Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Deep Eutectic Solvents
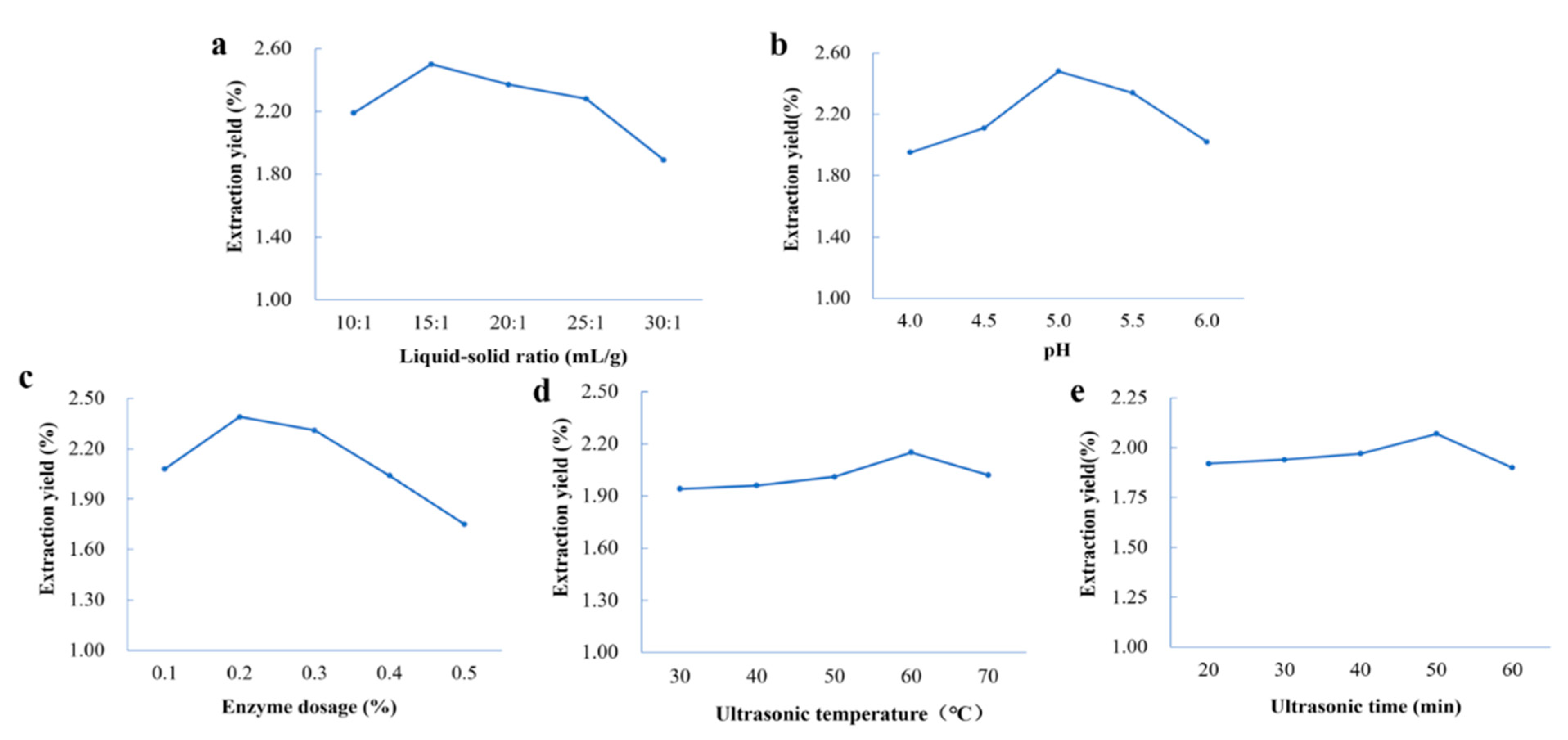
2.2. Single-Factor Experimental Analysis of Extraction of Coumarins from P. decursivum
2.2.1. Effect of the Liquid–Solid Ratio
2.2.2. Effect of the pH
2.2.3. Effect of the Enzyme Dosage
2.2.4. Effect of Ultrasonic Temperature
2.2.5. Effect of Ultrasonic Time
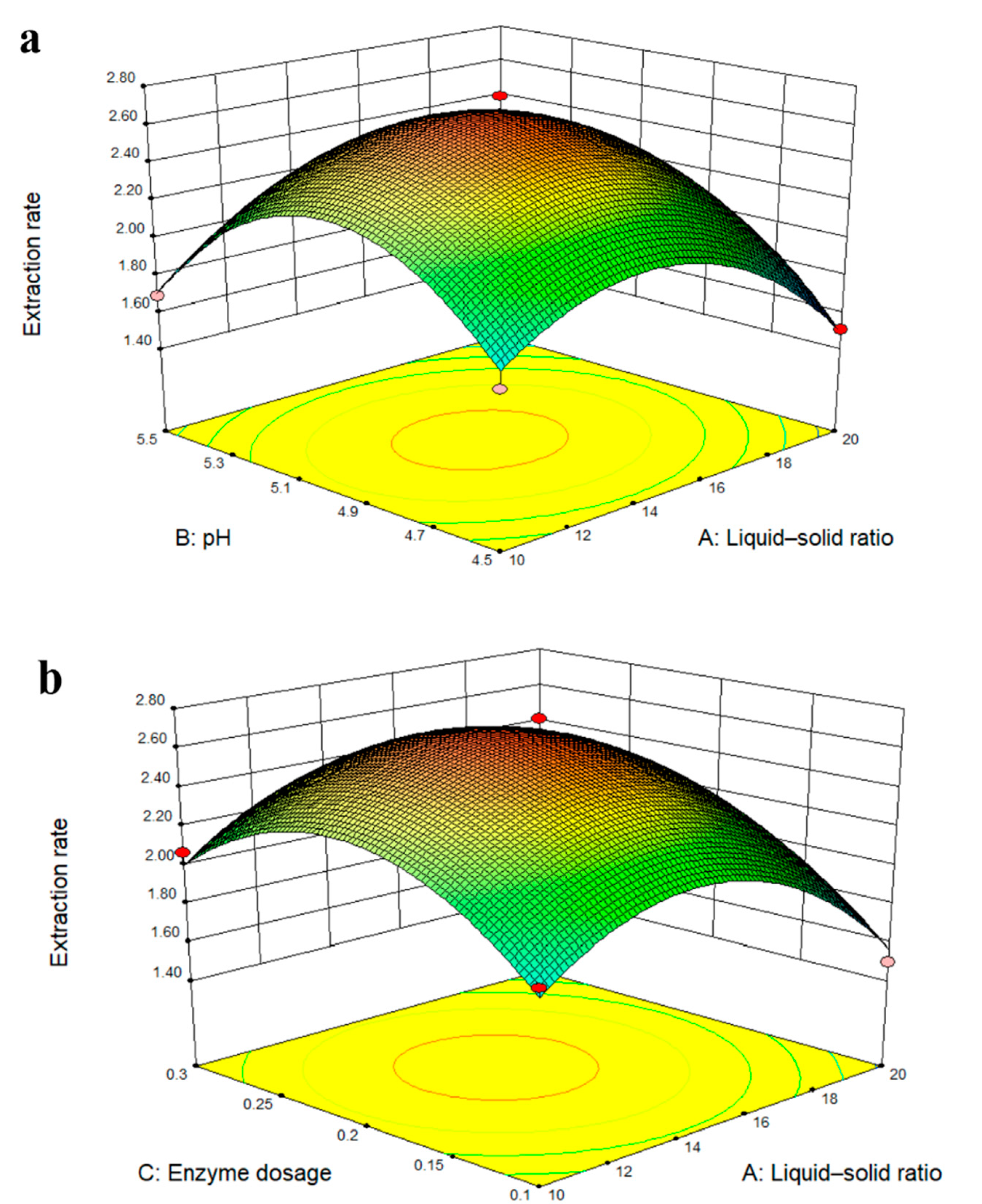
2.3. Optimization of the Extraction Conditions Using a BBD RSM
2.4. Response Surface Analysis Diagram
2.5. Validation of the Prediction Model
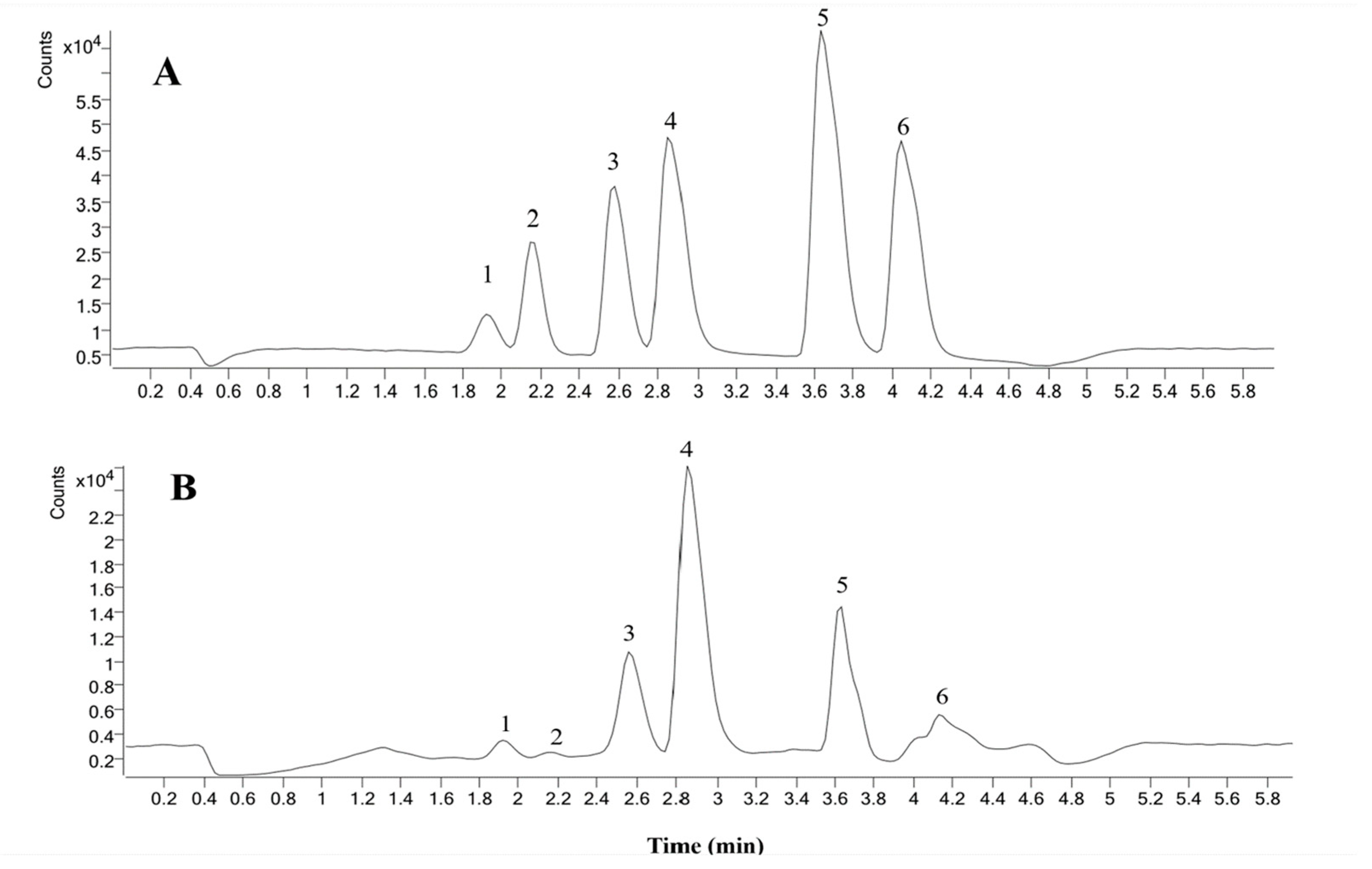
2.6. Contents Analysis of Six Coumarins of P. decursivum
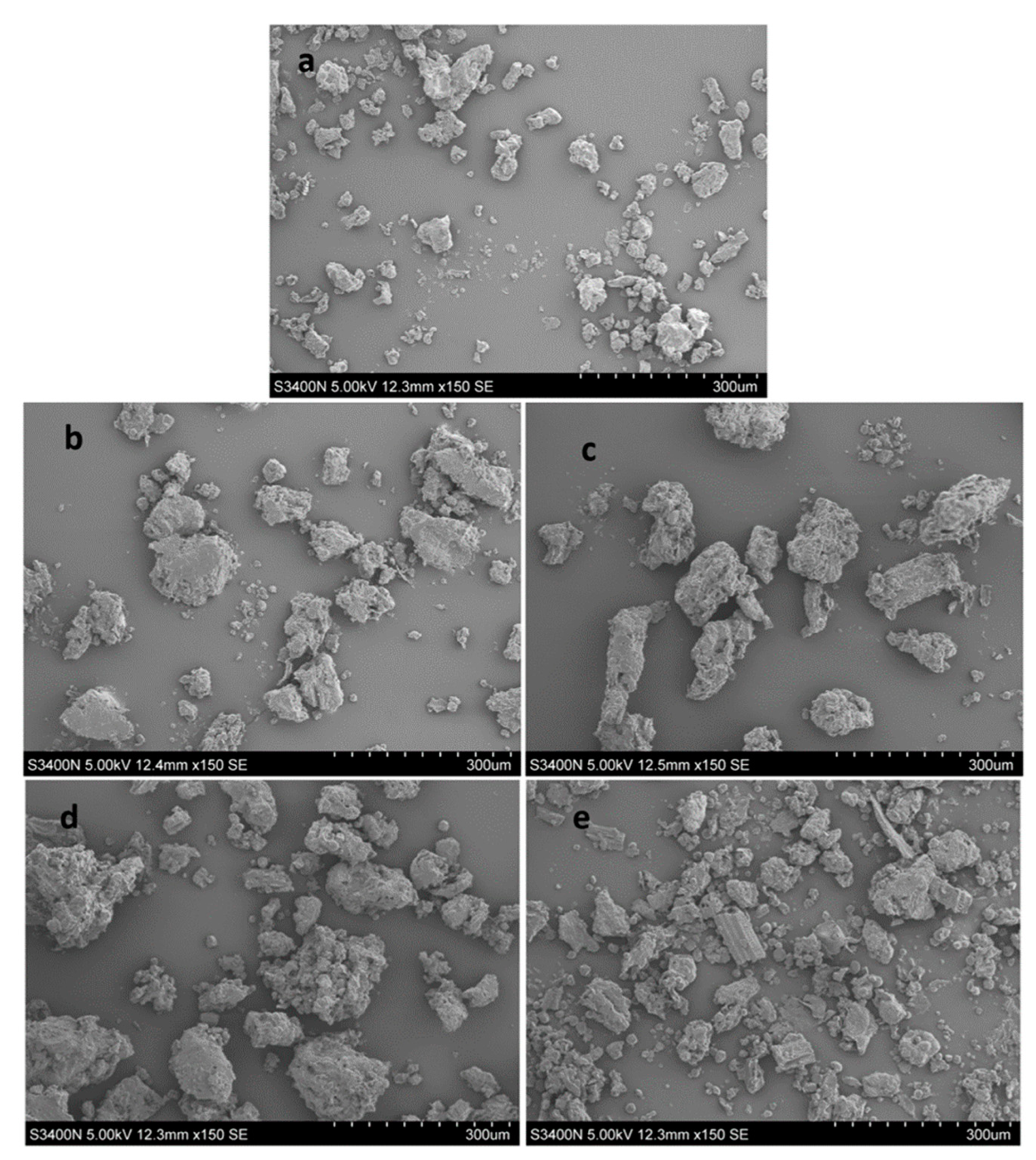
2.7. Microstructure of the Plant Powder
2.8. Antioxidant Capacity of the Extracts
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Establishment of a Method for the Determination of Total Coumarins in P. decursivum Using UV-VIS Spectroscopy
3.3.1. Preparation of the Reference Solution
3.3.2. UV-VIS Spectroscopy Conditions
3.3.3. Drawing the Standard Curve
3.4. Total Coumarins from P. decursivum Using Enzyme-Assisted DES Extraction
3.4.1. Preparation of the DESs
3.4.2. Enzyme Pretreatment
3.4.3. Ultrasonic Extraction
3.5. Validation of the Method for the Determination of Total Coumarins in P. decursivum Using UV-VIS Spectroscopy
3.6. Single-Factor Experimental Design
3.7. Determination of Six Coumarins in P. decursivum Using HPLC-MS
3.7.1. Chromatographic and Mass Spectrometric Conditions
3.7.2. Preparation of the Reference Solution
3.7.3. Preparation of the Test Solution
3.7.4. Investigation of the Linear Relationship
3.7.5. Methodological Validation
3.8. Microstructure of the Plant Powders
3.9. Determination of the Antioxidant Activity of Plant Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1, pp. 352–353. [Google Scholar]
- Zhou, Y. Identification of Radix Peucedani and its adulterant Radix Peucedani Terebinthaceum. China Pharm. 2008, 14, 62–63. [Google Scholar]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Song, Z.Q.; Li, B.; Tian, K.Y.; Hong, L.; Wu, W.; Zhang, H.Y. Research progress on chemical constituents and pharmacological activities of Peucedani Radix and Peucedani Decursivi Radix. Chin. Tradit. Herb. Drugs 2022, 53, 948–964. [Google Scholar]
- Hoult, J.R.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Chen, C.C.; Hong, W.Y.; Wei, H.D.; Wang, J.M. Simultaneous determination of five active components in Peucedanum decursivum (Miq.) Maxim by HPLC with dual wavelength. J. Chin. Med. Mater. 2022, 2, 412–415. [Google Scholar]
- Medlej, M.K.; Cherri, B.; Nasser, G.; Zaviska, F.; Hijazi, A.; Li, S.; Pochat-Bohatier, C. Optimization of polysaccharides extraction from a wild species of Ornithogalum combining ultrasound and maceration and their anti-oxidant properties. Int. J. Biol. Macromol. 2020, 161, 958–968. [Google Scholar] [CrossRef]
- Tong, X.; Yang, Z.; Wan, H.; Li, C. Greener extraction process and enhanced in vivo bioavailability of bioactive components from Carthamus tinctorius L. by natural deep eutectic solvents. Food Chem. 2021, 348, 129090. [Google Scholar] [CrossRef]
- Wang, B.L.; Chen, Y.X.; Xie, J.X.; Zhao, Y.H. Ultrasound-assisted cellulase optimization of extraction process of total flavonoids from Fagopyrum dibotrys (D. Don) Hara and its antioxidant activity research. Cereals Oils 2021, 34, 123–128. [Google Scholar]
- Wan, X.H.; Chen, X.M.; Ma, S. Application of new extraction methods of flavonoids. Chin. Tradit. Herb. Drugs 2019, 50, 3691–3699. [Google Scholar]
- Balasubramaniam, G.; Ayyappan, P.; Sathvika, S.; Antony, U. Effect of enzyme pretreatment in the ultrasound assisted extraction of finger millet polyphenols. J. Food Sci. Technol. 2019, 56, 1583–1594. [Google Scholar] [CrossRef]
- Xi, A.; Zhan, Y.J.; Wang, L. Study on enzymatic-assisted ultrasonic extraction of total flavonoids from Trollius altaicus. China Food Addit. 2020, 31, 68–75. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, K.; Bagh, F.S.G.; Mjalli, F.S. Prediction of refractive index and density of deep eutectic solvents using atomic contributions. Fluid Phase Equilib. 2013, 354, 304–311. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Lu, X.H.; Feng, X.; Shi, Y.J.; Ji, X.Y. Properties and applications of choline-based deep eutectic solvents. Prog. Chem. 2013, 25, 881–892. [Google Scholar]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Guo, W.; Hou, Y.; Wu, W. Separation of phenol from model oils with quaternary ammonium salts via forming deep eutectic solvents. Green Chem. 2013, 15, 226–229. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.L. Tailor-made hydrophobic deep eutectic solvents for cleaner extraction of polyprenyl acetates from Ginkgo biloba leaves. J. Clean. Prod. 2017, 152, 399–405. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Choi, Y.H.; Spronsen, J.V.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AINashef, I.M.; Mirghani, M.E.S. Assessment of cytotoxicity and toxicity for phosphonium based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AINashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Thermal Instability of Choline Chloride-Based Deep Eutectic Solvents and Its Influence on Their Toxicity—Important Limitations of DESs as Sustainable Materials. Ind. Eng. Chem. Res. 2022, 61, 11288–11300. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Deep eutectic solvents microbial toxicity: Current state of art and critical evaluation of testing methods. J. Hazard. Mater. 2022, 425, 127963. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Pandey, S. Densities and viscosities of (choline chloride + urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Chen, W.J.; Xue, Z.M.; Xue, J.F.; Wang, J.Y.; Jiang, X.H.; Zhao, T.C.; Mu, T. Investigation on the Thermal Stability of Deep Eutectic Solvents. Acta Phys. Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Rathod, V.K. Green extraction of Glycyrrhizic acid from Glycyrrhiza glabra using choline chloride based natural deep eutectic solvents (NADESs). Process Biochem. 2021, 102, 22–32. [Google Scholar] [CrossRef]
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crop. Prod. 2019, 140, 111724. [Google Scholar] [CrossRef]
- Zuo, J.; Ma, P.; Geng, S.; Kong, Y.; Li, X.; Fan, Z.; Zhang, Y.; Dong, A.; Zhou, Q. Optimization of the extraction process of flavonoids from Trollius ledebouri with natural deep eutectic solvents. J. Sep. Sci. 2022, 45, 717–727. [Google Scholar] [CrossRef]
- Savic Gajic, I.; Savic, I.; Boskov, I.; Žerajić, S.; Markovic, I.; Gajic, D. Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae Pseudoacaciae) flowers and comparison with conventional methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef]
- Deng, Y.Q. Preparation and application of ionic liquids. Bull. Chin. Acad. Sci. 2005, 4, 297–300. [Google Scholar]
- Vanda, H.; Dai, Y.; Wilson, E.G. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C. R. Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Fernández, M.D.L.; Boiteux, J.; Espino, M. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, F.; Su, E. Improving flavonoid Extraction from Ginkgo biloba leaves by prefermentation processing. J. Agric. Food Chem. 2013, 61, 5783–5791. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Curko, N.; Tomasevié, M. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C.; Huang, T.C. Comparison of modified ultrasound-assisted and traditional extraction methods for the extraction of baicalin and baicalein from Radix Scutellariae. Ind. Crop. Prod. 2013, 45, 182–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.H.; Ding, M.M.; Zhang, R.R. Optimization of extraction process and in vitro activities of polysaccharides from Umbilicaria muehlenbergii (Ascomycota). Mycosystema 2021, 40, 48–59. [Google Scholar]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress a key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Zou, Z.R. Research progress on flavonoids and coumarins from Chimonanthus plants and its pharmacological activities. Chin. Tradit. Herb. Drugs 2018, 49, 3425–3431. [Google Scholar]
- Xu, Z.; Cai, Y.; Ma, Q.; Zhao, Z.; Yang, D.; Xu, X. Optimization of extraction of bioactive compounds from Baphicacanthus cusia leaves by hydrophobic deep eutectic solvents. Molecules 2021, 26, 1729. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Fan, X.H.; Chang, Y.H.; Wang, L.T.; Zhu, Y.W.; Dong, M.Z.; Lv, M.J.; An, J.Y.; Yang, Q.; Jiao, J.; Meng, D.; et al. A simple and efficient sample preparation for taxanes in Taxus chinensis needles with natural menthol-based aqueous deep eutectic solvent. J. Sep. Sci. 2020, 43, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.T.; Pei, D.; Yu, P.L.; Wei, J.T.; Wang, N.L.; Di, D.L.; Liu, Y.W. Aqueous two-phase systems based on deep eutectic solvents and their application in green separation processes. J. Sep. Sci. 2020, 43, 348–359. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, M.; Zhang, K.; Wang, L.; Gao, M.; Xia, Z.; Gao, D. Extraction and determination of bioactive flavonoids from Abelmoschus manihot (Linn.) Medicus flowers using deep eutectic solvents coupled with high-performance liquid chromatography. J. Sep. Sci. 2019, 42, 2044–2052. [Google Scholar] [CrossRef]
- Zhang, K.; Li, S.; Liu, C.; Wang, Q.; Wang, Y.; Fan, J. A hydrophobic deep eutectic solvent-based vortex-assisted dispersive liquid-liquid microextraction combined with HPLC for the determination of nitrite in water and biological samples. J. Sep. Sci. 2019, 42, 574–581. [Google Scholar] [CrossRef]
- Najafi, A.; Hashemi, M. Vortex-assisted natural deep eutectic solvent microextraction using response surface methodology optimization for determination of orthophosphate in water samples by molybdenum blue method. J. Sep. Sci. 2019, 42, 3102–3109. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Lin, Y.S.; Zhang, J.; Fan, X.N. Optimization of cellulase-assisted flash extraction of total coumarins from Peucedani radix by response surface methodology. Food Ind. 2017, 38, 121–124. [Google Scholar]
- Zhang, X.S.; Liu, H.Y.; Zhou, Y.; Wang, S.J.; Zhou, W.H.; Nie, F. Properties and applications of choline-based deep eutectic solvents. China Food Addit. 2018, 2, 55–66. [Google Scholar]
- Zhang, D.-Y.; Wan, Y.; Hao, J.-Y. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crop. Prod. 2018, 122, 298–307. [Google Scholar] [CrossRef]



| Single Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A (Liquid–solid ratio, mg/mL) | 10 | 15 | 20 |
| B (pH) | 4.5 | 5.0 | 5.5 |
| C (Enzyme dosage, %) | 0.1% | 0.2% | 0.3% |
| No. | A (Liquid–Solid Ratio, mg/mL) | B (pH) | C (Enzyme Dosage, %) | Y (Extraction Yield, %) |
|---|---|---|---|---|
| 1 | 1 | 0 | −1 | 1.50 |
| 2 | 0 | 0 | 0 | 2.65 |
| 3 | −1 | 0 | −1 | 1.89 |
| 4 | 0 | 0 | 0 | 2.63 |
| 5 | 0 | −1 | −1 | 1.51 |
| 6 | 1 | 0 | 1 | 1.78 |
| 7 | 1 | −1 | 0 | 1.51 |
| 8 | 0 | −1 | 1 | 1.95 |
| 9 | 1 | 1 | 0 | 1.68 |
| 10 | 0 | 1 | −1 | 1.68 |
| 11 | 0 | 0 | 0 | 2.75 |
| 12 | −1 | −1 | 0 | 1.75 |
| 13 | 0 | 1 | 1 | 1.61 |
| 14 | −1 | 1 | 0 | 1.69 |
| 15 | 0 | 0 | 0 | 2.63 |
| 16 | 0 | 0 | 0 | 2.66 |
| 17 | −1 | 0 | 1 | 2.07 |
| Variables | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 3.48 | 9 | 0.39 | 61.80 | <0.001 | Significant |
| A: liquid–solid ratio | 0.11 | 1 | 0.11 | 17.26 | 0.0043 | |
| B: pH | 0.00045 | 1 | 0.00045 | 0.072 | 0.7964 | |
| C: enzyme dosage | 0.086 | 1 | 0.086 | 13.75 | 0.0076 | |
| AB | 0.013 | 1 | 0.013 | 2.11 | 0.1895 | |
| AC | 0.0025 | 1 | 0.0025 | 0.40 | 0.5476 | |
| BC | 0.065 | 1 | 0.065 | 10.38 | 0.0146 | |
| A2 | 0.82 | 1 | 0.82 | 131.33 | <0.001 | |
| B2 | 1.34 | 1 | 1.34 | 214.21 | <0.001 | |
| C2 | 0.71 | 1 | 0.71 | 114.11 | <0.001 | |
| Residual | 0.044 | 7 | 0.006264 | |||
| Lack of fit | 0.034 | 3 | 0.011 | 4.56 | 0.0884 | Not significant |
| Pure error | 0.00992 | 4 | 0.00248 | |||
| Cor total | 3.53 | 16 | ||||
| R2 | 0.9876 | |||||
| Adj R2 | 0.9716 |
| Extraction Method | IC50 (mg/mL) |
|---|---|
| Enzyme-assisted methanol | 24.088 |
| Enzyme-assisted 70% ethanol | 25.512 |
| Enzyme-assisted DESs | 21.046 |
| No. | Hydrogen Bond Acceptors (HBAs) | Hydrogen Bond Donors (HBDs) | HBA/HBD Ratio | Appearance at Room Temperature |
|---|---|---|---|---|
| 1 | Choline chloride | Lactic acid | 1:2 | Light-yellow transparent liquid |
| 2 | Choline chloride | Lactic acid | 1:3 | Light-yellow transparent liquid |
| 3 | Choline chloride | Lactic acid | 1:4 | Light-yellow transparent liquid |
| 4 | Choline chloride | Glycerol | 1:2 | Transparent liquid |
| 5 | Choline chloride | Glycerol | 1:3 | Transparent liquid |
| 6 | Choline chloride | Glycerol | 1:4 | Transparent liquid |
| 7 | Choline chloride | 1,3-Butanediol | 1:2 | Transparent liquid |
| 8 | Choline chloride | 1,3-Butanediol | 1:3 | Transparent liquid |
| 9 | Choline chloride | 1,3-Butanediol | 1:4 | Transparent liquid |
| 10 | Choline chloride | 1,4-Butanediol | 1:2 | Transparent liquid |
| 11 | Choline chloride | 1,4-Butanediol | 1:3 | Transparent liquid |
| 12 | Choline chloride | 1,4-Butanediol | 1:4 | Transparent liquid |
| 13 | Choline chloride | Urea | 1:2 | Transparent liquid |
| 14 | Choline chloride | Urea | 1:3 | Transparent liquid |
| 15 | Choline chloride | Urea | 1:4 | Transparent liquid |
| Analyte | Calibration Curve | R2 | Linearity Range (μg/mL) | Precision (RSD%) | Repeatability (RSD%) | Stability (RSD%) | Sample Recovery (RSD%) |
|---|---|---|---|---|---|---|---|
| Umbelliferone | y = 4.690579x − 306.776623 | 0.9947 | 0.16–6 | 0.68 | 0.71 | 0.60 | 1.49 |
| Nodakenin | y = 15.913140x − 506.921696 | 0.9996 | 0.16–6 | 1.19 | 0.97 | 0.79 | 1.40 |
| Xanthotoxin | y = 23.414009x − 2979.434097 | 0.9969 | 0.16–6 | 0.45 | 0.59 | 1.04 | 0.68 |
| Bergapten | y = 40.140902x − 2041.418550 | 0.9966 | 0.16–6 | 1.02 | 1.04 | 1.22 | 1.56 |
| Imperatorin | y = 75.809103x − 3549.463841 | 0.9986 | 0.16–6 | 0.68 | 0.89 | 0.84 | 1.38 |
| Decursin | y = 46.962356x − 6888.543659 | 0.9941 | 0.16–6 | 0.60 | 1.41 | 1.24 | 2.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Q. Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment. Molecules 2022, 27, 5715. https://doi.org/10.3390/molecules27175715
Li Z, Li Q. Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment. Molecules. 2022; 27(17):5715. https://doi.org/10.3390/molecules27175715
Chicago/Turabian StyleLi, Zeyu, and Qian Li. 2022. "Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment" Molecules 27, no. 17: 5715. https://doi.org/10.3390/molecules27175715
APA StyleLi, Z., & Li, Q. (2022). Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment. Molecules, 27(17), 5715. https://doi.org/10.3390/molecules27175715






