A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera
Abstract
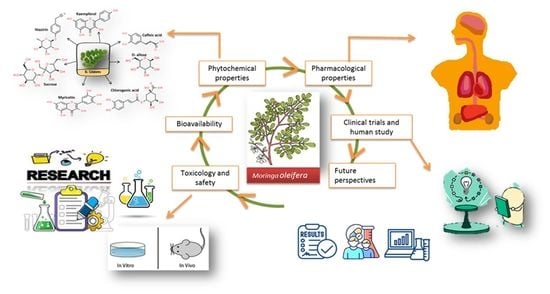
:1. Introduction
2. Methods of Literature Search
3. Toxicology and Safety
4. Origin, Geographical Distribution, Plant Description and Growth Condition of M. oleifera
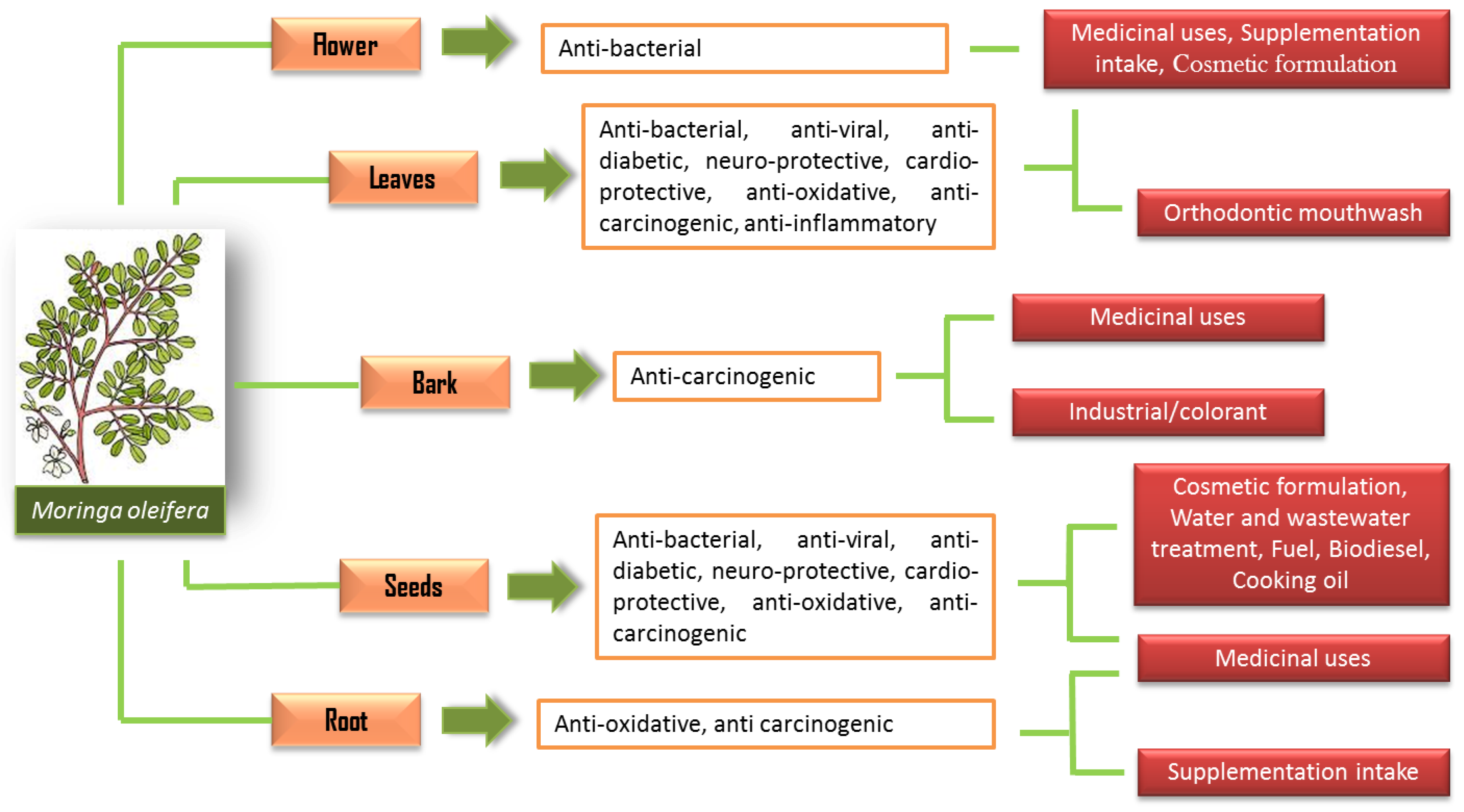
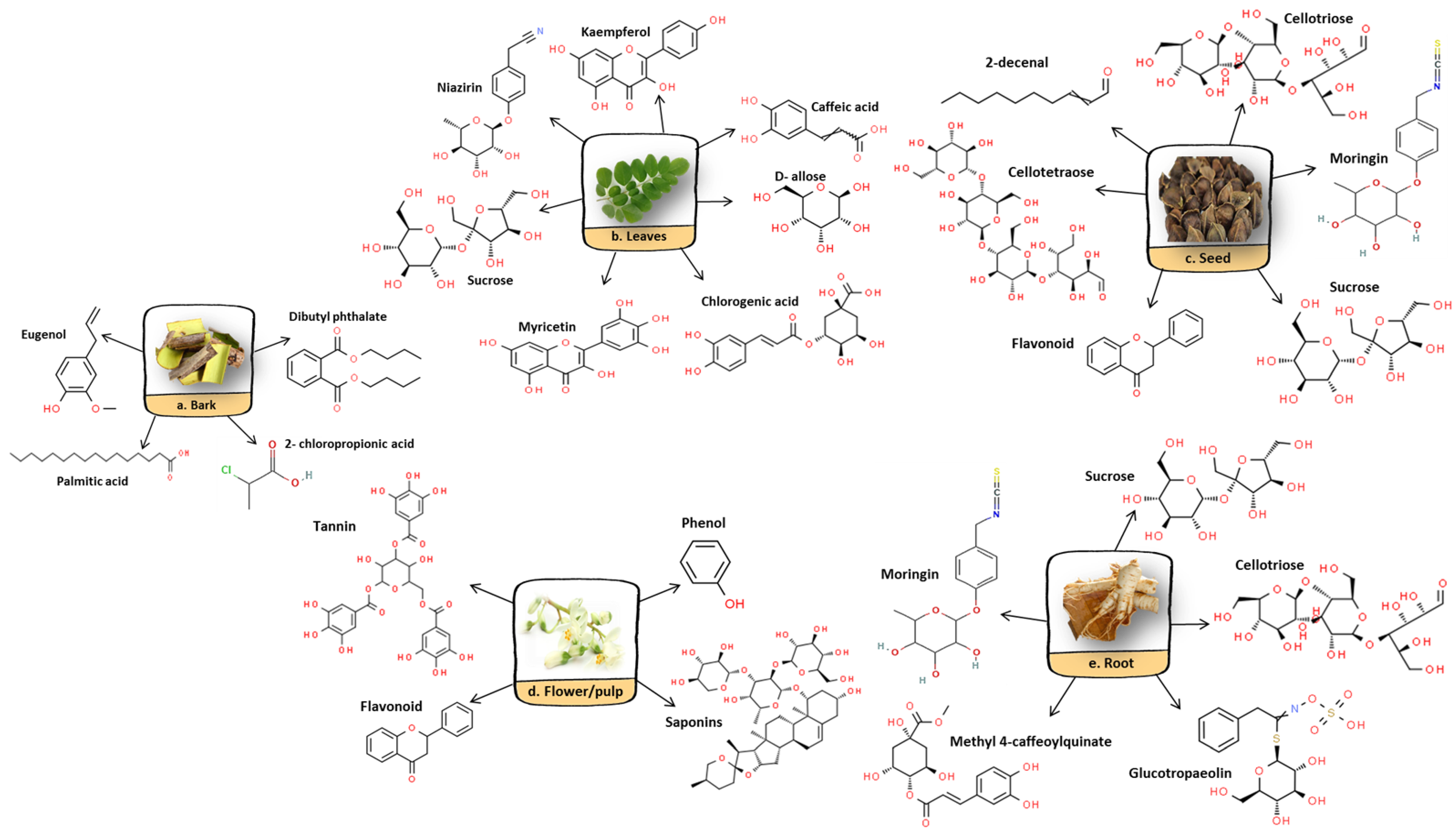
5. Phytochemical Properties of M. oleifera
6. Absorption, Metabolism and Excretion
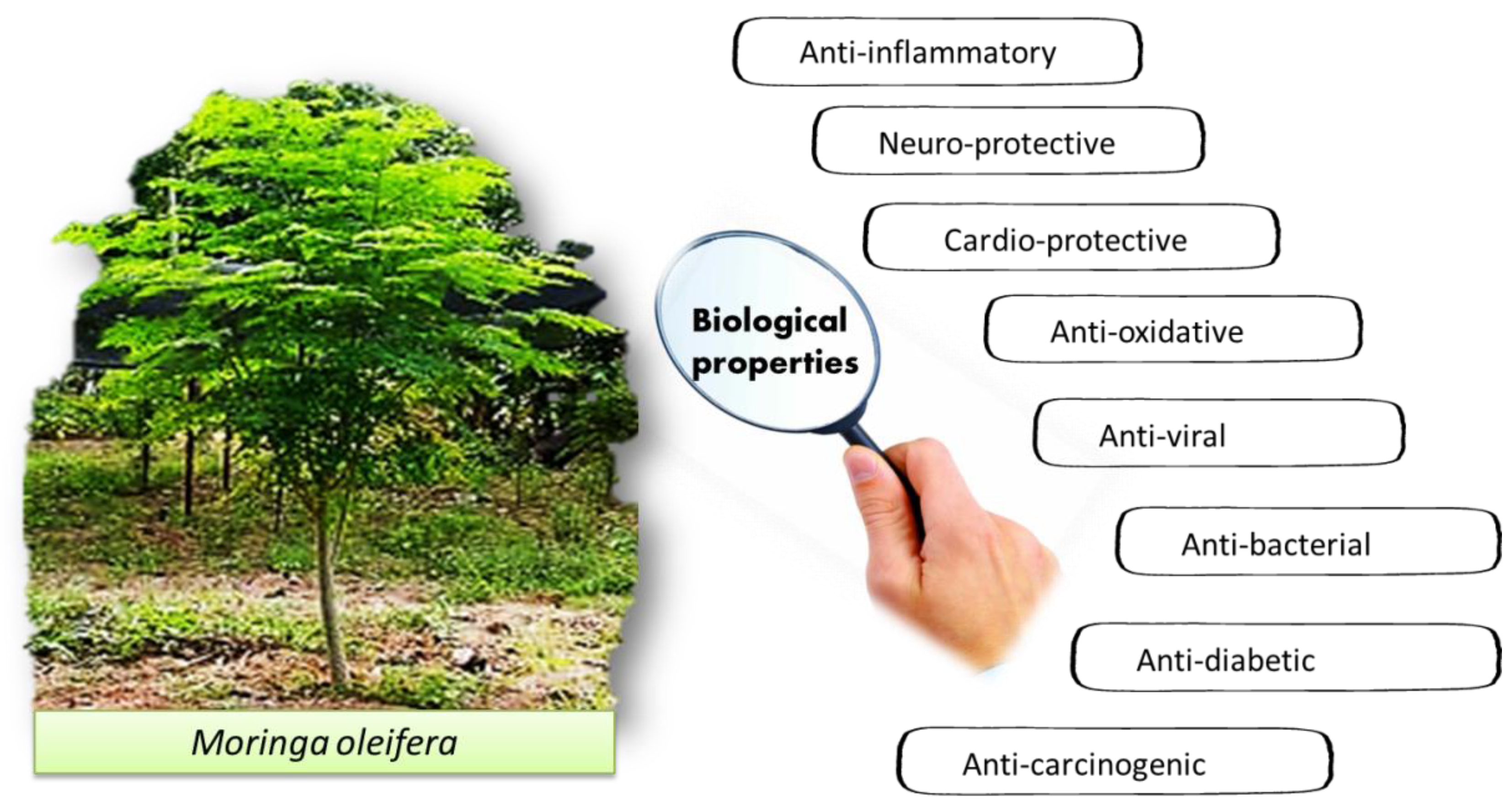
7. Pharmacological Properties of M. oleifera
7.1. Anti-Oxidative Effects of M. oleifera
7.2. Antiviral Effects of M. oleifera
7.3. Antibacterial Effects of M. oleifera
7.4. Anti-Diabetic Effects of M. oleifera
7.5. Anti-Carcinogenic Effects of M. oleifera
7.6. Cardio-Protective Effects of M. oleifera
8. Clinical Trials and Human Studies
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luetragoon, T.; Sranujit, R.P.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit the Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules 2020, 25, 191. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic Plants: Antioxidant Capacity and Polyphenol Characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Mahmood, K.T.; Mugal, T.; Ul Haq, I. Moringa oleifera: A natural gift—A review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef]
- Asare, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; Otu-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 139, 265–272. [Google Scholar] [CrossRef]
- Araújo, L.C.C.; Aguiar, J.S.; Napoleão, T.H.; Mota, F.V.B.; Barros, A.L.S.; Moura, M.C.; Coriolano, M.C.; Coelho, L.C.B.B.; Silva, T.G.; Paiva, P.M.G. Evaluation of cytotoxic and anti-inflammatory activities of extracts and lectins from Moringa oleifera seeds. PLoS ONE 2013, 8, e81973. [Google Scholar] [CrossRef]
- Nworu, S.C.; Okoye, L.E.; Ezeifeka, O.G. Esimone Extracts of Moringa oleifera Lam. showing inhibitory activity against early steps in the infectivity of HIV-1 lentiviral particles in a viral vector-based screening. Afr. J. Biotechnol. 2013, 12, 4866–4873. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Alam, S.S.; Fatima, M.; Altaf, I.; Khan, F.; Afzal, A. Comparative anti-influenza potential of Moringa oleifera leaves and amantadine In vitro. Pak. Postgrad. Med. J. 2017, 28, 127–131. [Google Scholar]
- Aldakheel, R.K.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Gondal, M.A.; Mostafa, A.; Baykal, A. Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Jafarain, A.; Asghari, G.; Ghassami, E. Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells. Adv. Biomed. Res. 2014, 3, 194–199. [Google Scholar] [CrossRef]
- Adedapo, A.; Mogbojuri, O.; Emikpe, B. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J. Med. Plant Res. 2009, 3, 586–591. [Google Scholar]
- Osman, H.; Shayoub, M.; Babiker, E.; Faiza, A.; Ahmed, M.M.; Osman, B.; Ali, M.E.; Taha, K. Assessment of acute toxicity and LD50 of Moringa oleifera ethanolic leave extract in albino rats and rabbits. J. Med. Biol. Sci. Res. 2015, 1, 38–43. [Google Scholar]
- Okumu, M.O.; Mbaria, J.M.; Kanja, L.W.; Gakuya, D.W.; Kiama, S.G.; Ochola, F.O.; Okumu, P.O. Acute toxicity of the aqueous-methanolic Moringa oleifera (Lam) leaf extract on female Wistar albino rats. Int. J. Basic Clin. Pharmacol. 2016, 5, 1856–1861. [Google Scholar] [CrossRef]
- Olayemi, A.T.; Olanrewaju, M.J.; Oloruntoba, A.C. Toxicological evaluation of Moringa oleifera Lam seeds and leaves in Wistar rats. Pharmacogn. Commun. 2016, 6, 100–111. [Google Scholar] [CrossRef]
- Moodley, I. Acute toxicity of Moringa oleifera leaf powder in rats. J. Med. Plants Stud. 2017, 5, 180–185. [Google Scholar]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Saleem, M.; Akhtar, M.F.; Baig, M.M.F.A.; Rasul, A. HPLC analysis, cytotoxicity, and safety study of Moringa oleifera Lam. (wild type) leaf extract. J. Food Biochem. 2020, 44, e13400. [Google Scholar] [CrossRef]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Ind. Crop. Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Palada, M.C. Moringa (Moringa oleifera Lam.): A versatile tree crop with horticultural hotential in the subtropical United States. HortScience 1996, 31, 794–797. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of Drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Sudeshraj, R.; Sureshkumar, S. Phytonutrients: Moringa oleifera leaf extracts an incredible health super food supplement. J. Pharm. Innov. 2019, 8, 29–33. [Google Scholar]
- Prabu, S.L.; Umamaheswari, A.; Puratchikody, A. Phytopharmacological potential of the natural gift Moringa oleifera Lam and its therapeutic application: An overview. Asian Pac. J. Trop. Med. 2019, 12, 485–498. [Google Scholar] [CrossRef]
- Mall, T.; Tripathi, S. Moringa oleifera: A Miracle Multipurpose Potential Plant in Health Management and Climate Change Mitigation from Bahraich (UP) India—An Overview. Int. J. Curr. Res. Biosci. Plant Biol. 2018, 4, 52–66. [Google Scholar] [CrossRef]
- Hamed, H.S.; El-Sayed, Y.S. Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.). Fish Physiol. Biochem. 2018, 45, 71–82. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; de Abreu Oliveira, J.T.; de Fátima Urano Carvalho, A. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008, 21, 431–437. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Plants Food Hum. Nutri. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Gallaher, D.D.; Gallaher, C.M.; Natukunda, S.; Schoenfuss, T.C.; Mupere, E.; Cusick, S.E. Iron Bioavailability from Moringa oleifera leaves is very low. FASEB J. 2017, 31, 786. [Google Scholar] [CrossRef]
- Idohou-Dossou, N.; Diouf, A.; Gueye, A.; Guiro, A.; Wade, S. Impact of daily consumption of Moringa (Moringa oleifera) dry leaf powder on iron status of Senegalese lactating women. Afr. J. Food, Agric. Nutr. Dev. 2011, 11, 4985–4999. [Google Scholar] [CrossRef]
- Brown, R.C.; Klein, A.; Simmons, W.K.; Hurrell, R.F. The influence of jamaican herb teas and other polyphenol-containing beverages on iron absorption in the rat. Nutr. Res. 1990, 10, 343–353. [Google Scholar] [CrossRef]
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of oral administration of Moringa oleifera Lam on glucose tol-erance in Goto-Kakizaki and Wistar rats. J. Clin. Biochem. Nutri. 2007, 40, 229–233. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 84, 289–295. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and phytic acid contribute to the low iron bioavail-ability from common beans in young women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Thurber, M.D.; Fahey, J.W. Adoption of Moringa oleifera to Combat Under-Nutrition Viewed Through the Lens of the “Diffusion of Innovations” Theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Pankaja, N.; Prakash, J. Availability of calcium from kilkeerai (Amaranthus tricolor) and drumstick (Moringa oleifera) greens in weanling rats. Die Nahr. 1994, 38, 199–203. [Google Scholar] [CrossRef]
- Akter, S.; Netzel, M.; Tinggi, U.; Fletcher, M.; Osborne, S.; Sultanbawa, Y. Interactions Between Phytochemicals and Minerals in Terminalia ferdinandiana and Implications for Mineral Bioavailability. Front. Nutr. 2020, 7, 598219. [Google Scholar] [CrossRef]
- Kumar, H.D. Management of nutritional and health needs of malnourished and vegetarian people in India. In Complementary and Alternative Approaches to Biomedicine; Springer Nature: Berlin/Heidelberg, Germany, 2004; pp. 311–312. [Google Scholar]
- Lyons, G.; Gondwe, C.; Banuelos, G.; Mendoza, C.; Haug, A.; Christophersen, O.; Ebert, A. Drumstick tree (Moringa oleifera) leaves as a source of dietary selenium, sulphur and pro-vitamin A. Acta Hortic. 2017, 1158, 287–292. [Google Scholar] [CrossRef]
- Nambiar, V.S.; Seshadri, S. Bioavailability trials of β-carotene from fresh and dehydrated drumstick leaves (Moringa oleifera) in a rat model. Plant Foods Hum. Nutr. 2001, 56, 83–95. [Google Scholar] [CrossRef]
- Pullakhandam, R.; Failla, M.L. Micellarization and Intestinal Cell Uptake of β-Carotene and Lutein from Drumstick (Moringa oleifera) Leaves. J. Med. Food 2007, 10, 252–257. [Google Scholar] [CrossRef]
- Mune, M.A.M.; Nyobe, E.C.; Bassogog, C.B.; Minka, S.R.; Yildiz, F. A comparison on the nutritional quality of proteins from Moringa oleifera leaves and seeds. Cogent Food Agric. 2016, 2, 1213618. [Google Scholar] [CrossRef]
- Liu, W.; Ma, H.; DaSilva, N.; Rose, K.N.; Johnson, S.L.; Zhang, L.; Wan, C.; Dain, J.A.; Seeram, N.P. Development of a neuroprotective potential algorithm for medicinal plants. Neurochem. Int. 2016, 100, 164–177. [Google Scholar] [CrossRef]
- Oseni, O.; Ogunmoyole, T.; Idowu, K. Lipid profile and cardio-protective effects of aqueous extract of Moringa oleifera (lam) leaf on bromate- induced cardiotoxicity on Wistar albino rats. Eur. J. Adv. Res. Biol. Life Sci. 2015, 3, 52–66. [Google Scholar]
- Sugahara, S.; Chiyo, A.; Fukuoka, K.; Ueda, Y.; Tokunaga, Y.; Nishida, Y.; Kinoshita, H.; Matsuda, Y.; Igoshi, K.; Ono, M.; et al. Unique antioxidant effects of herbal leaf tea and stem tea from Moringa oleifera L. especially on superoxide anion radical generation systems. Biosci. Biotechnol. Biochem. 2018, 82, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tafida, G. Antibacterial activity of Moringa oleifera (lam) leaves extracts against some selected bacteria. Int. J. Pharm. Pharmaceu. Sci. 2014, 6, 52–54. [Google Scholar]
- Dodiya, B.; Amin, B. Antibacterial activity and phytochemical screening of different parts of Moringa oleifera against selected gram positive and gram negative bacteria. Res. J. Pharm. Biol. Chem. Sci. 2015, 3, 421–425. [Google Scholar]
- Abdalla, A.M.; Alwasilah, H.Y.; Mahjoub, R.A.H.; Mohammed, H.I.; Yagoub, M. Evaluation of antimicrobial activ-ity of Moringa oleifera leaf extracts against pathogenic bacteria isolated from urinary tract infected patients. J. Adv. Lab. Res. 2016, 7, 47–51. [Google Scholar]
- Isitua, C.; Ibeh, I.; Olayinka, J. Antibacterial activity of Moringa Oleifera Lam leaves on enteric human pathogens. Indian J. Appl. Res. 2016, 6, 553–557. [Google Scholar]
- Abdallah, E.M. Antibacterial Properties of Leaf Extracts of Moringa oleifera Lam. Growing in Sudan. J. Adv. Med. Pharm. Sci. 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Jung, I.L.; Lee, J.H.; Kang, S.C. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol. Lett. 2015, 10, 1597–1604. [Google Scholar] [CrossRef]
- Ekong, M.B.; Ekpo, M.M.; Akpanyung, E.O.; Nwaokonko, D.U. Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degeneration. Metab. Brain Dis. 2017, 32, 1437–1447. [Google Scholar] [CrossRef]
- Hashim, F.J.; Vichitphan, S.; Boonsiri, P.; Vichitphan, K. Neuroprotective Assessment of Moringa oleifera Leaves Extract against Oxidative-Stress-Induced Cytotoxicity in SHSY5Y Neuroblastoma Cells. Plants 2021, 10, 889. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeong, C.H.; Lee, J.K. Neuroprotective effects of Moringa oleifera leaf extracts. Food Life 2022, 2022, 19–26. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, G.; Guo, M. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Imran, I.; Altaf, I.; Ashraf, M.; Javeed, A.; Munir, N.; Bashir, R. In vitro evaluation of antiviral activity of leaf extracts of Azadirachta indica, Moringa oleifera, and Morus alba against the foot and mouth disease virus on BHK-21 cell line. Sci. Asia 2016, 42, 392. [Google Scholar] [CrossRef]
- Elgamily, H.; Moussa, A.; Elboraey, A.; El-Sayed, H.; Al-Moghazy, M.; Abdalla, A. Microbiological Assessment of Moringa Oleifera Extracts and Its Incorporation in Novel Dental Remedies against Some Oral Pathogens. Maced. J. Med. Sci. 2016, 4, 585–590. [Google Scholar] [CrossRef]
- Arredondo-Valdés, R.; Hernández-Castillo, F.D.; Rocandio-Rodríguez, M.; Anguiano-Cabello, J.C.; Rosas-Mejía, M.; Vanoye-Eligio, V.; Ordaz-Silva, S.; López-Sánchez, I.V.; Carrazco-Peña, L.D.; Chacon-Hernandez, J.C. In vitro antibacterial activity of Moringa oleifera ethanolic extract against tomato phytopathogenic bacteria. J. Exp. Bot. 2021, 90, 895–906. [Google Scholar] [CrossRef]
- Aja, P.M.; Igwenyi, I.; Orji, O. Evaluation of anti-diabetic effect and liver function indices of ethanol extracts of Moringa oleifera and Cajanus cajan leaves in alloxan induced diabetic Albino rats. Glob. Vet. 2015, 14, 439–447. [Google Scholar] [CrossRef]
- Irfan, H.M.; Asmawi, M.Z.; Khan, N.A.K.; Sadikun, A.; Mordi, M.N. Anti-diabetic activity-guided screening of aqueous-ethanol Moringa oleifera extracts and fractions: Identification of marker compounds. Trop. J. Pharm. Res. 2017, 16, 543–552. [Google Scholar] [CrossRef]
- Saleh, S.S.; Sarhat, E.R. Effects of Ethanolic Moringa Oleifera Extract on Melatonin, Liver and Kidney Function Tests in Alloxan-Induced Diabetic Rats. Indian J. Forensic Med. Toxicol. 2019, 13, 1015–1019. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; AlBalawi, S.M.; Athar, T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Abutu, P.; Amuda, O.; Osinaya, O.; Babatunde, B. Regulatory activity of ethanol leaves extract of Moringa Oleifera on benzene induced leukemia in Wister Rat using TNF-α analysis. Am. J. Biomed. Res. 2020, 7, 6–11. [Google Scholar] [CrossRef]
- Wang, F.; Long, S.; Zhang, J.; Yu, J.; Xiong, Y.; Zhou, W.; Qiu, J.; Jiang, H. Antioxidant activities and anti-proliferative effects of Moringa oleifera L. extracts with head and neck cancer. Food Biosci. 2020, 37, 100691. [Google Scholar] [CrossRef]
- Idoga, E.S.; Ambali, S.F.; Ayo, J.O.; Mohammed, A. Assessment of antioxidant and neuroprotective activities of methanol extract of Moringa oleifera Lam. leaves in subchronic chlorpyrifos-intoxicated rats. Comp. Clin. Pathol. 2018, 27, 917–925. [Google Scholar] [CrossRef]
- FadiaTaufik, M.; Rashed, A.; Oshkondali, S.T.; Alacrouk, S.A.; Sleman, K. Antibacterial activities of Moringa oleifera leaf extract on some human pathogenic bacteria. Saudi J. Med. Pharm. Sci. 2021, 7, 426–431. [Google Scholar] [CrossRef]
- Kwami, W.S.; Saeed, H.A.; Mohammed Hamad, M.N. Screening the Antibacterial Activity of Moringa oleifera Leaves and Seeds Extract against Selected Members of Bacteria. Saudi J. Pathol. Microbiol. 2020, 5, 370–373. [Google Scholar] [CrossRef]
- Shailemo, D.H.P.; Kwaambwa, H.M.; Kandawa-Schulz, M.; Msagati, T.A.M. Antibacterial Activity of Moringa ovalifolia and Moringa oleifera Methanol, N-Hexane and Water Seeds and Bark Extracts against Pathogens That Are Implicated in Water Borne Diseases. Green Sustain. Chem. 2016, 6, 71–77. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881. [Google Scholar] [CrossRef]
- Randriamboavonjy Joseph Iharinjaka Rio, M.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera seeds attenuate vas-cular oxidative and nitrosative stresses in spontaneously hypertensive rats. Oxid. Med. Cell. Longev. 2017, 2017, 4129459. [Google Scholar] [CrossRef]
- Li, Y.-J.; Ji, Q.-Q.; Wang, Z.; Shen, L.-H.; He, B. Moringa oleifera seeds mitigate myocardial injury and prevent ventricular failure induced by myocardial infarction. Am. J. Transl. Res. 2020, 12, 4511–4521. [Google Scholar]
- Ijaz, A.; Javed, I.; Aslam, B.; Khan, J.A.; Khaliq, T.; Khan, M.Z.; Iqbal, Z.; Naeem, M.A.; Ashraf, M.M. Nephroprotective and antioxidant effects of Moringa oleifera (Sohanjna) in paracetamol induced nephrotoxic Albino rabbits. Pak. Vet. J. 2016, 36, 292–296. [Google Scholar]
- Nadro, M.; Audu, A.; Glen, E. Anti-diabetic effects of aqueous extract and oil of Moringa oleifera seed on liver and kid-ney functions in streptozotocin-induced diabetes in rat. Am. J. Biochem. 2018, 8, 69–74. [Google Scholar] [CrossRef]
- Jahan, I.A.; Hossain, M.H.; Ahmed, K.S.; Sultana, Z.; Biswas, P.K.; Nada, K. Antioxidant activity of Moringa oleifera seed extracts. Orient. Pharm. Exp. Med. 2018, 18, 299–307. [Google Scholar] [CrossRef]
- Bello, S.; Jamiu, A. Antibacterial activity of Moringa oleifera seed extracts on Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Niger. J. Microb. 2017, 31, 3873–3881. [Google Scholar]
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 25. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, W.-S.; Suo, D.-Q.; Li, Y.; Peng, L.; Xu, L.-X.; Zeng, K.-Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharmacol. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Hugar, S.; Shivapraksha, S.; Biradar, S.; Shivakumar, B. Evaluation of Moringa oleifera seeds for the cardio protective efficacy. World J. Pharm. Res. 2018, 7, 1461–1473. [Google Scholar] [CrossRef]
- Walter, A.; Samuel, W.; Peter, A.; Joseph, O. Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases. Afric. J. Microb. Res. 2011, 5, 153–157. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Total Antioxidant Capacity of Fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Nutr. Sci. 2013, 4, 35234. [Google Scholar] [CrossRef]
- Khattab, R.; Goldberg, E.; Lin, L.; Thiyam, U. Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem. 2010, 122, 1266–1272. [Google Scholar] [CrossRef]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.; Dey, A. Moringa oleifera Lam. and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2020, 129, 272–282. [Google Scholar] [CrossRef]
- WHO. Small Pox Is Dead; WHO Report; World Health Organization: Geneva, Switzerland, 1980; Volume 23, pp. 1–40.
- Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Zhang, M.; Liang, N.; Li, C.; He, Z. Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 2021, 95, 107561. [Google Scholar] [CrossRef]
- Goswami, D.; Mukherjee, P.K.; Kar, A.; Ojha, D.; Roy, S.; Chattopadhyay, D. Screening of ethnomedicinal plants of diverse culture for antiviral potentials. Indian J. Tradit. Knowl. 2016, 15, 474–481. [Google Scholar]
- Kurokawa, M.; Wadhwani, A.; Kai, H.; Hidaka, M.; Yoshida, H.; Sugita, C.; Watanabe, W.; Matsuno, K.; Hagiwara, A. Activation of Cellular Immunity in Herpes Simplex Virus Type 1-Infected Mice by the Oral Administration of Aqueous Extract of Moringa oleifera Lam. Leaves. Phytother. Res. 2016, 30, 797–804. [Google Scholar] [CrossRef]
- Lipipun, V.; Kurokawa, M.; Suttisri, R.; Taweechotipatr, P.; Pramyothin, P.; Hattori, M.; Shiraki, K. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 2003, 60, 175–180. [Google Scholar] [CrossRef]
- Younus, I.; Siddiq, A.; Assad, T.; Badar, S.; Jameel, S.; Ashraf, M. Screening antiviral activity of Moringa oliefera L. leaves against foot and mouth disease virus. Glob. Vet. 2015, 15, 409–413. [Google Scholar] [CrossRef]
- Feustel, S.; Ayón-Pérez, F.; Sandoval-Rodriguez, A.; Rodríguez-Echevarría, R.; Contreras-Salinas, H.; Armendáriz-Borunda, J.; Sánchez-Orozco, L.V. Protective Effects of Moringa oleifera on HBV Genotypes C and H Transiently Transfected Huh7 Cells. J. Immunol. Res. 2017, 2017, 6063850. [Google Scholar] [CrossRef]
- Waiyaput, W.; Payungporn, S.; Issara-Amphorn, J.; Panjaworayan, N.T.-T. Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells. Complement. Altern. Med. 2012, 12, 246. [Google Scholar] [CrossRef]
- Monera, T.; Maponga, C. Moringa oleifera supplementation by patients on antiretroviral therapy. J. Int. AIDS Soc. 2010, 13, P188. [Google Scholar] [CrossRef]
- Murakami, A.; Kitazono, Y.; Jiwajinda, S.; Koshimizu, K.; Ohigashi, H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor- promoter-induced epstein-barr virus activa-tion. Planta Med. 1998, 64, 319–323. [Google Scholar] [CrossRef]
- Eze, D.C.; Okwor, E.C.; Okoye, J.O.A.; Onah, D.N. Immunologic effects of Moringa oleifera methanolic leaf extract in chickens infected with Newcastle disease virus (kudu 113) strain. Afr. J. Pharm. Pharmacol. 2013, 7, 2231–2237. [Google Scholar] [CrossRef]
- Tolba, H.M.N.; Elmaaty, A.A.; Farag, G.K.; Mansour, D.A.; Elakkad, H.A. Immunological effect of Moringa Oleifera leaf extract on vaccinated and non-vaccinated Hubbard chickens experimentally infected with Newcastle virus. Saudi J. Biol. Sci. 2022, 29, 420–426. [Google Scholar] [CrossRef]
- Fajri, M. The potential of Moringa oleifera as immune booster against COVID 19. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 022008. [Google Scholar] [CrossRef]
- Nair, D.A.; James, T. Computational screening of phytocompounds from Moringa oleifera leaf as potential inhibitors of SARS-CoV-2 Mpro. Res. Square. 2002, 1–14. [Google Scholar] [CrossRef]
- Shaji, D. Computational identification of drug lead compounds for COVID-19. ChemRxiv 2020, 1–15. [Google Scholar] [CrossRef]
- Eremwanarue, O.; Shittu, H. Antimicrobial activity of Moringa oleifera leaf extracts on multiple drug resistant bacterial isolates from urine samples in Benin City. Niger. J. Biotechnol. 2018, 35, 16–26. [Google Scholar] [CrossRef]
- Jacques, A.S.; Arnaud, S.S.S.; Fréjus, O.O.H.; Jacques, D.T. Review on biological and immunomodulatory properties of Moringa oleifera in animal and human nutrition. J. Pharmacogn. Phytother. 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Ain, N.; Bashah, K.; Noor, M.M.A.T. Antihyperglycemic and androgenic properties of Moringa oleifera leaves aqueous extract attenuate sexual dysfunction in diabetes-induced male rats. Malays. Appl. Biol. 2021, 50, 99–105. [Google Scholar]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2014, 59, 1013–1024. [Google Scholar] [CrossRef]
- Chin, C.-Y.; Jalil, J.; Ng, P.Y.; Ng, S.-F. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef]
- Awodele, O.; Oreagba, I.A.; Odoma, S.; da Silva, J.A.T.; Osunkalu, V.O. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J. Ethnopharmacol. 2012, 139, 330–336. [Google Scholar] [CrossRef]
- Khor, K.Z.; Lim, V.; Moses, E.J.; Samad, N.A. The In Vitro and In Vivo Anticancer Properties of Moringa oleifera. Evid.-Based Complement. Altern. Med. 2018, 2018, 1071243. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Pathak, S.K.; Banerjee, A.; Pathak, S.; Bhattacharyya, A.; Yang, Z.; Talarico, S.; Kundu, M.; Basu, J. Execution of Macrophage Apoptosis by PE_PGRS33 of Mycobacterium tuberculosis Is Mediated by Toll-like Receptor 2-dependent Release of Tumor Necrosis Factor-α. J. Biol. Chem. 2007, 282, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose Response 2019, 17, 1559325819852243. [Google Scholar] [CrossRef]
- Zern, T.L.; Fernandez, M.L. Cardioprotective Effects of Dietary Polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef]
- Kumolosasi, E.; Wei, C.C.; Abdullah, A.Z.; Abd Manap, N.S.; Lee, W.L.; Yousuf, M.H.; Ying, L.S.; Buang, F.; Said, M.M.; Mohamad, H.F.; et al. Antihyperten-sive activities of standardised Moringa oleifera Lam. (Merunggai) extracts in spontaneously hypertensive rats. Sains Malays 2021, 50, 769–778. [Google Scholar] [CrossRef]
- Seriki, S.A.; Omolaso, B.; Adegbite, O.A.; Audu, A.I. Effect of Moringa oleifera on lipid profile, blood pressure and body mass index in human. Eur. J. Pharm. Med. Res. 2015, 2, 94–99. [Google Scholar]
- Adegbite, O.; Omolaso, B.; Seriki, S.; Shatima, C. Effects of Moringa oleifera leaves on hematological indices in humans. J. Hematol. Oncol. Pharm. 2016, 3, 1107–1113. [Google Scholar]
- Fombang, E.N.; Saa, R.W. Antihyperglycemic Activity of Moringa oleifera Lam Leaf Functional Tea in Rat Models and Human Subjects. Food Nutr. Sci. 2016, 7, 1021–1032. [Google Scholar] [CrossRef]
- Anthanont, P.; Lumlerdkij, N.; Akarasereenont, P.; Vannasaeng, S.; Sriwijitkamol, A. Moringa Oleifera Leaf Increases Insulin Secretion after Single Dose Administration: A Preliminary Study in Healthy Subjects. J. Med. Assoc. Thail. 2016, 99, 308–313. [Google Scholar]
- Gómez-Martínez, S.; Díaz-Prieto, L.E.; Castro, I.V.; Jurado, C.; Iturmendi, N.; Martín-Ridaura, M.C.; Calle, N.; Dueñas, M.; Picón, M.J.; Marcos, A.; et al. Moringa oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients 2022, 14, 57. [Google Scholar] [CrossRef] [PubMed]

| Extracts | Concentration/Doses | Experimental Animal/Cell Line | Finding | Citation |
|---|---|---|---|---|
| In vitro studies | ||||
| Aqueous leaf extract | 80.0, 40.0, 20.0, 10.0, 5.0 mg/mL of M. oleifera extract | Human peripheral blood mononuclear cells (PBMCs) | Lactate dehydrogenase (LDH) released upon cell damage or lysis indicates processes that occur during apoptosis and necrosis. Thus, the number of cells corresponds to the intracellular activity of LDH. As extracts increased above 20 mg/mL, the amount of LDH was released proportionally, indicating its cytotoxicity. | [9] |
| Aqueous seed extract |
| PBMCs, human pulmonary mucoepidermoid carcinoma (NCI-H292), human colon adenocarcinoma (HT-29), human larynx epidermoid carcinoma (HEp-2) | The IC50 of 144 μg/mL for PBMCs treated with diluted seed extract (used to treat drinking water) indicates that it was not cytotoxic to PBMCs and had low cytotoxic activity toward other cancer cell lines. None of the evaluations showed hemolytic activity, indicating no damage to the plasma membrane of erythrocytes. | [10] |
| Aqueous (AM), methanolic (MM) and petroleum ether (EM) leaf extracts | 5, 25 and 62.5 µg/mL | Human embryonic kidney cells expressing SV40 large T-antigen (293 T), Henrietta Lacks cells | The 50% cytotoxic concentration (TC50) was 41.58 µg AM/mL for aqueous extract, 38.88 µg MM/mL for methanolic extract and 32.33 µg EM/mL for ether extract in the assay performed by MTT assay. | [11] |
| Ethanolic leaf extracts | 400 to 0.02 µg/mL (serial-fold dilution) | Fibroblast cell line | The cytotoxic concentration 50 (CC50) was at 100 µg/mL as cell survival percentage decreased to 50%. The CC50 for twelve concentrations (0.02 to 50 µg/mL) was lower and safe. Two concentrations (200 µg/mL and 400 µg/mL) were above CC50 and were cytotoxic to fibroblast cells. | [12] |
| Ethanolic seed extract | 30, 50, 66, 83 and 100 µg/mL | Human colorectal carcinoma cells (HCT-116), Normal human cell lines (HEK-293) | Non-treated groups (cancer cells MOS treatment) showed 100% cell viability, whereas cancer cells treated with MOS extract showed a significant decrease, suggesting that the treatment led to significant drop in cancer cell viability. No inhibitory action observed in HEK-293 cells (non-cancerous cells), postulating that the extract is non-cytotoxic to normal cells. | [13] |
| Ethanolic leaf extracts | 100 to 500 μg/mL | Human cervix carcinoma cell line (Hela) | Positive control significantly reduced the cell viability to less than 25%. Extracts were considered cytotoxic when they reduced cell viability to less than 50%. Leaf extract significantly and concentration-dependently reduced the viability of Hela cells at concentrations above 260 μg/mL. | [14] |
| In vivo studies | ||||
| Aqueous leaf extract | 400, 800, 1600 and 2000 mg/kg | Rats (Male, Wistar rats) | 2000 mg/kg dose showed no fatality, except decrease in body weight in a dose-dependent manner. | [15] |
| Aqueous seed extract | 2000 mg/kg | Mice (Male, Balb/c) | No systemic toxicity, no significant changes in erythrocytes, platelets, hemoglobin and hematocrit observed. | [10] |
| Aqueous leaf extract | Low dose (1000 mg/mL), high dose (3000 mg/mL) | Rats (Male, Sprague Dawley) | M. oleifera shows acute toxicity at supra- supplementation levels of ≥3000 mg/kg bw. | [9] |
| Ethanolic leaf extracts | 150 mg/mL dose every 5 min interval | White albino rats and rabbits (local breed) | Lethal dose for acute toxicity; 6616.67 mg/kg for rats and 26043.67 mg/kg for rabbits. | [16] |
| Aqueous-methanolic leaf extract | 2000 mg/kg | Rats (Female, Wistar strain albino rats) | Increased levels of aspartate aminotransferase (AST) and total bilirubin. Decreased in alanine aminotransferase (ALT) level. Non-significant increase in hepatic index and mild distortions in liver cells via section analysis. | [17] |
| Crude methanol seed and leaf extracts | 100, 200, 400 and 1000 mg/kg | Albino rats | No mortality at 1000 mg/kg dose. Seed extracts showed more potential in a long-term application. | [18] |
| Dried leaf powder | 5, 50, 300 and 2000 mg/kg | Rats (Male and female, Sprague Dawley nulliparous and non-pregnant) | No adverse effect observed in clinical signs or gross pathology. | [19] |
| Parts of the Plants | Extraction Solvent | Drying Method | Extraction Method | Analytical and Bioassay Approach | Phytochemical Content | Tested Biological Assay | Reference |
|---|---|---|---|---|---|---|---|
| Leaves | Leaf powder | Dried under the shade | NA | High-performance liquid chromatograph-photodiode array (HPLC-PDA) |
| Anti-diabetic (in vivo) | [39] |
| Aqueous | NA | NA | Total phenolic content (TPC), 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), ferric reducing antioxidant power assay (FRAP) |
| Anti-glycating agent NPA: neuroprotective potential algorithm | [50] | |
| Air-dried | NA | NA | NA | Cardio-protective (in vivo) | [51] | ||
| NA | Hot-water extraction | TPC, TFC, HPLC-UV |
| Anti-oxidative (in vitro) | [52] | ||
| Washed, dried and ground to powder using a mechanical grinder | Maceration | Standard phytochemical methods |
| Antiviral (in vitro) | [11] | ||
| Air-dried under shade | 12 h constantly shaken with 30 min intervals | NA | NA | Antibacterial (in vitro) | [53] | ||
| Air-dried | Rotary shaker for 24 h | Standard phytochemical screening method |
| [54] | |||
| Dried in shade | Overnight infusion | NA | NA | [55] | |||
| Air-dried | Maceration in water, 50 °C for 8 h | [56] | |||||
| NA | Intermittent shaking for 2 weeks | [57] | |||||
| NA | Cold-water extraction at 4 °C, vigorous and vortexes | NA | NA | Anti-carcinogenic (in vitro) | [58] | ||
| Ethanol | Air-dried under shade | Soxhlet extraction in 80% ethanol, 24 h | NA | NA | Neuro-protective (in vitro and in vivo) | [59] | |
| Dried at 65 °C using a hot-air oven | Stirred for 8 h (ratio 1:4) | DPPH, FRAP |
| [60] | |||
| NA | Maceration with 80% ethanol at 70 ℃, 3 h | HPLC, TPC |
| [61] | |||
| Dried under sun, pulverized by disintegrator | Ultrasonic extraction using water bath (200 W, 40 KHz) for 30 min in 90% ethanol | TFC LC-MS (HPLC-UV/ESI-MS/MS) |
| Anti-oxidative (in vitro) | [62] | ||
| NA | Soxhlet extraction | NA | NA | Antiviral (in vitro) | [63] | ||
| Air-dried | Macerated for 72 h and stirred for 24 h in 95% ethanol | NA | NA | Antibacterial (in vitro) | [64] | ||
| Air-dried under shade | 12 h constantly shaken with 30 min intervals | [53] | |||||
| Air-dried | Rotary shaker for 24 h, 70% ethanol | Standard phytochemical screening method |
| [54] | |||
| Air-dried | Maceration in water, 50 °C for 8 h | NA | NA | [56] | |||
| Vegetal tissue dehydrated using conventional oven for 3 days, 60 °C until constant weight | Stirred for 3 days in dark with ab. ethanol | Standard phytochemical methods |
| [65] | |||
| Air-dried at room temperature | Maceration with 98% ethanol, room temperature for 24 h | NA | NA | Anti-diabetic (in vivo) | [66] | ||
| Dried in shade | Maceration with 95, 75, 50 and 25% (v/v) ethanol | HPLC-DAD |
| [67] | |||
| Air-dried | Maceration | NA | NA | [68] | |||
| NA | Maceration for 6–8 h | GC-MS |
| Anti-carcinogenic (in vitro and in vivo) | [69] | ||
| Air-dried at room temperature for over 3 weeks | Stirred for 48 h | NA | NA | [70] | |||
| NA | Ultrasound bath extraction (40 kHz, 300 W) for 1.5 h at 50 °C | TPC, TFC, FRAP |
| [71] | |||
| Methanol | Air-dried | Cold extraction | Standard phytochemical methods, HPLC-DAD and HPLC-EC, UV-visible spectroscopy (for vitamin content determination) |
| Neuro-protective (in vivo) | [72] | |
| NA | Maceration | Standard phytochemical screening method |
| Antiviral (in vitro) | [11] | ||
| Air-dried under shade | 12 h constantly shaken with 30 min intervals | NA | NA | Antibacterial (in vitro) | [53] | ||
| Air-dried | Rotary shaker for 24 h, 80% methanol | Standard phytochemical screening method |
| [54] | |||
| Dried in shade | Soxhlet extraction for 3 h | NA | NA | [55] | |||
| Air-dried at room temperature | Maceration | Standard phytochemical screening method |
| [73] | |||
| Air-dried | Soxhlet extraction for 8 h, in 80% methanol | NA | NA | [74] | |||
| Chloroform | NA | Intermittent shaking for 2 weeks | NA | NA | Antibacterial (in vitro) | [57] | |
| Ethyl acetate | |||||||
| Butanol | |||||||
| Petroleum ether | NA | Maceration | Standard phytochemical methods |
| Antiviral (in vitro) | [11] | |
| Flower/pulp | Aqueous | Air-dried | Rotary shaker for 24 h | Standard phytochemical screening method | Flower
| Antibacterial (in vitro) | [54] |
| Ethanol | Rotary shaker for 24 h, 70% ethanol |
| |||||
| Methanol | Rotary shaker for 24 h, 80% methanol | Flower
| |||||
| Petroleum ether | Rotary shaker for 24 h | Flower
| |||||
| Stem | Aqueous | Dried in shade | Maceration for 72 h, room temperature | NA | NA | Antibacterial (in vitro) | [75] |
| NA | Hot-water extraction | TPC, TFC, HPLC-UV |
| Anti-oxidative (in vitro) | [52] | ||
| Ethanol | NA | Ultrasound bath extraction (40 kHz, 300 W) for 1.5 h at 50 °C | NA | NA | Anti-carcinogenic (in vitro) | [71] | |
| Methanol | Dried in shade | Maceration for 72 h in 99.9% methanol, room temperature | NA | NA | Antibacterial (in vitro) | [75] | |
| n-Hexane | Maceration for 72 h in 98.9% n-hexane, room temperature | ||||||
| Bark | Ethanol | NA | Maceration for 6–8 h | GC-MS |
| Anti-carcinogenic (in vitro) | [69] |
| Root | Ethanol | Dried under sun, pulverized by disintegrator | Ultrasonic extraction using water bath (200 W, 40 KHz) for 30 min in 90% ethanol | TFC LC-MS (HPLC-UV/ESI-MS/MS) |
| Anti-oxidative (in vitro) | [62] |
| NA | Ultrasound bath extraction (40 kHz, 300 W) for 1.5 h at 50 °C | NA | NA | Anti-carcinogenic (in vitro) | [71] | ||
| Seed | Seed powder | Dried and ground to powder | NA | NA | NA | Cardio-protective (in vivo) | [76,77,78] |
| Dried in shade | Anti-oxidative (in vivo) | [79] | |||||
| Seed oil | Air-dried in oven | Soxhlet extraction using hexane, heated at low temperature | NA | NA | Anti-diabetic (in vivo) | [80] | |
| Aqueous | Dried in shade | Stirred for 48 h, room temperature | TPC, TFC, Total tannin |
| Anti-oxidative (bioassays) | [81] | |
| Dried in shade | Maceration for 72 h, room temperature | NA | NA | Antibacterial (in vitro) | [75] | ||
| Rotary shaker for 2 days, cold and hot water | [82] | ||||||
| Air-dried for 2 days | Shaking on horizontal shaker for 3 days | [83] | |||||
| NA | Maceration for 72 h at room temperature | NA | NA | Anti-diabetic (in vivo) | [80] | ||
| Ethanol | Dried under sun, pulverized by disintegrator | Ultrasonic extraction using water bath (200 W, 40 KHz) for 30 min in 90% ethanol | TFC LC-MS (HPLC-UV/ESI-MS/MS) |
| Anti-oxidative (in vitro) | [62] | |
| Dried in shade | Rotary shaker for 2 days | NA | NA | Antibacterial (in vitro) | [82] | ||
| NA | Ultrasound bath extraction (40 kHz, 300 W) for 1.5 h at 50 °C | NA | NA | Anti-carcinogenic (in vitro) | [71] | ||
| NA | Maceration for 6–8 h | GC-MS |
| [69] | |||
| NA | Maceration in 70% ethanol at 85 °C, 2 h | NA | NA | Neuro-protective (in vivo) | [84] | ||
| Methanol | Dried in shade | Soxhlet extraction | Standard phytochemical screening method |
| Cardio-protective (in vivo) | [85] | |
| Dried in shade | Stirred for 48 h, room temperature | TPC, TFC, Total tannin |
| Anti-oxidative (bioassays) | [81] | ||
| De-shelled, dried at 23 to 25 °C for 5 days | Shaken for 4 h and macerated overnight | NA | NA | Antibacterial (in vitro) | [86] | ||
| Dried in shade | Maceration for 72 h in 99.9% methanol, room temperature | [75] | |||||
| Rotary shaker for 2 days | [82] | ||||||
| Air-dried for 2 days | Shaking on horizontal shaker for 3 days | [83] | |||||
| Air-dried | Soxhlet extraction for 8 h | [74] | |||||
| Acetone | Dried in shade | Stirred for 48 h, room temperature | TPC, TFC, Total tannin |
| Anti-oxidative (bioassays) | [81] | |
| Air-dried for 2 days | Shaking on horizontal shaker for 3 days | NA | NA | Antibacterial (in vitro) | [83] | ||
| Petroleum ether | Air-dried | Soxhlet extraction for 4 h, 40–60 °C | [74] | ||||
| Hexane | Dried in shade | Maceration for 72 h in 98.9% n-hexane, room temperature | [75] |
| Extracts | Application | Finding | Citation |
|---|---|---|---|
| 70% Ethanol, 80% methanol, petroleum ether and aqueous extracts of M. oleifera leaves, flower, pulp and seed | Method: Agar-well diffusion method Bacteria species: E. coli and S. aureus | Maximum zone of inhibition: Leaves: 80% methanol extract against E. coli (28 mm) and S. aureus (26 mm). Flower: 70% ethanol extract against E. coli (23 mm) and S. aureus (17 mm). Pulp: 80% methanol extract against E. coli (15.33 mm). Aqueous extracts against S. aureus (18.33 mm). Seed: 80% methanol extract against E. coli (18.33 mm). 70% ethanol extracts against S. aereus (15.66 mm). | [54] |
| Aqueous, petroleum ether and methanolic (20, 40, 60%) extracts of M. oleifera leaf | Method: In vitro, cup–plate method and disc diffusion method Bacteria species: S. aureus, E. coli, Klebsiella pneumonia, P. aeruginosa and Proteus vulgaris | Methanolic extracts (20, 40, 60%) had high inhibitory effects on S. aureus, K. pneumoniae standard strains and S. aureus, S. saprophyticus and E.coli isolated from urinary tract infection. Aqueous extract only showed effects on P. vulgaris standard strain. Petroleum ether extracts showed no inhibitory activity at all. | [55] |
| Methanol (99.9%), n-hexane (98.9%) and aqueous extracts of M. oleifera and M. ovalifolia seeds and bark | Method: Paper-disc diffusion method Bacteria species: E. coli, Enterococcus faecalis and Bacillus cereus | M. oleifera extracts showed higher inhibitory activities than M. ovolifolia. Seed extracts of M. oleifera exhibit a wider range of antibacterial activity than M. ovalifolia. M. oleifera bark extracts showed higher antibacterial activity than M. ovalifolia against all tested species. n-hexane extracts for both M. ovalifolia and M. oleifera showed similar inhibitory activities but were generally lower than other solvents. | [75] |
| Aqueous and ethanolic M. oleifera leaf | Method: Agar diffusion and microbroth dilution methods Bacteria species: S. aureus, Streptococcus pyogenes, Bacillus cereus, E. coli, P. aeruginosa, Shigella sonnei, Shigella dysenteriae, Shigella flexneri, Shigella boydii and Proteus mirabilis | All tested bacterial isolates were observed to be susceptible to both extracts at 100 µg concentration, but susceptibility decreased as the extract concentration reduced. M.oleifera leaf extract showed broad spectrum of antibacterial activities as it works on both Gram-positive and -negative bacteria. Ethanol extract exhibited higher inhibition and minimal inhibitory concentrations. | [56] |
| Chloroform, ethyl acetate, butanol and aqueous extracts M. oleifera leaf | Method: In vitro, agar-well diffusion method Bacteria species: E. coli, P. vulgaris, K. pneumoniae, Salmonella enterica, P. aeruginosa, S. aureus, Staphylococcus epidermidis and B. cereus | Ethyl acetate extract observed the highest antibacterial activity against S. epidermidis, S. aureus, P. aeruginosa and B. cereus. Butanol extract reacted against S. epidermidis and S. aureus. Aqueous and chloroform extract only showed activity against S. epidermidis and S. aureus, respectively. | [57] |
| Methanolic M. oleifera leaf | Method: In vitro, agar-well diffusion method Bacteria species: E. coli and Klebsiella | The extracts showed activities against both bacteria in dose-dependent manner, as such highest activities were observed at dose of 200 mg/L. Methanol extract of M. oleifera was shown to have minimum inhibitory concentration (MIC) value against Kleibsilla at 45 mg %. | [73] |
| Aqueous (hot and cold), ethanolic and methanolic extracts of M. oleifera seeds | Method: In vitro, disc diffusion method and broth dilution method Bacteria species: S. aureus, E. coli and P. aeruginosa | The MIC for different extracts was observed as follows: aqueous (cold) extract showed no MIC, but aqueous (hot) extract was 100 mg/mL. The MIC for ethanolic and methanolic extracts is also capped at 100 mg/mL, except for E. coli and S. aureus with MIC of 50 mg/mL. The minimum bactericidal concentration (MBC) of aqueous (hot), ethanolic and methanolic extracts on tested bacteria was 200 mg/mL, but methanolic extract showed MBC of 100 mg/mL on E. coli. | [82] |
| Methanol, acetone and aqueous extracts of M. oleifera seeds | Method: In vitro, agar-well diffusion technique and MIC and MBC Bacteria species: E. coli, Shigella typhii and Shigella dysenteriae | Acetone extracts showed highest antibacterial activity against S. typhii and least sensitivity with the aqueous extract. The most observed MIC value was 6.25 mg/mL, then 12.5 mg/mL. Acetone extract is the most potent in exhibiting inhibitory activities at very low concentration for Shigella typhii | [83] |
| 80% methanolic extracts of M. oleifera leaf and seeds | Method: In vitro, agar-well diffusion method Bacteria species: E. coli, s. typhi, salmonella paratyphi- A, salmonella paratyphi-B, shigella dysentriae, s. aureus, streptococcus feacalis, p. aeruginosa, Proteus mirabilis and k. Pneumoniae | Both leaf and seed extracts exhibit antibacterial activity against all bacterial ATCC strains, but for leaf extract, highest activity was observed on S. typhi (ATCC19430) while the seed extracts showed on E. coli (ATCC25922). Both extracts also showed activities in clinically isolated bacterial strains, but for leaf extract, highest activity was observed against S. aureus, and for seed extract, highest activities was observed against K. pneumoniae, P. mirabilis and S. typhi. Overall, the results show higher antibacterial activity in leaf extract as compared to seed extract. | [74] |
| Ethanolic extracts of M. oleifera leaf | Method: Bacteria inhibition microplate assay Bacteria species: Agrobacterium tumefeciens (At), Clavibacter michiganensis subsp. michiganensis (Cmm), Pseudomonas syringae pv. tomato (Pst), Ralstonia solanacearum (Rs) and Xanthomonas axonopodis (Xa) | Results show that the extract exhibits inhibitory effects on the growth of phytopathogenic bacteria At, Cmm, Pst, Rs and Xa in dose-dependent manner. Higher inhibition was observed at higher concentration of extract. At was found to be most susceptible to the exact treatment while Rs was more resistant. Ethanolic extracts of M. oleifera leaf showed prominent bio-bactericide potential. | [65] |
| Status | Official Title | Intervention/ Treatment | Outcome Description | Study Design |
|---|---|---|---|---|
| Completed Duration: December 2012–August 2014 | Safety and efficacy of Chandrakanthi Choornam in Oligospermia—A preclinical and clinical study | Drug: Chandrakanthi Choornam (CKC)—formulation consisting of 25 ingredients Conditions: Oligospermia | Determination of sperm concentration, motility, and proportion of sperm morphology, as well as the effects on hormonal level. | Primary Purpose: Treatment Allocation: N/A Interventional Model: Single Group Assignment Masking: None (Open Label) |
| Completed Duration: January 2013–September 2013 | Effect of M. oleifera (Moringa, Drumstick/Horseradish Tree) on the pharmacokinetics of Efavirenz and Nevirapine in-vivo. | Dietary Supplement: M. oleifera Drug: Efavirenz (600 mg) and Nevirapine (200 mg) *Oral Tablet Conditions: HIV | Pharmacokinetic endpoints include area under the curve (AUC), time to maximum plasma concentration (tmax), peak plasma concentration (Cmax), trough concentration (Cmin), clearance (CL), volume of distribution (Vd/F) and half-life (T1/2). | Observational Model: Case-Crossover Time Perspective: Prospective Biospecimen Retention: SAMPLES_WITH_DNA Biospeciman Description: Whole Blood, Plasma, Urine |
| Completed Duration: January 2013–April 2014 | Impact of dried M. oleifera leaves as value added supplement in enhancing hemoglobin status of reproductive aged females of low socio-economic group | Dietary Supplement: Dried M. oleifera leaves Conditions: Anemia, iron deficiency | Improvement in hemoglobin status and body mass index. | Primary Purpose: Supportive Care Allocation: Non-Randomized Interventional Model: Single Group Assignment Masking: None (Open Label) |
| Completed Duration: August 2014–November 2014 | The effects of M. oleifera supplements on hsCRP and HgbA1c levels of patients in Hospital Ng Maynila Medical Center diabetic clinic: A prospective cohort study | Dietary Supplement: M. oleifera Conditions: Diabetes | Post treatment means of inflammatory markers HsCRP and hgba1c. | Primary Purpose: Supportive Care Allocation: N/A Interventional Model: Single Group Assignment Masking: None (Open Label) |
| Completed Duration: January 2014–April 2022 | An observational clinical study to determine the effect of multi-modal Ayurvedic treatment in the patients of chronic kidney disease | Other: Ayurveda treatment Conditions: Diabetic nephropathy, hypertensive nephropathy | The determination of the signs and symptoms as well as the improvement in metabolic profiles. | Primary Purpose: Treatment Allocation: N/A Interventional Model: Single Group Assignment Masking: None (Open Label) |
| Completed Duration: July 2015–May 2016 | Effect of M. oleifera on bone density in post-menopausal Women | Dietary Supplement: M. oleifera Dietary Supplement: Cabbage placebo Conditions: Osteoporosis, osteopenia, postmenopausal, osteoporosis | Bone density determined by dual-energy X-ray absorptiometry. | Primary Purpose: Basic Science Allocation: Randomized Interventional Model: Parallel Assignment Masking: Double |
| Completed Duration: March 2016–April 2016 | Evaluation of the effect of M. oleifera tea on metformin steady state plasma level in type 2 diabetes mellitus patients—A pre and post non-randomised trial | Dietary Supplement: M. oleifera tea Conditions: Type 2 diabetes mellitus | Changes in fasting blood glucose, 2 h post prandial blood glucose, metformin plasma concentration, estimated glomerular filtration rate. | Primary Purpose: Treatment Allocation: N/A Interventional Model: Single Group Assignment Masking: None (Open Label) |
| Completed Duration: November 2016–May 2017 | Short term cardiovascular and renal effects of M. oleifera extracts and Stevia Rebaudiana Bertoni as add-on therapy in a population of type II diabetes individuals | Dietary Supplement: MOROLSTEVER1 (Capsules of M. oleifera and Stevia Rebaudiana Bertoni) Conditions: Type 2 diabetes mellitus | Determination of diastolic function, early diastolic filling velocity, urinary excretion of albumin and changes in blood pressure. | Primary Purpose: Treatment Allocation: N/A Interventional Model: Single Group Assignment Masking: None (Open Label) |
| Completed Duration: December 2017–August 2020 | Evaluation of the effect of Artemisia annua and M. oleifera on immunological response in HAART HIV patients | Dietary Supplement: Artemisia Annua, M. oleifera Conditions: HIV infections | Determination of CD4 counts, viral load, complete blood count, immunoglobins, antiretroviral plasma drug level, patients’ perceptions on mental and physical quality of life, liver function biomarkers, side effects or adverse drug reactions, renal function biomarkers. | Primary Purpose: Treatment Allocation: Randomized Interventional Model: Parallel Assignment Masking: Double |
| Completed Duration: September 2018–March 2019 | MORINGA; Delivering nutrition and economic value to the people of Malawi | Other: Control corn soya diet Other: Test corn, moringa diet Conditions: Malnourishment | Determination of phytochemical metabolite concentration in systemic circulation. | Primary Purpose: Prevention Allocation: Randomized Interventional Model: Crossover Assignment Masking: None (Open Label) |
| Completed Duration: January 2019–January 2021 | Effects of M. oleifera Leaves on glycemia, lipemia and inflammatory profile: Nutritional intervention study in prediabetic patients | Dietary Supplement: Moringa Dietary Supplement: Placebo Conditions: Pre-diabetes | Determination of fasting blood glucose and glycated hemoglobin (HbA1C), conversion rate from prediabetes to normal, concentration of total serum cholesterol, lipoprotein–cholesterol, inflammatory markers and metabolic hormones, antioxidant capacity and microbiota composition. | Primary Purpose: Prevention Allocation: Randomized Interventional Model: Parallel Assignment Masking: Quadruple |
| Completed Duration: January 2020–March 2021 | An evaluation of the effects of a non-caffeinated energy dietary supplement on cognitive and physical performance: A randomized double-blind placebo-controlled study | Dietary Supplement: Phytovive, placebo, caffeine Conditions: Health behavior | Evaluation of cognition, mood and physical performance. | Primary Purpose: Basic Science Allocation: Randomized Interventional Model: Parallel Assignment Masking: Double |
| Unknown status Duration: February 2020–July 2020 | Effect of aerobic training and M. oleifera on dyslipidemia and cardiac endurance | Other: Aerobic training and M. oleifera Conditions: Dyslipidemias | Change in level of HDL, LDL and triglycerides, impact on cardiac endurance. | Primary Purpose: Health Services Research Allocation: Randomized Interventional Model: Parallel Assignment Masking: None (Open Label) |
| Unknown status Duration: February 2020–August 2020 | Randomized clinical study investigating the effect of M. oleifera infusion on bioclinical parameters of health | Dietary Supplement: M. oleifera tea Conditions: Metabolic syndrome | Change in blood glucose level, LDL cholesterol and triglyceride level as well as antioxidants of blood (e.g., superoxide dismutase, glutathione peroxidase, total blood antioxidant capacity). | Primary Purpose: Other Allocation: Randomized Interventional Model: Crossover Assignment Masking: Single |
| Completed Duration: March 2021–June 2022 | Effect of M. oleifera capsule in increasing breast milk volume in early postpartum patients: A double blind randomized controlled trial | Drug: M. oleifera leaf Drug: Placebo Conditions: Postpartum women | Determination of breast milk volume at postpartum day 3, as well as percentage of good satisfaction, quality of life scores, side effects and compliance. | Primary Purpose: Other Allocation: Randomized Interventional Model: Parallel Assignment Masking: Quadruple |
| Completed Duration: July 2021–November 2021 | Investigating the impact of M. oleifera leaf supplementation on growth, nutrition, lactation, and inflammation in Kenyan breastfeeding mothers and children | Dietary Supplement: M. oleifera (high dose and low dose) Dietary Supplement: Placebo Conditions: Malnutrition, wasting, growth failure | Changes in physical descriptions (body weight, height, mid-upper arm and head circumference) and metabolite levels (vitamin A, C-reactive protein, soluble transferrin–ferritin index, fecal neopterin, fecal myeloperoxidase, alpha-1-antitrypsin). The prevalence of diarrhea and changes in breast milk (output, vitamin A, retinol, vitamin E, catalase, lactoferrin) were also determined. | Primary Purpose: Prevention Allocation: Randomized Interventional Model: Single Group Assignment Masking: Single |
| Active, not recruiting Duration: December 2021–October 2022 | Effects of M. oleifera mouthwash in patients undergoing fixed orthodontic appliance treatment: A parallel arm, triple blinded, randomized controlled trial | Other: M. oleifera mouthwash Other: Placebo mouthwash Conditions: Gingivitis, periodontitis, plaque formation, enamel demineralization/white spot lesion, discoloration and bacterial load in dental plaque (orthodontic appliance complication) | Periodontal probing depth, plaque index, white spot lesions, modified gingival index, discoloration of teeth, bacterial load in plaque. | Primary Purpose: Prevention Allocation: Randomized Interventional Model: Parallel Assignment Masking: Quadruple |
| Completed Duration: February 2021– December 2021 | Antifungal potential of M. oleifera-loaded nanoparticles against otomycosis; Preparation, characterization, and Clinical Evaluation | Drug: M. oleifera leaf 10 mg/100 mL Drug: Ear drop Conditions: Otomycosis | The number of participants recovered with clear endoscopic examination and identification of different microorganisms that infected ear were determined. | Primary Purpose: Treatment Allocation: Randomized Interventional Model: Parallel Assignment Masking: None (Open Label) |
| Active, not recruiting Duration: April 2021–February 2022 | Child health, agriculture, and integrated nutrition (CHAIN): A randomized trial to close the nutrient gap in rural Zimbabwe | Dietary Supplement: Small-quantity lipid-based nutrient supplement, provitamin A biofortified maize, NUA-45 biofortified sugar beans, M. oleifera, whole egg powder and white maize meal Conditions: Stunting | Determinations of protein, iron, zinc, folate and energy intake, height and weight for age score, hemoglobin level, microbiome maturity, environmental enteric dysfunction, innate immune cell phenotype and function, plasma essential amino acids and choline, as well as urinary metabolic secretion. | Primary Purpose: Prevention Allocation: Randomized Interventional Model: Parallel Assignment Masking: Single |
| Recruiting Duration: August 2021–March 2022 | A randomized, double-blind, cross-over, placebo-controlled study to explore the effect of M. oleifera (E-HS-01) on flow mediated dilatation and hemodynamics | Other: M. oleifera (E-HS-01) Other: Placebo Conditions: Endothelial function | Determination of flow-mediated dilatation and blood flow velocity. | Primary Purpose: Other Allocation: Randomized Interventional Model: Crossover Assignment Masking: Quadruple |
| Recruiting Duration: April 2022–July 2022 | Evaluation of the anti-plaque and anti-gingivitis effects of Moringa plant extract and fluoride toothpastes among a group of Egyptian children: A randomized clinical trial | Drug: M. oleifera leaf Conditions: Oral disease | Determination of gingival index and plaque index. | Primary Purpose: Treatment Allocation: Randomized Interventional Model: Parallel Assignment Masking: Double |
| Recruiting Duration: May 2022–May 2023 | Investigating the effect of M. oleifera leaf powder on breastmilk quantity and quality: A double blinded randomized placebo-controlled trial | Dietary Supplement: Moringa leaf powder Other: Placebo Conditions: Breastfeeding | Differences in milk output, species prevalence of infant microbiome and maternal milk microbiome, maternal milk fat and protein, infant fecal and maternal milk microbiome composition, maternal milk vitamin A. | Primary Purpose: Treatment Allocation: Randomized Interventional Model: Parallel Assignment Masking: Quadruple |
| Not yet recruiting Duration: July 2022- December 2023 | Antibacterial, antiplaque and anticariogenic effect of M. oleifera mouthwash compared to chlorhexidine mouthwash: A randomized clinical trial | Drug: M. oleifera, chlorhexidine mouthwash, base formula Conditions: Plaque, dental antimicrobial, mouthwash cytotoxicity | Determinations of gingival index (GI) and white spot lesions. | Primary Purpose: Prevention Allocation: Randomized Interventional Model: Parallel Assignment Masking: Double |
| Not yet recruiting Duration: August 2022–April 2023 | Effect of M. oleifera leaf extract versus sodium hypochlorite as root canal irrigant on postoperative pain and bacterial reduction in mandibular premolars with necrotic pulps: A randomized clinical trial | Dietary Supplement: M. oleifera leaf Drug: Sodium hypochlorite Conditions: Necrotic pulp | Determinations of postoperative pain and intracanal bacterial load change. | Primary Purpose: Treatment Allocation: Randomized Interventional Model: Parallel Assignment Masking: Double |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azlan, U.K.; Mediani, A.; Rohani, E.R.; Tong, X.; Han, R.; Misnan, N.M.; Jam, F.A.; Bunawan, H.; Sarian, M.N.; Hamezah, H.S. A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera. Molecules 2022, 27, 5765. https://doi.org/10.3390/molecules27185765
Azlan UK, Mediani A, Rohani ER, Tong X, Han R, Misnan NM, Jam FA, Bunawan H, Sarian MN, Hamezah HS. A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera. Molecules. 2022; 27(18):5765. https://doi.org/10.3390/molecules27185765
Chicago/Turabian StyleAzlan, Ummi Kalthum, Ahmed Mediani, Emelda Rosseleena Rohani, Xiaohui Tong, Rongchun Han, Norazlan Mohmad Misnan, Faidruz Azura Jam, Hamidun Bunawan, Murni Nazira Sarian, and Hamizah Shahirah Hamezah. 2022. "A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera" Molecules 27, no. 18: 5765. https://doi.org/10.3390/molecules27185765
APA StyleAzlan, U. K., Mediani, A., Rohani, E. R., Tong, X., Han, R., Misnan, N. M., Jam, F. A., Bunawan, H., Sarian, M. N., & Hamezah, H. S. (2022). A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera. Molecules, 27(18), 5765. https://doi.org/10.3390/molecules27185765