1. Introduction
Zinc borate ranks among the top ten boron-containing chemicals in terms of global production and use. Of the several known zinc borate phases, ZnB
3O
4(OH)
3, having the resolved oxide formula 2ZnO·3B
2O
3·3H
2O, is by far the most commercially important and has been in industrial scale production for more than half a century [
1]. Tens of thousands of tons of this compound are used annually in industrial applications, where it is known as an article of commerce as 2ZnO·3B
2O
3·3.5H
2O. This slightly incorrect commercial formula is a result of an early error in characterization, but it is correctly formulated as the trihydrate for its oxide formula [
2]. The compound actually contains no free water of hydration, nor free ZnO and B
2O
3, which are abstract components of the oxide formula conventionwhich factors out hydroxyl groups as apparent water. While the minor inaccuracy in the commercial formula has no bearing on the properties or efficacy of this compound, the more accurate and concise formula ZnB
3O
4(OH)
3 is primarily used herein.
This is the only zinc borate compound that currently carries biocidal registrations, including with the U.S. EPA and the Canadian PMRA, for certain brands. It is used extensively as a fire retardant synergist in polymers and to lend durability to engineered wood building materials, wood-plastic composites, and related products. In polymers, it is used in combination with other additives to reduce flammability, suppress smoke, and improve electrical properties [
3,
4]. In building materials, it provides protection against attack by decay fungi and boring insects. In particular, oriented strand board (OSB) siding and sheathing products containing zinc borate preservative have a long record of excellent durability in service extending back more than 30 years [
5,
6,
7,
8]. The hydrolysis chemistry of zinc borate has considerable relevance to its industrial applications.
Boric acid is well known to inhibit wood destroying organisms and is a registered biocide in many regions, including USA, Canada, and the EU. However, due to its relatively high water solubility and low dehydration onset temperature (<100 °C), it is often impractical to incorporate boric acid directly into polymers and composite building materials, especially when heat is applied during manufacture. On the other hand, borates that are completely insoluble are not expected to be efficacious as biocides since it is necessary for some amount of borate to be mobile and biologically available in order to control undesirable organisms. Zinc borate exhibits both low solubility and a high dehydration onset temperature, ca. 290 °C, making it suitable for use in many manufacturing processes. Despite its industrial use in many applications for more than half a century, we are unaware of any detailed reports on the hydrolysis chemistry of zinc borate. Herein, we describe measurements of the solubility of zinc borate ZnB3O4(OH)3 and its mode of hydrolysis, which provides a likely mechanism for its efficacy in enhancing durability with respect to biodegradation.
3. Results and Discussion
Zinc borate is produced by the reaction of zinc oxide with excess boric acid at temperatures above 70 °C in aqueous suspension, usually in the presence of product seed, as shown in Equation (1) [
9]. If the mother liquor does not contain a sufficient concentration of excess of boric acid, or if the temperature is not sufficiently high, other zinc borate phase may form, such as Zn[B
3O
3(OH)
5]·H
2O (2ZnO·3B
2O
3·7H
2O) or 3ZnO·5B
2O
3·14H
2O. The structure and chemical formula of ZnB
3O
4(OH)
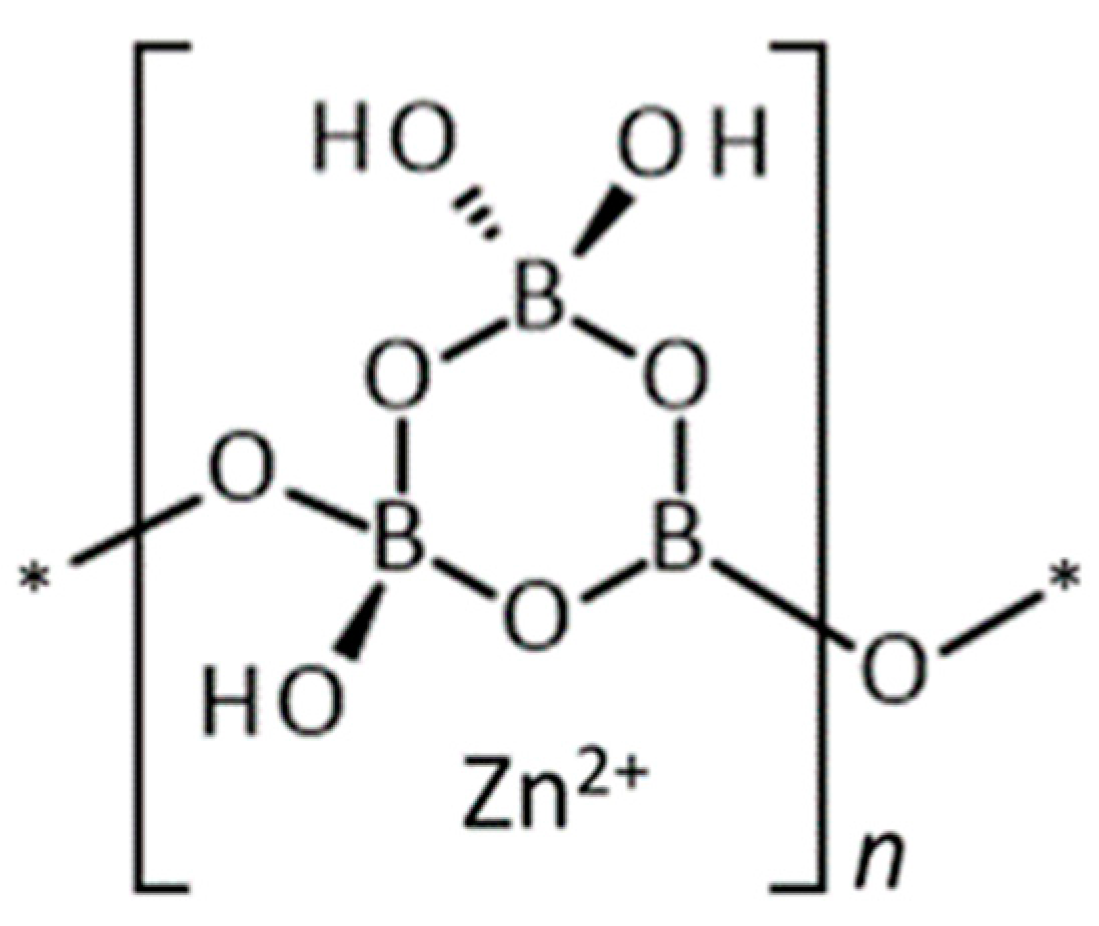
3 is well defined, based on its single crystal X-ray diffraction structure, shown schematically in
Figure 1 [
2]. It is an inoborate consisting of chains of triborate rings with interstitial tetrahedral Zn
2+ cations coordinated to hydroxyl and boroxyl oxygen atoms. The structure is further interconnected by hydrogen bonds. Inoborates generally exhibit relatively slow dissolution rates compared to nesoborates which contain insular borate anions. The structural chemistry of metal borates has been reviewed [
10]. Although the formula 2ZnO·3B
2O
3·3.5H
2O used in commerce was shown long ago to be incorrect and properly should be given as 2ZnO·3B
2O
3·3H
2O, these two formulas have different CAS numbers, causing some confusion in the regulatory domain.
When zinc borate is added to water at neutral pH in the absence of excess boric acid it exhibits incongruent solubility, hydrolyzing to less soluble zinc hydroxide and a more soluble boric acid, according to Equation (2). Hydrolysis at room temperature proceeds until the boron concentration of the supernatant solution reaches a concentration of about 830 ppm B (77 mM B). The zinc concentration in solution at equilibrium is about 25 ppm (0.38 mM Zn), owing to the much lower solubility of zinc hydroxide. A boron concentration of 830 ppm is equivalent to a 0.475 wt% solution of boric acid. The solution pH remained near 7 during hydrolysis. This can be regarded as a buffered system.
Solubilities of borate compounds are traditionally expressed as the sum of the anhydrous components of their oxide formulas in solution. Therefore, the solubility of ZnB3O4(OH)3, with oxide formula 2ZnO·3B2O3·3H2O, is expressed as the sum of the equivalent B2O3 and ZnO concentrations calculated from measured boron and zinc concentrations. Using this convention, the room temperature solubility of ZnB3O4(OH)3 is 0.270 wt%, comprised of 0.0267% B2O3 and 0.003% ZnO.
Polyborate species, most notably [B
5O
6(OH)
4]
−, [B
3O
3(OH)
4]
−, [B
3O
3(OH)
5]
2−, and [B
4O
5(OH)
4]
2−, exist in rapid equilibrium in relatively concentrated borate solutions with population distributions being largely a function of pH. However, at low concentrations the predominate borate species in solution are limited to monomeric B(OH)
3 (pK
a = 9.2) and its conjugate base [B(OH)
4]
− [
11]. Since the solution resulting from zinc borate hydrolysis has a relatively low boron concentration (<0.5% boric acid eqivalent) and pH
7, B(OH)
3 is expected to be the dominant chemical species present with only minor amounts of polyborates. Owing to the amphoteric nature of zinc, the solubilty is zinc borate is substational greatly in both acidic and basic solutions.
Concentrated aqueous slurries of zinc borate ZnB
3O
4(OH)
3 are infinitely stable. A 10 wt% zinc borate suspension hydrolyzes to an extent of <4% at room temperature. Since ZnB
3O
4(OH)
3 is observed to form rapidly only above about 70 °C, one might expect it to slowly convert at room temperature to another zinc borate phase, such as Zn[B
3O
3(OH)
5]·H
2O, which forms below 70 °C. However, apparently because of seeding effects, this does not occur. After continuously stirring a 10 wt% suspension of ZnB
3O
4(OH)
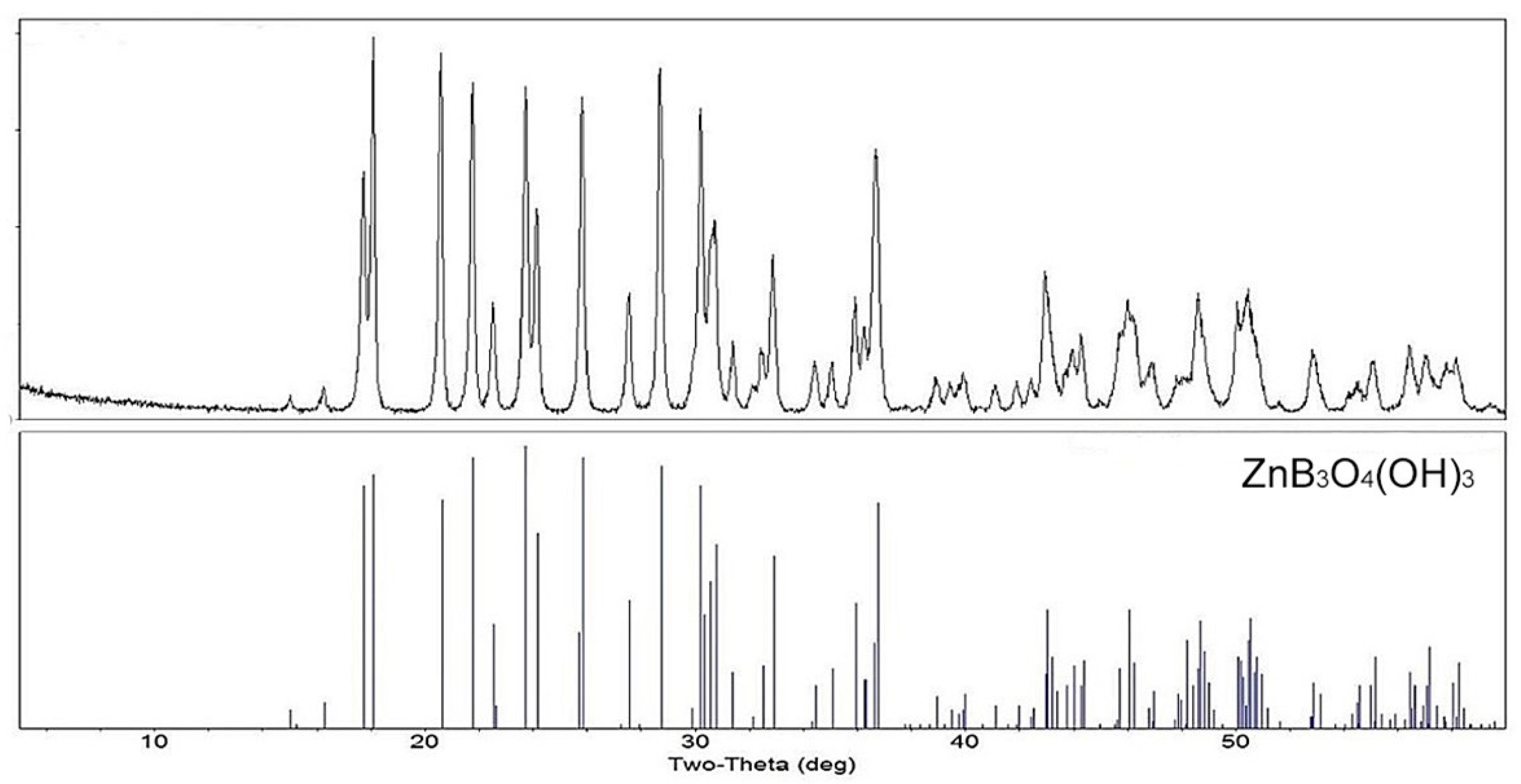
3 at room temperature for more than one year the PXRD pattern of the suspended solids showed no indiction of the presence of other phases. This indicates that hydrolysis, at least in concentrated suspensions, is reversible. The PXRD pattern for ZnB
3O
4(OH)
3 is shown in
Figure 2.
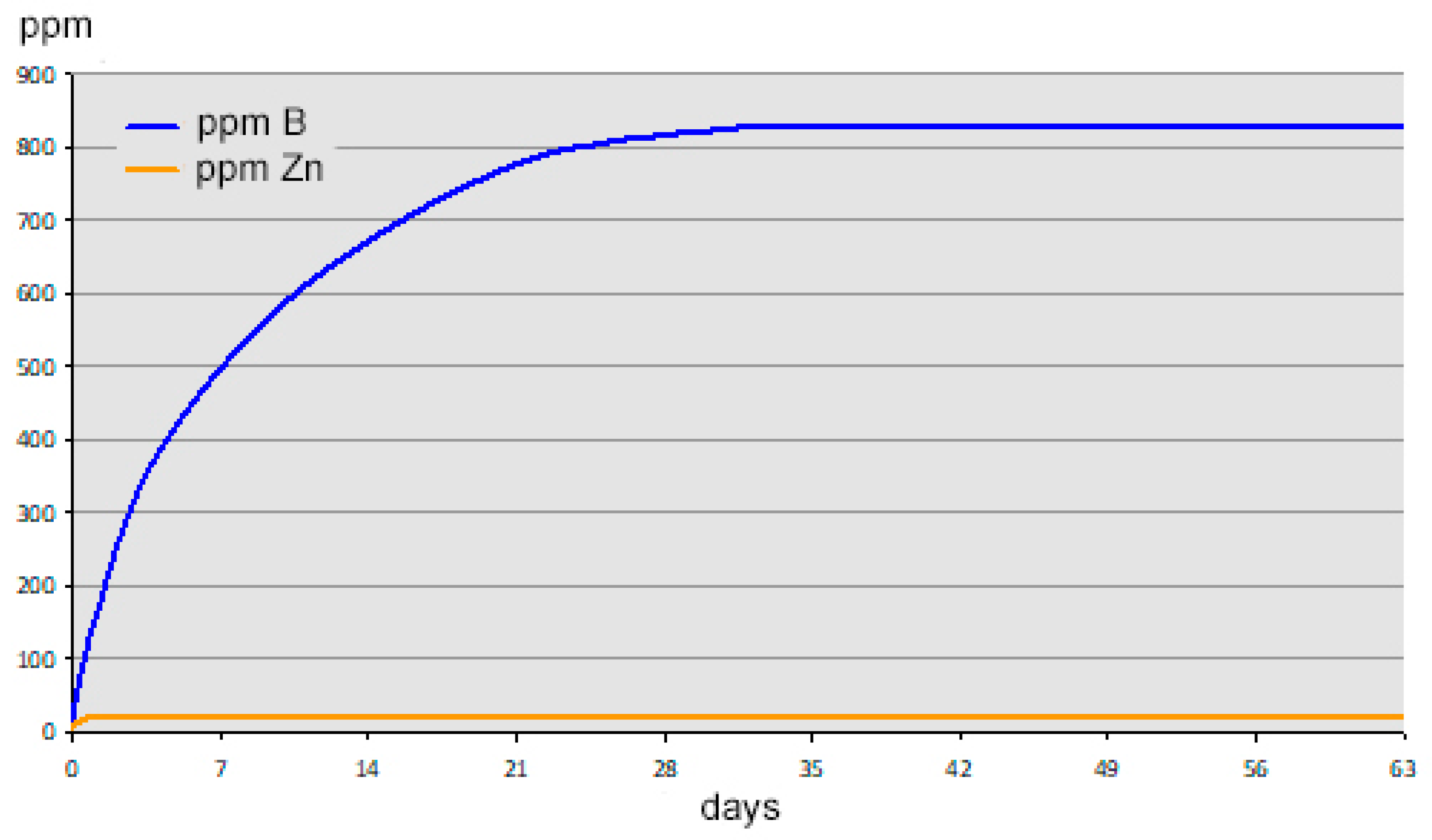
When the boron and zinc concentrations of the supernatant solution in a 10 wt% aqueous suspension of zinc borate were monitored over time, it was found that approximately one month is required to reach equilibrium, as shown by the graph in
Figure 3. As expected, more dilute suspensions achieve equilibrium more rapidly. A 0.05 wt% suspension of ZnB
3O
4(OH)
3 completely hydrolyzes within 24 h.
ZnB
3O
4(OH)
3 was found to be a stable phase when a 10 wt% aqueous suspension was maintained at the boiling point under reflux for prolonged periods of time. However, more dilute slurries were observed to convert to other phases. For example, continuous boiling under reflux of a 5 wt% aqueous suspension of ZnB
3O
4(OH)
3 (2ZnO·3B
2O
3·3H
2O) for one month resulted in complete conversion to the crystalline phase 6ZnO·5B
2O
3·3H
2O. This is represented using oxide formulas by Equation (3) as the structural formula of 6ZnO·5B
2O
3·3H
2O is not currently known. This phase was first reported by Lehmann, et al. who prepared it by heating a mixture of zinc oxide with boric acid in a 1:6–8 mole ratio with water in a sealed container for 16 h at 165 °C [
12]. The formation of 6ZnO·5B
2O
3·3H
2O from ZnB
3O
4(OH)
3 in boiling water is preceded after one week by quantitative formation of the crystalline phase Zn
2BO
3(OH) (4ZnO·B
2O
3·H
2O) according to Equation (4), which left in situ further converts to 6ZnO·5B
2O
3·3H
2O. Methods were developed previously to manufacture Zn
2BO
3(OH) rapidly on large commercial scale since it is valued for its usually high dehydration onset temperature of ca. 411 °C when used as a polymer additive [
13,
14]. It is notable that Zn
2BO
3(OH) (4ZnO·B
2O
3·H
2O), with B
2O
3/ZnO ratio 0.25, can be considered to be more hydrolyzed than 6ZnO·5B
2O
3·3H
2O, with B
2O
3/ZnO ratio 0.83, and must reabsorb boric acid from solution to further convert to the latter.
Complete hydrolysis, according to Equation (2), of 100.0 g of zinc borate results in 87.2 g of boric acid and 46.7 g zinc hydroxide and requires 33.9 g of water. Thus, pure zinc borate can be considered to have a boric acid equivalence (%BAE) of 87.2% upon complete hydrolysis. This boric acid content is only released at relatively high dilution. Because of the low solubility of the Zn(OH)
2 hydrolysis product, zinc borate does not appear to dissolve in water. This has led to occasional statements in commercial literature that zinc borate is insoluble in water. The extent of hydrolysis ZnB
3O
4(OH)
3 as a function of varying suspension concentrations is illustrated in
Figure 4. A 50 wt% aqueous suspension of zinc borate hydrolyzes to the extent of about 0.4%, whereas a 0.05 wt% suspension completely hydrolyzes
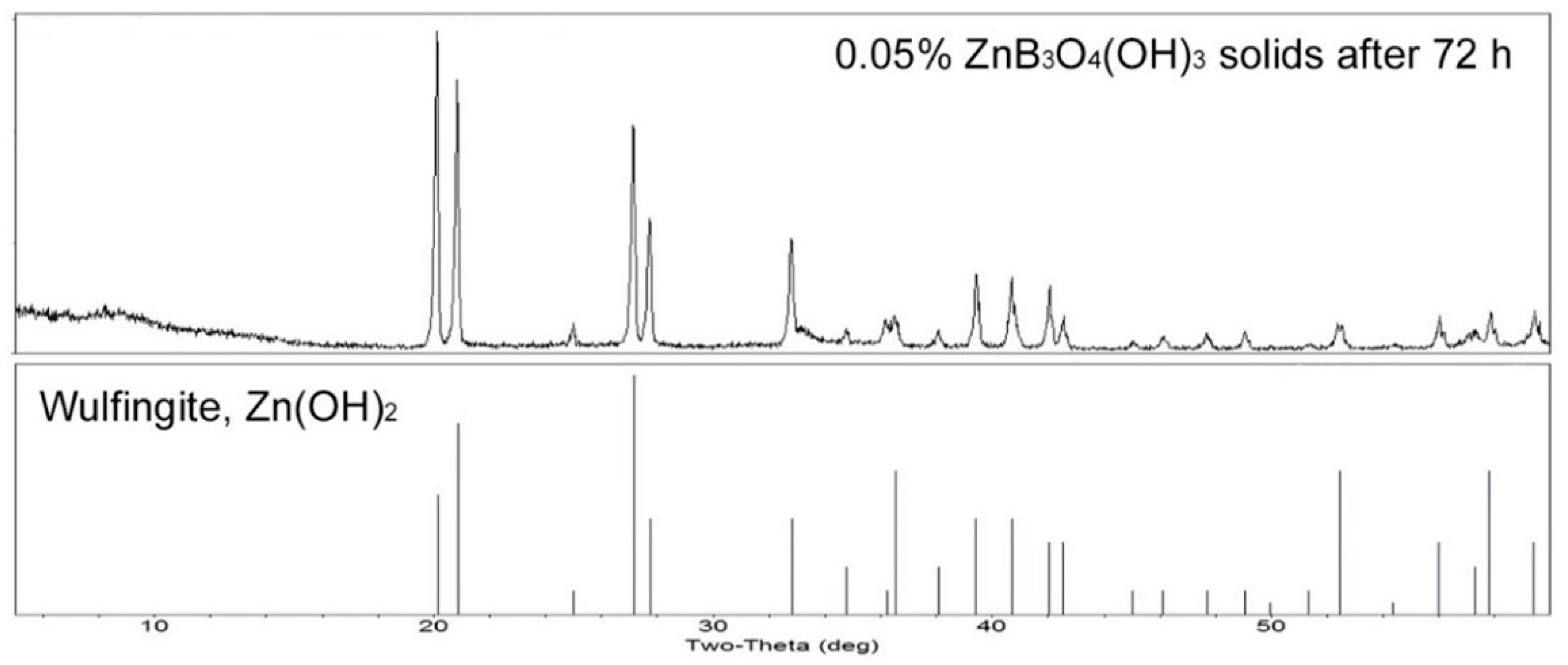
When the hydrolysis procedure described in
Section 2.2 was carried out with a 0.10% zinc borate aqueous suspension, the solids remaining after 72 h were found to be primarily the wulfingite phase of zinc hydoxide with a minor amount of yet unhydrolyzed zinc borate, as indicated by the XRD pattern shown in
Figure 5. Synthetic wulfingite, also known as ε-Zn(OH)
2, is the most stable of the five polymorphs of Zn(OH)
2. It has a well-defined structure composed of tetrahedral Zn
2+ cations connected by corner sharing hydroxyl groups in the orthorhombic space group [
15].
PXRD analysis of the suspended solids in a 0.05 wt% suspension of ZnB
3O
4(OH)
3 after 72 h showed complete hydrolysis to Zn(OH)
2, as shown in
Figure 6. Analysis of the clear filtrate by ICP-OES indicated that it contained 77 ppm B, within error of the calculated value of 76 ppm expected for full hydrolysis of the zinc borate according to Equation (2). The zinc concentration of the filtrate, which had a pH value near 7, was found to be 10 ppm, indicating that ca. 94% of the zinc resulting from hydrolysis was in the form of insoluble Zn(OH)
2. At this concentration and pH, it is expected that boron is primarily present as free boric acid with only a small fraction exiting in the form its conjugate base, B(OH)
4−, and polyborate species.
A portion of the clear filtrate from the 0.05 wt% suspension that was filtered after 72 h was allowed to evaporate to dryness, leaving a white powder. This solid evaporite was submitted to PXRD analysis revealing the pattern of boric acid (sassolite phase) and no additional diffraction peaks, as shown in
Figure 7.