of NP847 and Sox2 after TBI and Its Influence on NSCs. Front. Cell. Neurosci. 2016, 10, 282. [Google Scholar] [CrossRef]
- Sundman, M.H.; Chen, N.-K.; Subbian, V.; Chou, Y.-H. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav. Immun. 2017, 66, 31–44. [Google Scholar] [CrossRef]
- Hang, C.-H.; Shi, J.-X.; Li, J.-S.; Wu, W.; Yin, H.-X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J. Gastroenterol. 2003, 9, 2776–2781. [Google Scholar] [CrossRef]
- Bansal, V.; Costantini, T.; Ryu, S.Y.; Peterson, C.; Loomis, W.; Putnam, J.; Elicieri, B.; Baird, A.; Coimbra, R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J. Trauma. 2010, 68, 1059–1064. [Google Scholar] [CrossRef]
- Chai, S.; Liu, K.; Feng, W.; Liu, T.; Wang, Q.; Zhou, R.; Chen, S.; Wang, L.; Chen, G.; Ming, T.; et al. Activation of G protein–coupled estrogen receptor protects intestine from ischemia/reperfusion injury in mice by protecting the crypt cell proliferation. Clin. Sci. 2019, 133, 449–464. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Engers, J.; Haque, M.; King, S.; Al-Omari, D.; Ma, T.Y. Matrix Metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-kappaB activation. PLoS ONE 2021, 16, e0249544. [Google Scholar] [CrossRef]
- Tóth, Š.; Jonecová, Z.; Čurgali, K.; Maretta, M.; Šoltés, J.; Švaňa, M.; Kalpadikis, T.; Caprnda, M.; Adamek, M.; Rodrigo, L.; et al. Quercetin attenuates the ischemia reperfusion induced COX-2 and MPO expression in the small intestine mucosa. Biomed. Pharmacother. 2017, 95, 346–354. [Google Scholar] [CrossRef]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ling, Y.; Deng, Q.; Qiu, Y.; Shen, J.; Lai, H.; Chen, Z.; Huang, C.; Liang, L.; Li, X.; et al. HMGB1-mediated neutrophil extracellular trap formation exacerbates intestinal ische-mia/reperfusion-induced acute lung injury. J. Immunol. 2022, 208, 968–978. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.-M.; Wu, L.-Y.; Liu, G.-J.; Xu, W.-D.; Zhang, X.-S.; Gao, Y.-Y.; Tao, T.; Zhou, Y.; Lu, Y.; et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J. Neuroinflammation 2020, 17, 188. [Google Scholar] [CrossRef]
- Du, D.; Tang, W.; Zhou, C.; Sun, X.; Wei, Z.; Zhong, J.; Huang, Z. Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neu-rological deficits after traumatic brain injury. Oxid. Med. Cell. Longev. 2021, 2021, 5816837. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Zhang, X.; Wu, X.; Zhao, Y.; Ren, J. STING-dependent induction of lipid peroxidation mediates intestinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2021, 163, 135–140. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- do Nascimento, L.C.P.; Neto, J.P.R.C.; de Andrade Braga, V.; Lagranha, C.J.; de Brito Alves, J.L. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxi-dative stress along the gut-kidney axis in rat offspring. Life Sci. 2020, 261, 118367. [Google Scholar] [CrossRef]
- Li, W.; Kong, A.-N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Z.; Li, R.; Wang, S.; Wang, W.; Tang, M.; Zhang, W. The role of ANXA5 in DBP-induced oxidative stress through ERK/Nrf2 pathway. Environ. Toxicol. Pharmacol. 2019, 72, 103236. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, C.; Wang, G.; Xu, R.; Zhu, Y. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia–reperfusion injury in mice. Agents Actions 2015, 64, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schwacha, M.G.; Chaudry, I.H.; Choudhry, M.A. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2− production in a two-hit model of alcohol intoxication and burn injury. J. Immunol. 2008, 180, 6933–6940. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Gao, Y.; Li, T.; Li, F.; Peng, R.; Wang, C.; Zhang, S.; Zhang, J. Recombinant Human Annexin A5 Alleviated Traumatic-Brain-Injury Induced Intestinal Injury by Regulating the Nrf2/HO-1/HMGB1 Pathway. Molecules 2022, 27, 5755. https://doi.org/10.3390/molecules27185755
Zhang H, Gao Y, Li T, Li F, Peng R, Wang C, Zhang S, Zhang J. Recombinant Human Annexin A5 Alleviated Traumatic-Brain-Injury Induced Intestinal Injury by Regulating the Nrf2/HO-1/HMGB1 Pathway. Molecules. 2022; 27(18):5755. https://doi.org/10.3390/molecules27185755
Chicago/Turabian StyleZhang, Hejun, Yalong Gao, Tuo Li, Fanjian Li, Ruilong Peng, Cong Wang, Shu Zhang, and Jianning Zhang. 2022. "Recombinant Human Annexin A5 Alleviated Traumatic-Brain-Injury Induced Intestinal Injury by Regulating the Nrf2/HO-1/HMGB1 Pathway" Molecules 27, no. 18: 5755. https://doi.org/10.3390/molecules27185755
APA StyleZhang, H., Gao, Y., Li, T., Li, F., Peng, R., Wang, C., Zhang, S., & Zhang, J. (2022). Recombinant Human Annexin A5 Alleviated Traumatic-Brain-Injury Induced Intestinal Injury by Regulating the Nrf2/HO-1/HMGB1 Pathway. Molecules, 27(18), 5755. https://doi.org/10.3390/molecules27185755





