Intermolecular Interactions of Edaravone in Aqueous Solutions of Ethaline and Glyceline Inferred from Experiments and Quantum Chemistry Computations
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Solubility Determination of Edaravone in DES
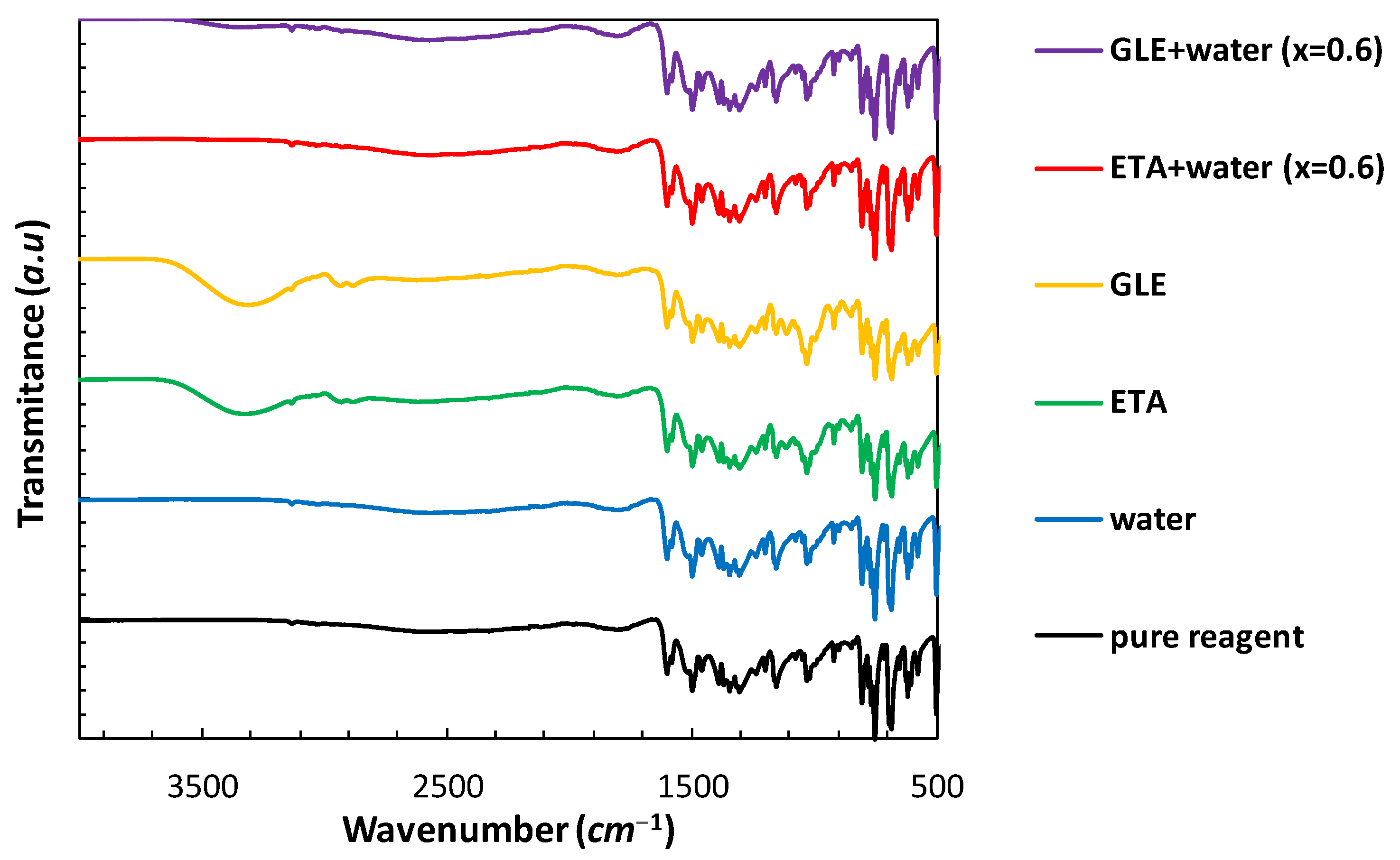
2.2. Instrumental Characteristics of Saturated Systems
2.3. Tautomersim of Edaravone
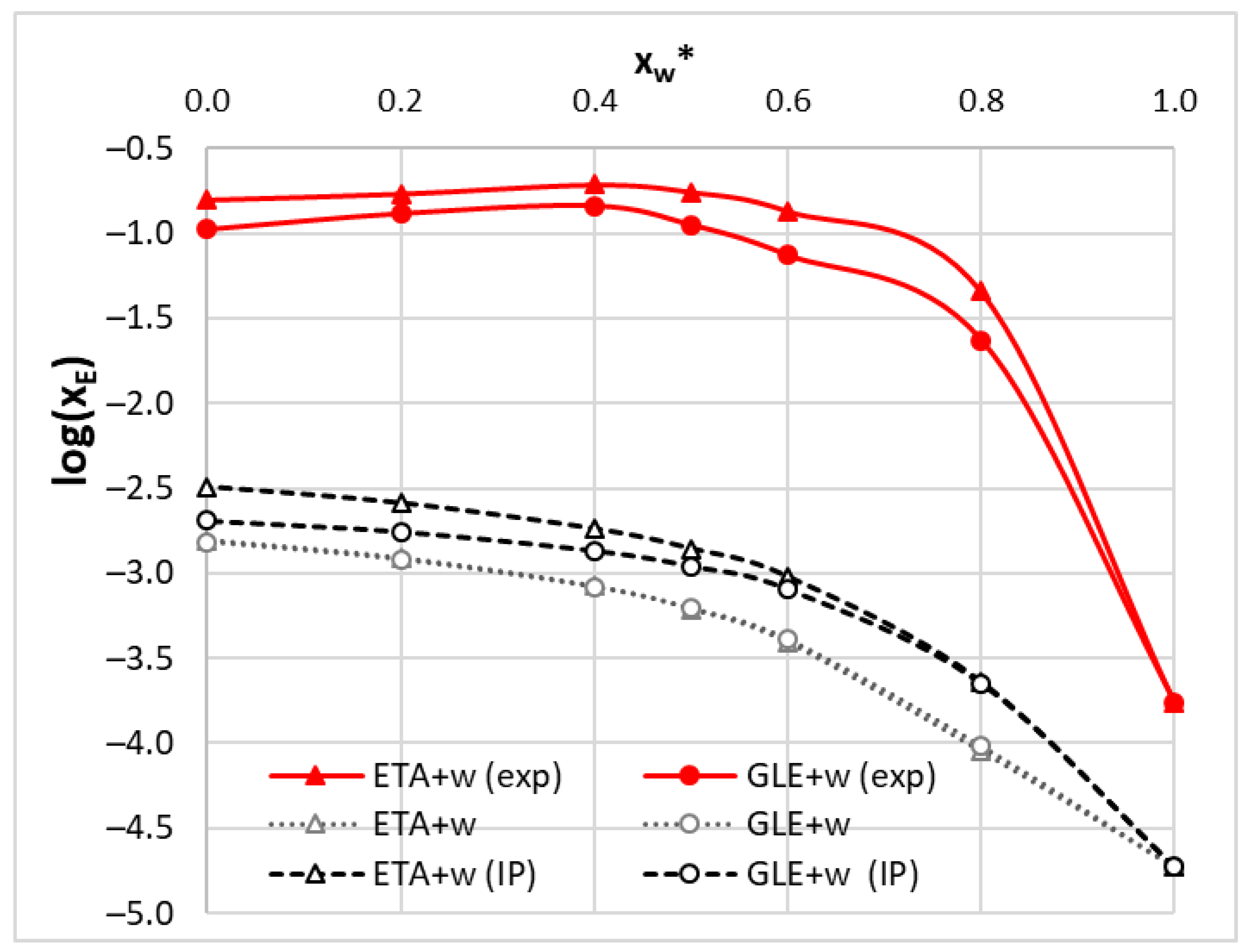
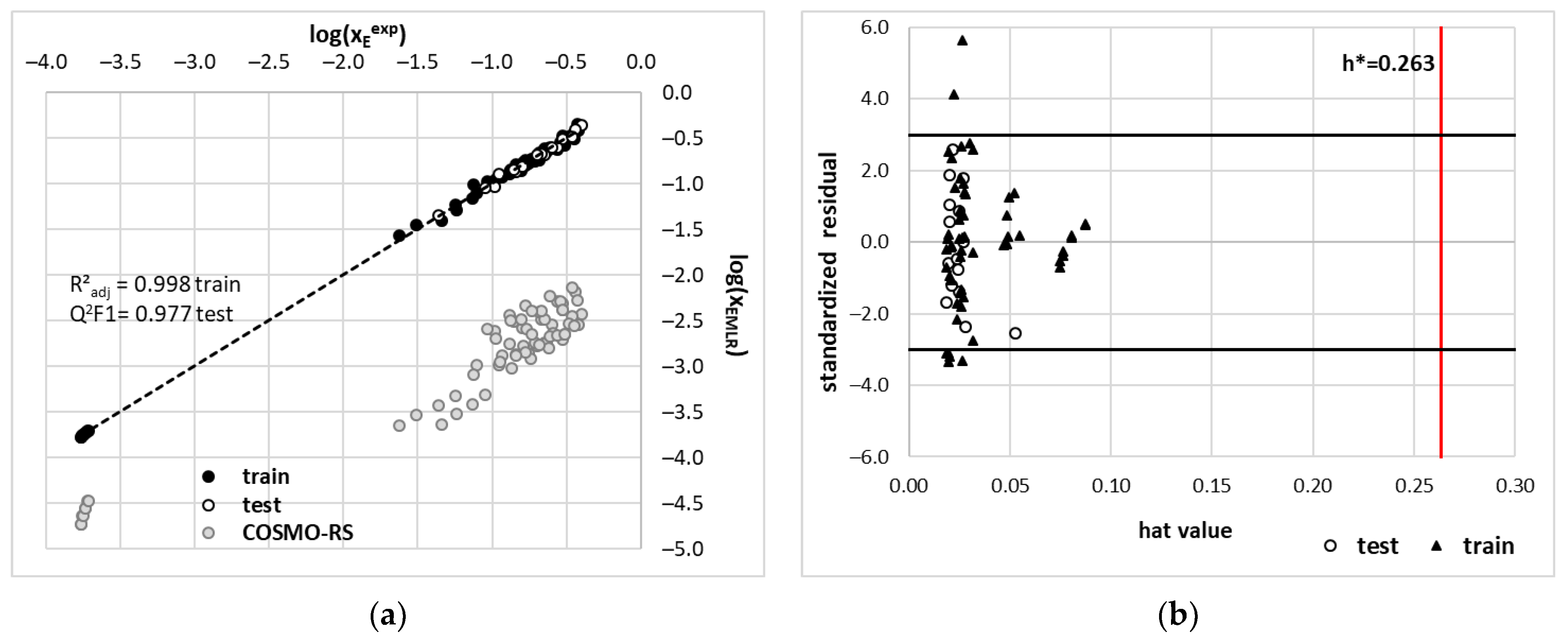
2.4. COSMO-RS Derived Edaravone Solubility in DES
2.5. Intermolecular Interactions in Eradavone-DES Systems
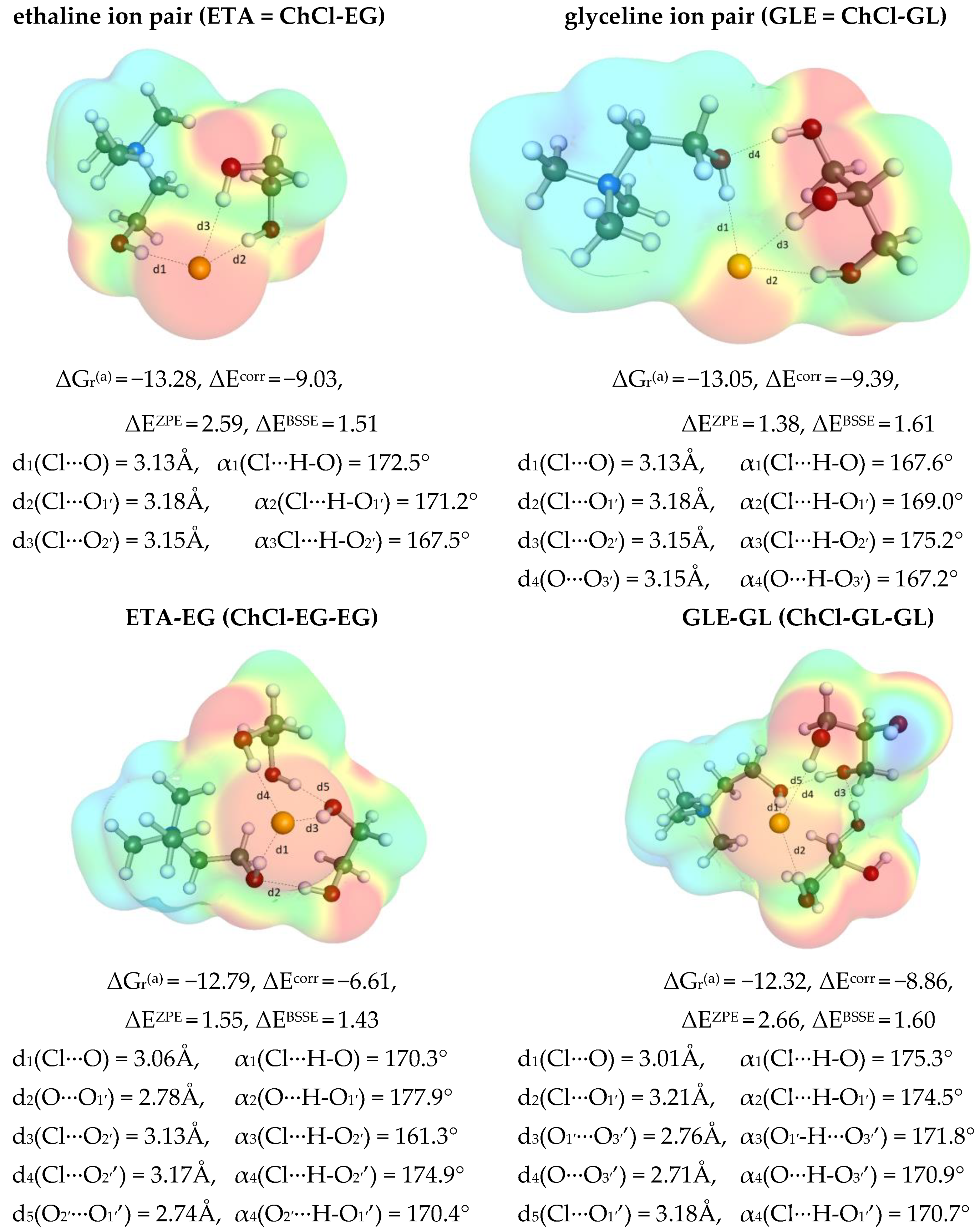
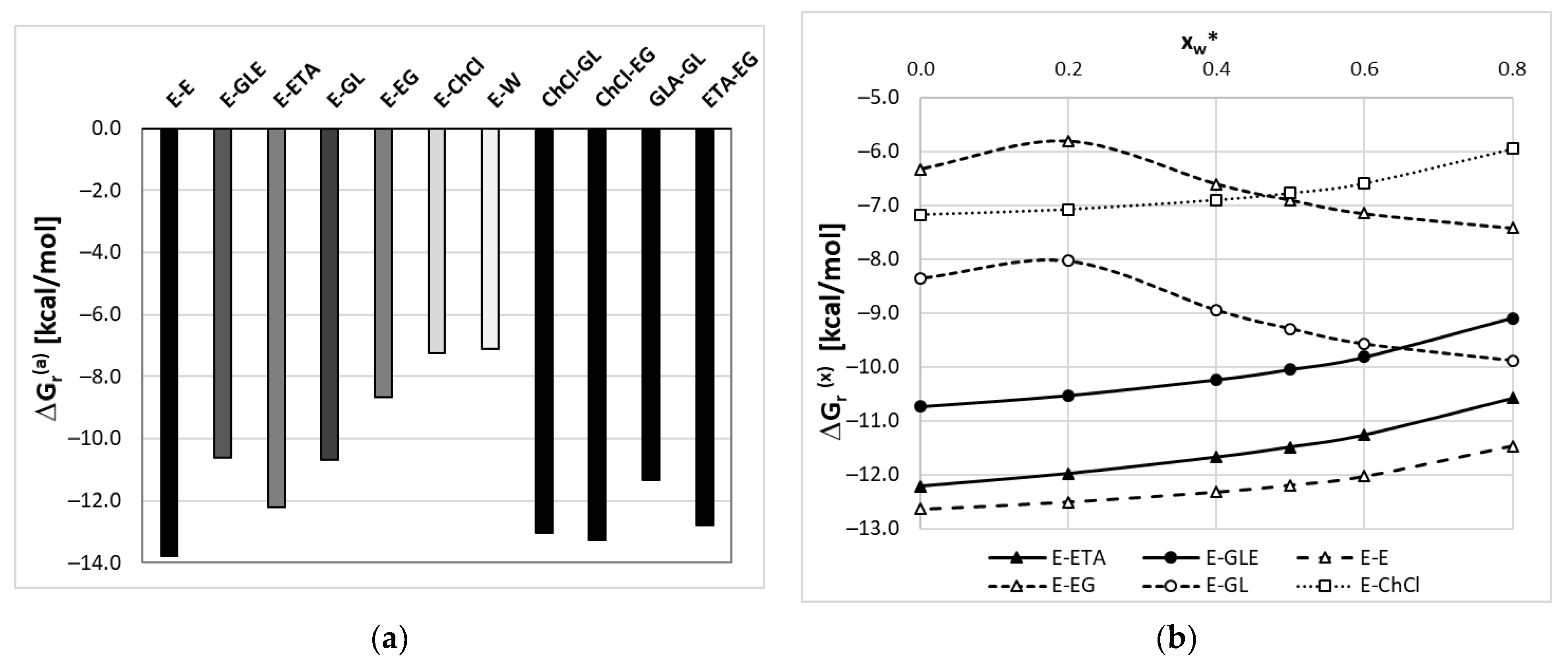
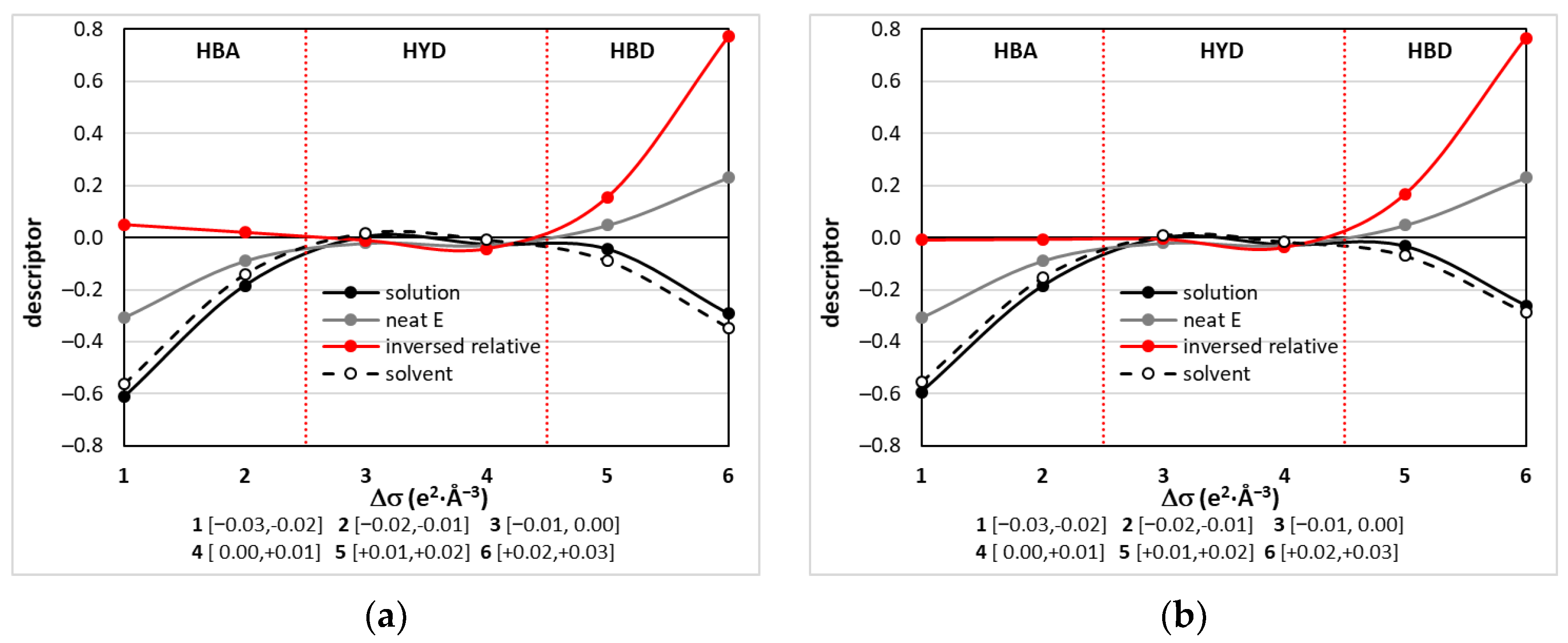
2.5.1. Mutual Affinity of DES Components
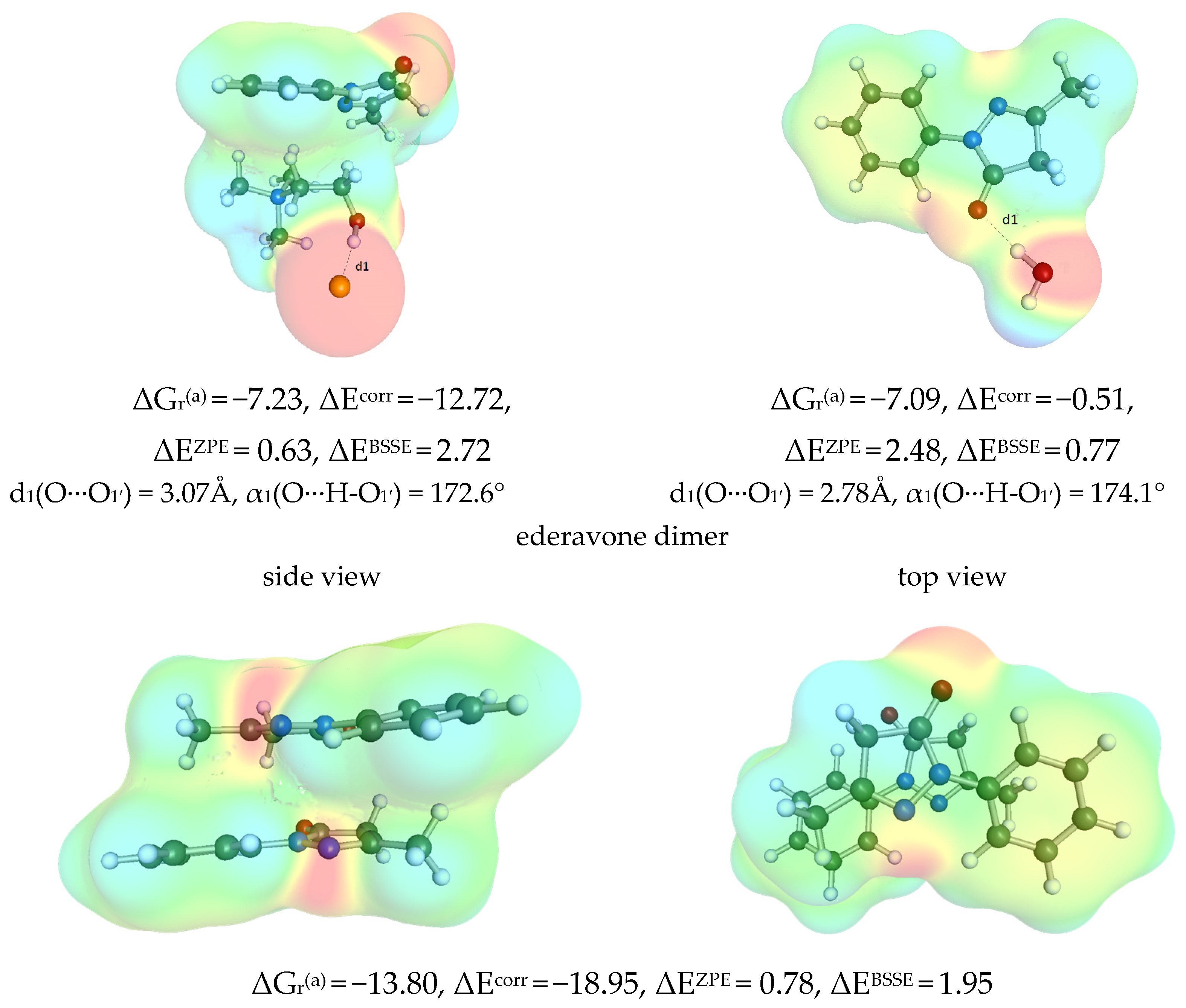
2.5.2. Edaravone Affinity to DES Components
2.6. Theoretical Model of Edaravone Solubility in DES
3. Materials and Methods
3.1. Solubility Measurements
3.1.1. Materials
3.1.2. Preparation of the Calibration Curve
3.1.3. Preparation of Edaravone Samples in DES and in DES-Water Systems
3.1.4. Edaravone Solubility Measurements
3.2. DSC and FTIR Measurements
3.3. QSPR Modeling
3.4. Molecular Descriptors Computations
3.4.1. Cosmo-RS Derived Solubility
3.4.2. Intermolecular Interactions Characteristics
3.4.3. Affinity Computations
3.4.4. Intermolecular Interactions as Molecular Descriptors
3.4.5. Cosmo-RS Derived σ-Potentials as Molecular Descriptors
3.5. Calculation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kalam, M.A.; Khan, A.A.; Alshamsan, A.; Haque, A.; Shakeel, F. Solubility of a poorly soluble immunosuppressant in different pure solvents: Measurement, correlation, thermodynamics and molecular interactions. J. Mol. Liq. 2018, 249, 53–60. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Xia, M.; Dai, W.; Zhu, B.; Mei, X. Improving the dissolution behaviors and bioavailability of abiraterone acetate via multicomponent crystal forms. Int. J. Pharm. 2022, 614, 121460. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Kumar, R.; Vipin Kumar, V.K.; Kumar, A.; Kumar Gaurav, G.; Naresh Gupta, K. A critical review on thermodynamic and hydrodynamic modeling and simulation of liquid antisolvent crystallization of pharmaceutical compounds. J. Mol. Liq. 2022, 362, 119663. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, C.-M.; Jo, A.-N.; Kim, J.-E. Studies on Preformulation and Formulation of JIN-001 Liquisolid Tablet with Enhanced Solubility. Pharmaceuticals 2022, 15, 412. [Google Scholar] [CrossRef]
- Anwer, M.K.; Muqtader, M.; Iqbal, M.; Ali, R.; Almutairy, B.K.; Alshetaili, A.; Alshahrani, S.M.; Aldawsari, M.F.; Alalaiwe, A.; Shakeel, F. Estimating the Solubility, Solution Thermodynamics, and Molecular Interaction of Aliskiren Hemifumarate in Alkylimidazolium Based Ionic Liquids. Molecules 2019, 24, 2807. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Imran, M.; Haq, N.; Alshehri, S.; Anwer, M.K. Synthesis, Characterization and Solubility Determination of 6-Phenyl-pyridazin-3(2H)-one in Different Pharmaceutical Solvents. Molecules 2019, 24, 3404. [Google Scholar] [CrossRef]
- Drugbank Edaravone. Available online: https://go.drugbank.com/drugs/DB12243 (accessed on 28 November 2022).
- Watanabe, T.; Tahara, M.; Todo, S. The Novel Antioxidant Edaravone: From Bench to Bedside. Cardiovasc. Ther. 2008, 26, 101–114. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A.; Kuhad, A. Edaravone: A new hope for deadly amyotrophic lateral sclerosis. Drugs Today 2018, 54, 349. [Google Scholar] [CrossRef]
- Mao, Y.-F.; Yan, N.; Xu, H.; Sun, J.-H.; Xiong, Y.-C.; Deng, X.-M. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res. 2009, 1248, 68–75. [Google Scholar] [CrossRef]
- Lin, M.; Katsumura, Y.; Hata, K.; Muroya, Y.; Nakagawa, K. Pulse radiolysis study on free radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one). J. Photochem. Photobiol. B Biol. 2007, 89, 36–43. [Google Scholar] [CrossRef]
- Li, Z. Equilibrium solubility of edaravone in some binary aqueous and non-aqueous solutions reconsidered: Extended Hildebrand solubility approach, transfer property and preferential solvation. J. Mol. Liq. 2021, 331, 115794. [Google Scholar] [CrossRef]
- Wu, X.; Yin, X.; Tang, T.; Zheng, H.; Xu, W.; Lin, Z.; Chen, X.; Li, R.; Zhao, J.; Han, D. Solubility of Edaravone in Four Mixed Solvents at 273.15–313.15 K and Correlation of Jouyban-Acree and CNIBS/R-K Models. J. Chem. Eng. Data 2020, 65, 1460–1467. [Google Scholar] [CrossRef]
- Li, R.; Yao, L.; Khan, A.; Zhao, B.; Wang, D.; Zhao, J.; Han, D. Co-solvence phenomenon and thermodynamic properties of edaravone in pure and mixed solvents. J. Chem. Thermodyn. 2019, 138, 304–312. [Google Scholar] [CrossRef]
- Qiu, J.; Huang, H.; He, H.; Liu, H.; Hu, S.; Han, J.; Yi, D.; An, M.; Guo, Y.; Wang, P. Solubility Determination and Thermodynamic Modeling of Edaravone in Different Solvent Systems and the Solvent Effect in Pure Solvents. J. Chem. Eng. Data 2020, 65, 3240–3251. [Google Scholar] [CrossRef]
- Abraham, R.J.; Cooper, M.A.; Aghamohammadi, A.; Afarinkia, K.; Liu, X. The Use of MM/QM Calculations of 13C Chemical Shifts in the Analysis of Edaravone Tautomers. J. Solut. Chem. 2022, 51, 1162–1167. [Google Scholar] [CrossRef]
- Fakhraian, H.; Nafari, Y. Preparative, mechanistic and tautomeric investigation of 1-phenyl and 1-methyl derivative of 3-methyl-5-pyrazolone. J. Chem. Sci. 2021, 133, 1–11. [Google Scholar] [CrossRef]
- Freyer, W.; Köppel, H.; Radeglia, R.; Malewski, G. /sup 1/H-N. M. R./sup 13/C-N. M. R., and I. R.-investigations concerning tautomerism of/sup 15/N-labeled 3-methyl-1-phenyl-. delta./sup 2/-pyrazolin-5-one. J. Prakt. Chem. 1983, 325, 238–250. [Google Scholar] [CrossRef]
- Liu, X.; Aghamohammadi, A.; Afarinkia, K.; Abraham, R.J.; Acree, W.E.; Abraham, M.H. Descriptors for Edaravone; studies on its structure, and prediction of properties. J. Mol. Liq. 2021, 332, 115821. [Google Scholar] [CrossRef]
- Souza, G.A.L.; Silva, L.Y.A.; Martinez, P.F.M. Vapour-liquid equilibria of systems containing deep eutectic solvent based on choline chloride and glycerol. J. Chem. Thermodyn. 2021, 158, 106444. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Peyrovedin, H.; Raeissi, S.; Duarte, A.R.C.; Shariati, A. Investigating the performance of novel green solvents in absorption refrigeration cycles: Energy and exergy analyses. Int. J. Refrig. 2020, 113, 174–186. [Google Scholar] [CrossRef]
- Abedin, R.; Heidarian, S.; Flake, J.C.; Hung, F.R. Computational Evaluation of Mixtures of Hydrofluorocarbons and Deep Eutectic Solvents for Absorption Refrigeration Systems. Langmuir 2017, 33, 11611–11625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Keshri, S.; Bharti, A.; Kumar, S.; Mogurampelly, S. Solubility of Gases in Choline Chloride-Based Deep Eutectic Solvents from Molecular Dynamics Simulation. Ind. Eng. Chem. Res. 2022, 61, 4659–4671. [Google Scholar] [CrossRef]
- Aissaoui, T.; AlNashef, I.M.; Qureshi, U.A.; Benguerba, Y. Potential applications of deep eutectic solvents in natural gas sweetening for CO2 capture. Rev. Chem. Eng. 2017, 33, 523–550. [Google Scholar] [CrossRef]
- Hallett, J.E.; Hayler, H.J.; Perkin, S. Nanolubrication in deep eutectic solvents. Phys. Chem. Chem. Phys. 2020, 22, 20253–20264. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Harris, R.C.; Ryder, K.S. Evaluating water miscible deep eutectic solvents (DESs) and ionic liquids as potential lubricants. Green Chem. 2014, 16, 4156–4161. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of Choline Chloride-Based Deep Eutectic Solvents: Experiments and Modeling. J. Chem. Eng. Data 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Ahmed, O.U. Ethaline and Glyceline binary eutectic mixtures: Characteristics and intermolecular interactions. Asia-Pac. J. Chem. Eng. 2017, 12, 313–320. [Google Scholar] [CrossRef]
- Wu, S.H.; Caparanga, A.R.; Leron, R.B.; Li, M.H. Vapor pressure of aqueous choline chloride-based deep eutectic solvents (ethaline, glyceline, maline and reline) at 30–70 °C. Thermochim. Acta 2012, 544, 1–5. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Ahmed, O.U. Physical properties and intermolecular interaction of eutectic solvents binary mixtures: Reline and ethaline. Asia-Pac. J. Chem. Eng. 2016, 11, 549–557. [Google Scholar] [CrossRef]
- Celebi, A.T.; Vlugt, T.J.H.; Moultos, O.A. Structural, Thermodynamic, and Transport Properties of Aqueous Reline and Ethaline Solutions from Molecular Dynamics Simulations. J. Phys. Chem. B 2019, 123, 11014–11025. [Google Scholar] [CrossRef] [PubMed]
- Abdel Jabbar, N.M.; Mjalli, F.S. Ultrasonic study of binary aqueous mixtures of three common eutectic solvents. Phys. Chem. Liq. 2019, 57, 1–18. [Google Scholar] [CrossRef]
- Kalhor, P.; Zheng, Y.Z.; Ashraf, H.; Cao, B.; Yu, Z.W. Influence of Hydration on the Structure and Interactions of Ethaline Deep-Eutectic Solvent: A Spectroscopic and Computational Study. ChemPhysChem 2020, 21, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Rublova, Y.; Kityk, A.; Danilov, F.; Protsenko, V. Mechanistic Study on Surface Tension of Binary and Ternary Mixtures Containing Choline Chloride, Ethylene Glycol and Water (Components of Aqueous Solutions of a Deep Eutectic Solvent, Ethaline). Z. Phys. Chem. 2020, 234, 399–413. [Google Scholar] [CrossRef]
- Jani, A.; Sohier, T.; Morineau, D. Phase behavior of aqueous solutions of ethaline deep eutectic solvent. J. Mol. Liq. 2020, 304, 112701. [Google Scholar] [CrossRef]
- Agieienko, V.; Buchner, R. Is ethaline a deep eutectic solvent? Phys. Chem. Chem. Phys. 2022, 24, 5265–5268. [Google Scholar] [CrossRef]
- Lapeña, D.; Lomba, L.; Artal, M.; Lafuente, C.; Giner, B. The NADES glyceline as a potential Green Solvent: A comprehensive study of its thermophysical properties and effect of water inclusion. J. Chem. Thermodyn. 2019, 128, 164–172. [Google Scholar] [CrossRef]
- Agieienko, V.; Neklyudov, V.; Buchner, R. Why Does Ethaline Apparently Behave as an Ideal Binary Mixture? J. Phys. Chem. Lett. 2022, 13, 10805–10809. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Lu, Y.; Li, G.; Fu, L.; Mu, T. Volatility of Deep Eutectic Solvent Choline Chloride: N -Methylacetamide at Ambient Temperature and Pressure. Ind. Eng. Chem. Res. 2019, 58, 7308–7317. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline, and reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef]
- Aslan Türker, D.; Doğan, M. Application of deep eutectic solvents as a green and biodegradable media for extraction of anthocyanin from black carrots. LWT 2021, 138, 110775. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, S.; Sharma, A.; Kumar, V.; Sharma, R.; Janghu, S.; Suthar, P. Deep eutectic solvents: The new generation sustainable and safe extraction systems for bioactive compounds in agri food sector: An update. J. Food Process. Preserv. 2022, 46, 16250. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Zaragoza, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Harifi-Mood, A.R. Ethaline deep eutectic solvent as a hydrophilic additive in modification of polyethersulfone membrane for antifouling and separation improvement. J. Membr. Sci. 2020, 614, 118528. [Google Scholar] [CrossRef]
- Kityk, A.; Protsenko, V.; Danilov, F.; Bobrova, L.; Hnatko, M.; Pavlik, V.; Šoltýs, J.; Labudová, M.; Rusková, M.; Pangallo, D. Design of Ti-6Al-4V alloy surface properties by galvanostatic electrochemical treatment in a deep eutectic solvent Ethaline. Surf. Coat. Technol. 2022, 429, 127936. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, R.; Radjenovic, P.M.; Liu, S.; Wu, D.; Li, J.; Mao, B.; Yan, J. Electrochemical impedance spectroscopy and Raman spectroscopy studies on electrochemical interface between Au(111) electrode and ethaline deep eutectic solvent. Electrochim. Acta 2021, 390, 138859. [Google Scholar] [CrossRef]
- Divsalar, A.; Ghobadi, R. The presence of deep eutectic solvents of reline and glyceline on interaction and side effect of anti-cancer drug of 5-fluorouracil: Bovine liver catalase as a target. J. Mol. Liq. 2021, 323, 114588. [Google Scholar] [CrossRef]
- Aryafard, M.; Abbasi, M.; Řeha, D.; Harifi-Mood, A.R.; Minofar, B. Experimental and theoretical investigation of solvatochromic properties and ion solvation structure in DESs of reline, glyceline, ethaline and their mixtures with PEG 400. J. Mol. Liq. 2019, 284, 59–67. [Google Scholar] [CrossRef]
- Jahanbakhsh-Bonab, P.; Esrafili, M.D.; Rastkar Ebrahimzadeh, A.; Jahanbin Sardroodi, J. Exploring the structural and transport properties of glyceline DES-Based boron nitride nanotube Nanofluid: The effects of nanotube diameter. J. Mol. Liq. 2021, 341, 117277. [Google Scholar] [CrossRef]
- Jeliński, T.; Cysewski, P. Quantification of Caffeine Interactions in Choline Chloride Natural Deep Eutectic Solvents: Solubility Measurements and COSMO-RS-DARE Interpretation. Int. J. Mol. Sci. 2022, 23, 7832. [Google Scholar] [CrossRef]
- Jeliński, T.; Stasiak, D.; Kosmalski, T.; Cysewski, P. Experimental and theoretical study on theobromine solubility enhancement in binary aqueous solutions and ternary designed solvents. Pharmaceutics 2021, 13, 1118. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, Y.; Kinoshita, S.; Kawaguchi, A.; Takei, K.; Palumbo, J.; Suzuki, M. Pharmacokinetic profile of edaravone: A comparison between Japanese and Caucasian populations. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shukla, S. Role of Edaravone as a Treatment Option for Patients with Amyotrophic Lateral Sclerosis. Pharmaceuticals 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, S.; Dubé, M.A. Reaction Monitoring of Glycerol Step-Growth Polymerization Using ATR-FTIR Spectroscopy. Macromol. React. Eng. 2012, 6, 85–92. [Google Scholar] [CrossRef]
- Ambure, P.; Gajewicz-Skretna, A.; Cordeiro, M.N.D.S.; Roy, K. New workflow for QSAR model development from small data sets: Small dataset curator and small dataset modeler. Integration of data curation, exhaustive double cross-validation, and a set of optimal model selection techniques. J. Chem. Inf. Model. 2019, 59, 10. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Das, R.N. Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128016336. [Google Scholar]
- Roy, K.; Kar, S.; Ambure, P. On a simple approach for determining applicability domain of QSAR models. Chemom. Intell. Lab. Syst. 2015, 145, 22–29. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V.; Bürger, T.; Lohrenz, J.C.W. Refinement and parametrization of COSMO-RS. J. Phys. Chem. A 1998, 102, 5074–5085. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS for aqueous solvation and interfaces. Fluid Phase Equilib. 2016, 407, 152–158. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Jeliński, T.; Kubsik, M.; Cysewski, P. Application of the Solute–Solvent Intermolecular Interactions as Indicator of Caffeine Solubility in Aqueous Binary Aprotic and Proton Acceptor Solvents: Measurements and Quantum Chemistry Computations. Materials 2022, 15, 2472. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P. Intermolecular interaction as a direct measure of water solubility advantage of meloxicam cocrystalized with carboxylic acids. J. Mol. Model. 2018, 24, 112. [Google Scholar] [CrossRef] [PubMed]
- Cysewski, P.; Jeliński, T. Optimization, thermodynamic characteristics and solubility predictions of natural deep eutectic solvents used for sulfonamide dissolution. Int. J. Pharm. 2019, 570, 118682. [Google Scholar] [CrossRef]
- Hellweg, A.; Eckert, F. Brick by brick computation of the gibbs free energy of reaction in solution using quantum chemistry and COSMO-RS. AIChE J. 2017, 63, 3944–3954. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Witte, J.; Neaton, J.B.; Head-Gordon, M. Effective empirical corrections for basis set superposition error in the def2-SVPD basis: gCP and DFT-C. J. Chem. Phys. 2017, 146, 234105. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M.; Rozalski, R. Experimental and theoretical screening for green solvents improving sulfamethizole solubility. Materials 2021, 14, 5915. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Eckert, F. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib. 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- COSMOtherm, Version 21.0.0; Dassault Systèmes; Biovia: San Diego, CA, USA, 2020.
- TURBOMOLE, Version 7.5.1; TURBOMOLE GmbH: Frankfurt, Germany, 2020.
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
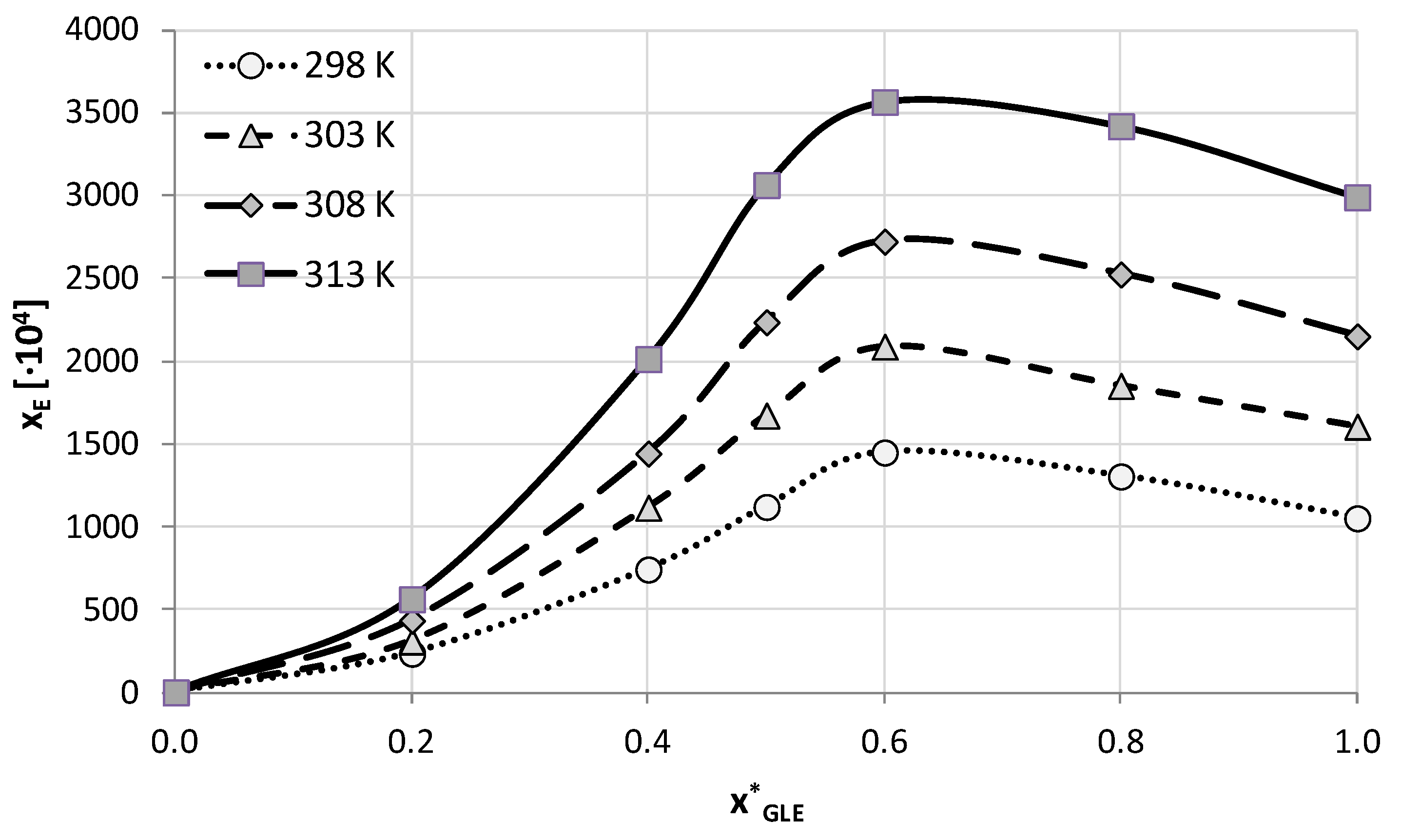
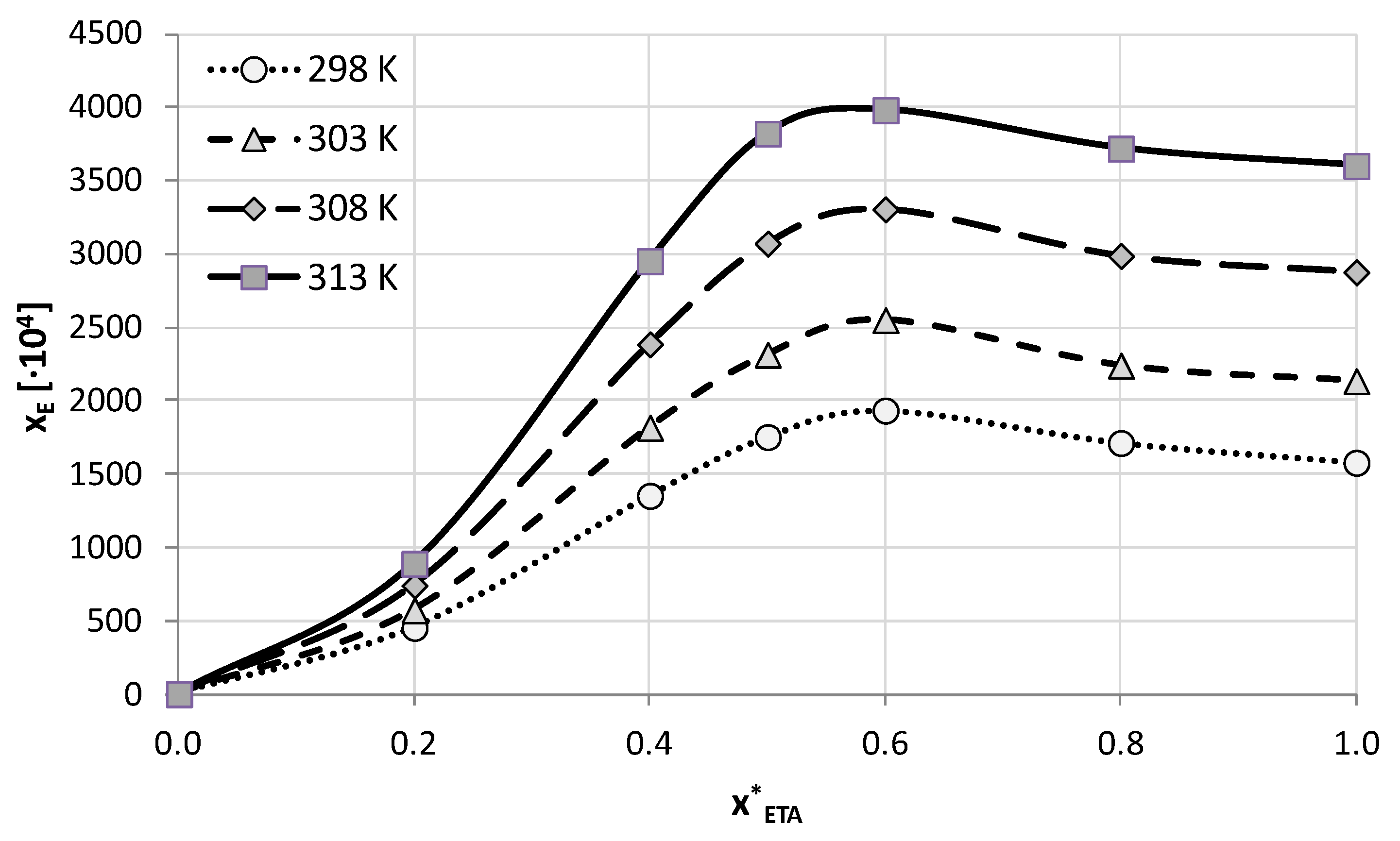




| T (K) | 1:1 Molar Ratio | 1:2 Molar Ratio | 1:4 Molar Ratio | |||
|---|---|---|---|---|---|---|
| xE (∙104) | cE (mg/mL) | xE (∙104) | cE (mg/mL) | xE (∙104) | cE (mg/mL) | |
| choline chloride + glycerol (GLE) | ||||||
| 298.15 | 786.83 (±3.34) | 138.18 (±1.21) | 1052.85 (±68.85) | 191.06 (±11.91) | 926.44 (±23.62) | 177.17 (±4.09) |
| 303.15 | 1173.65 (±39.25) | 202.99 (±6.76) | 1606.43 (±50.52) | 282.25 (±8.03) | 1321.28 (±36.17) | 246.26 (±5.86) |
| 308.15 | 1615.31 (±40.69) | 274.07 (±6.88) | 2151.57 (±64.56) | 369.09 (±11.3) | 1861.97 (±40.45) | 336.43 (±6.12) |
| 313.15 | 2429.30 (±72.71) | 398.02 (±10.92) | 2991.42 (±64.1) | 492.12 (±9.58) | 2739.72 (±65.05) | 470.20 (±9.06) |
| choline chloride + ethylene glycol (ETA) | ||||||
| 298.15 | 1031.93 (±62.91) | 189.66 (±10.5) | 1579.47 (±70.73) | 302.96 (±11.01) | 1306.68 (±55.75) | 280.14 (±10.35) |
| 303.15 | 1400.98 (±40.74) | 251.75 (±7.12) | 2135.68 (±42.46) | 392.28 (±6.53) | 1677.66 (±47.52) | 346.64 (±7.87) |
| 308.15 | 2089.82 (±49.36) | 359.76 (±7.92) | 2878.22 (±133.85) | 499.02 (±18.34) | 2434.99 (±114.12) | 467.15 (±16.58) |
| 313.15 | 2966.24 (±120.59) | 484.49 (±16.00) | 3602.21 (±117.41) | 592.85 (±14.78) | 3428.20 (±105.69) | 601.77 (±12.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cysewski, P.; Jeliński, T.; Przybyłek, M. Intermolecular Interactions of Edaravone in Aqueous Solutions of Ethaline and Glyceline Inferred from Experiments and Quantum Chemistry Computations. Molecules 2023, 28, 629. https://doi.org/10.3390/molecules28020629
Cysewski P, Jeliński T, Przybyłek M. Intermolecular Interactions of Edaravone in Aqueous Solutions of Ethaline and Glyceline Inferred from Experiments and Quantum Chemistry Computations. Molecules. 2023; 28(2):629. https://doi.org/10.3390/molecules28020629
Chicago/Turabian StyleCysewski, Piotr, Tomasz Jeliński, and Maciej Przybyłek. 2023. "Intermolecular Interactions of Edaravone in Aqueous Solutions of Ethaline and Glyceline Inferred from Experiments and Quantum Chemistry Computations" Molecules 28, no. 2: 629. https://doi.org/10.3390/molecules28020629
APA StyleCysewski, P., Jeliński, T., & Przybyłek, M. (2023). Intermolecular Interactions of Edaravone in Aqueous Solutions of Ethaline and Glyceline Inferred from Experiments and Quantum Chemistry Computations. Molecules, 28(2), 629. https://doi.org/10.3390/molecules28020629









