Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/mTOR Independent Pathway under Hypoxic Condition
Abstract
1. Introduction
2. Results
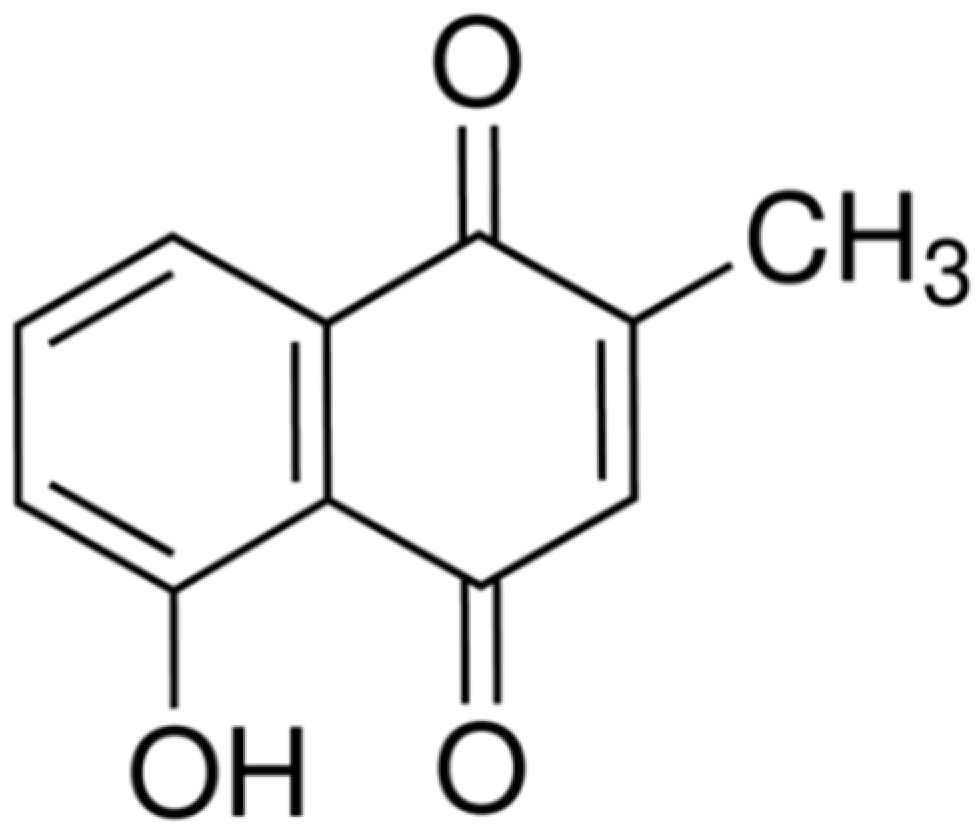
2.1. Cytotoxic Effects of Plumbagin on MCF-7 Cells
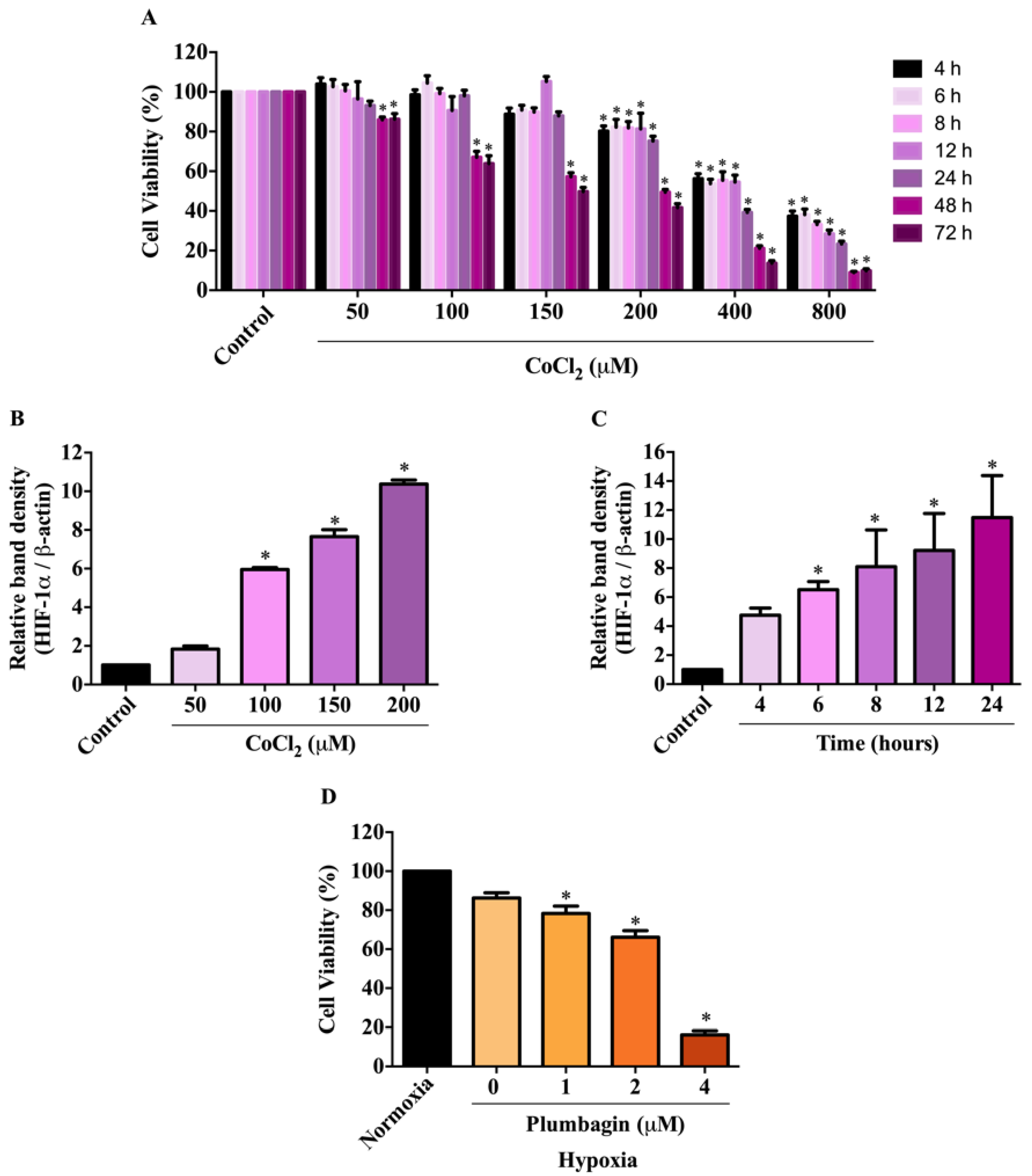
2.2. Effects of CoCl2-Induced Conditions on HIF-1α Expression and MCF-7 Cell Viability
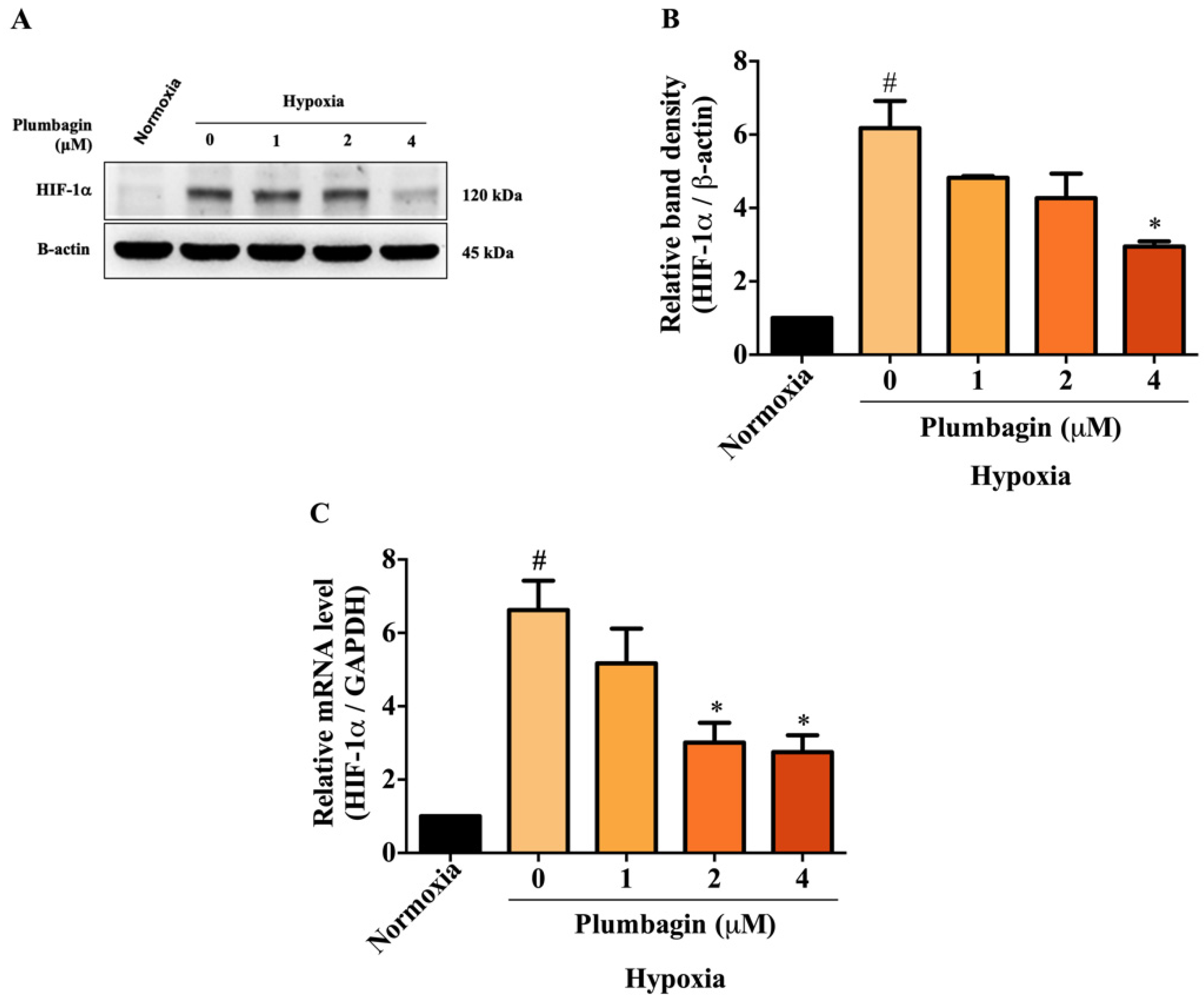
2.3. Plumbagin Inhibited HIF-1α mRNA and Protein Expression under Hypoxic Conditions
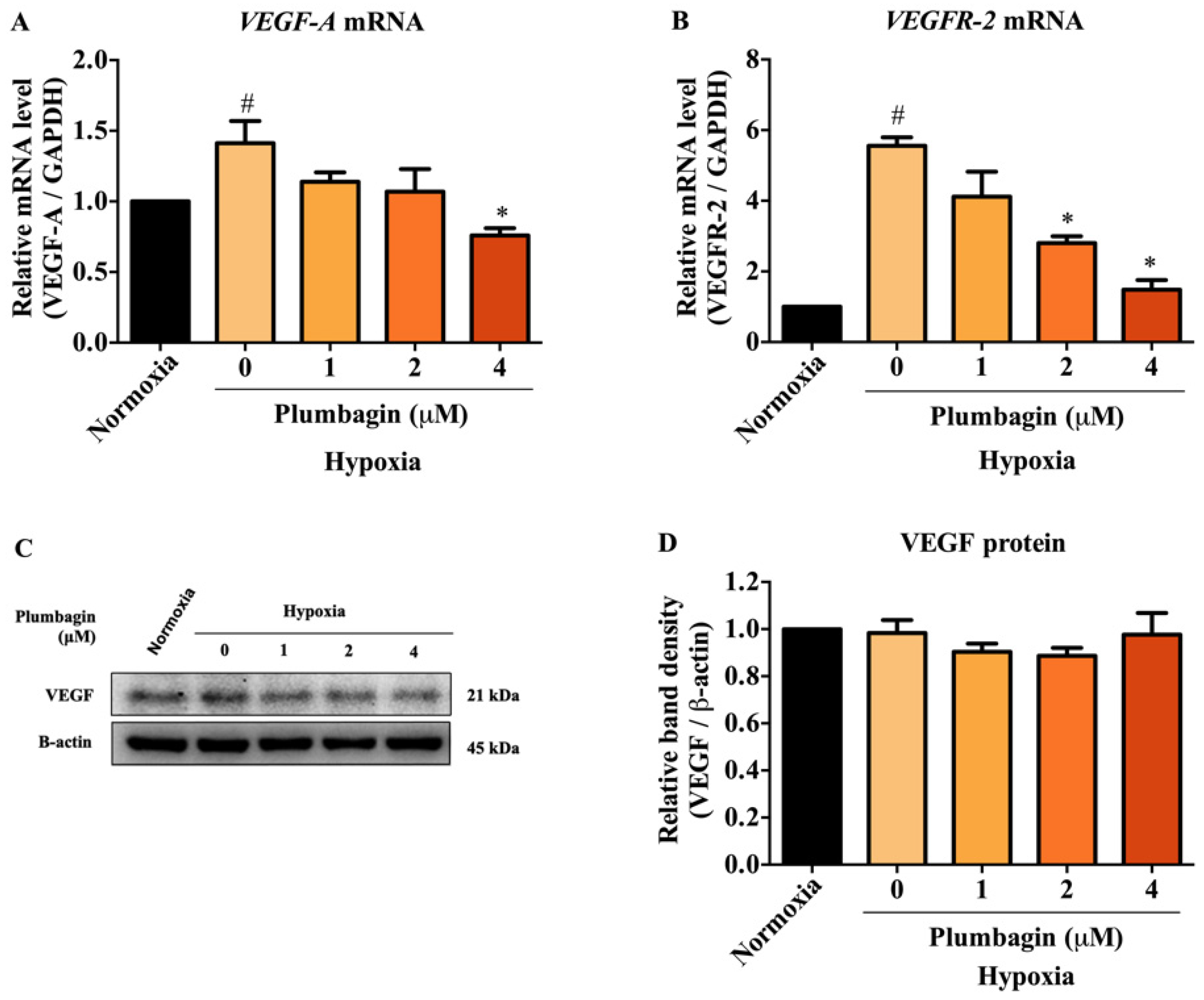
2.4. The mRNA and Protein Expression of HIF-1α Target Genes, Including VEGF-A and VEGFR-2, Were Downregulated by Plumbagin
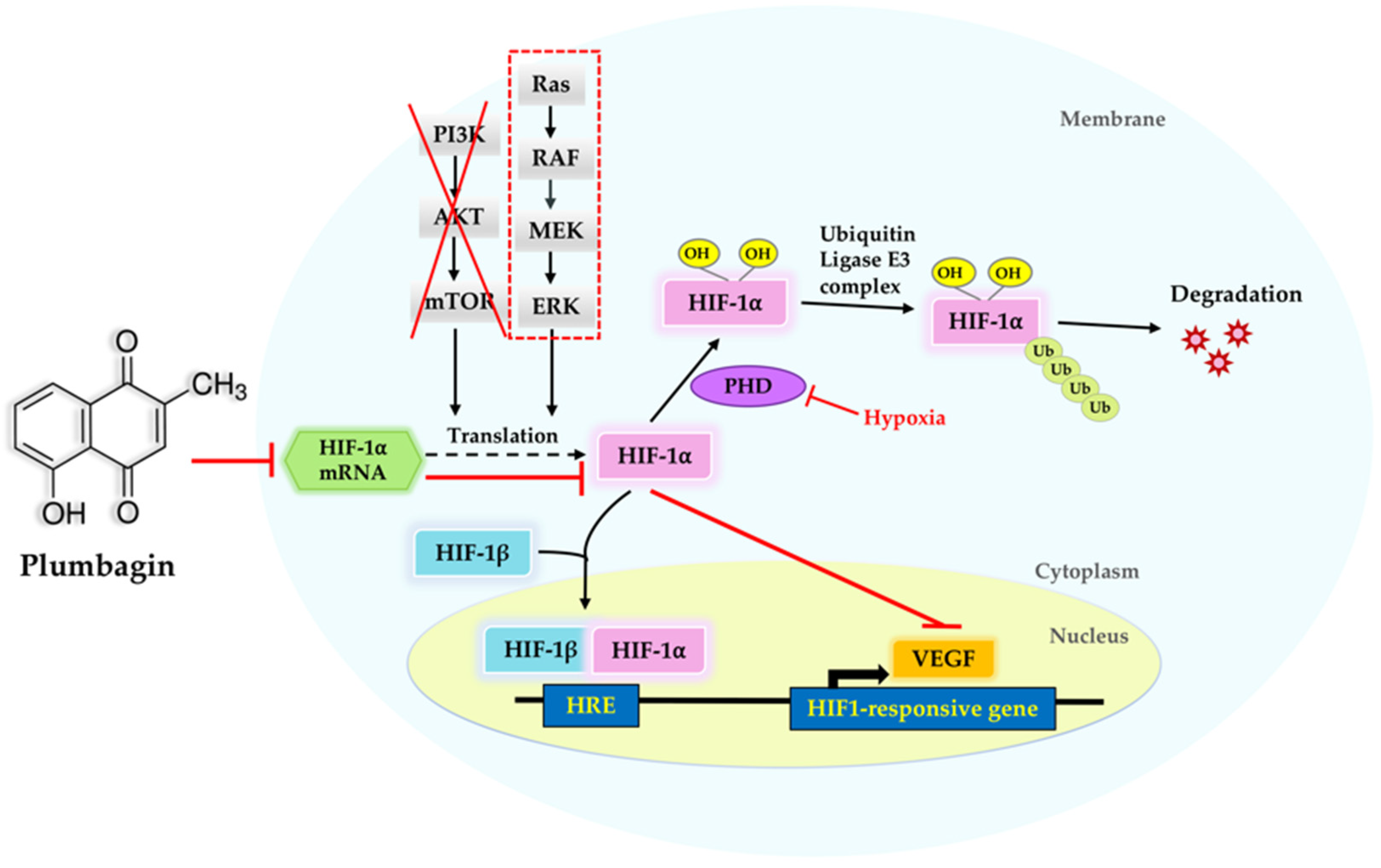
2.5. Suppression of HIF-1α by Plumbagin Was Not Regulated by the PI3K/Akt/mTOR Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Experimental Treatments
4.3. Cell Viability
4.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gilkes, D.M.; Semenza, G.L. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013, 9, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; Puente, P.D.I.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer. 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Kizaka-Kondoh, S.; Konse-Nagasawa, H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Sci. 2009, 100, 1366–1373. [Google Scholar] [CrossRef]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.M. An overview of the recent development of anticancer agents targeting the HIF-1 transcription factor. Cancers 2021, 13, 2813. [Google Scholar] [CrossRef]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef]
- Wu, H.; Dai, X.; Wang, E. Plumbagin inhibits cell proliferation and promotes apoptosis in multiple myeloma cells through inhibition of the PI3K/Akt-mTOR pathway. Oncol. Lett. 2016, 12, 3614–3618. [Google Scholar] [CrossRef]
- Li, Y.C.; He, S.M.; He, Z.X.; Li, M.; Yang, Y.; Pang, J.X.; Zhang, X.; Chow, K.; Zhou, Q.; Duan, W.; et al. Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells. Cancer Lett. 2014, 344, 239–259. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Li, X.X.; He, Z.X.; Pan, S.T.; Yang, Y.; Zhang, X.; Chow, K.; Yang, T.; Qiu, J.X.; Zhou, Q.; et al. Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des. Devel. Ther. 2015, 9, 1511–1554. [Google Scholar] [CrossRef]
- Wang, C.C.C.; Chiang, Y.M.; Sung, S.C.; Hsu, Y.L.; Chang, J.K.; Kuo, P.L. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008, 259, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Rajendran, P.; Li, F.; Ramachandran, L.; Hay, H.S.; Kannaiyan, R.; Swamy, S.N.; Vali, S.; Kapoor, S.; et al. Plumbagin inhibits invasion and migration of breast and gastric cancer cells by downregulating the expression of chemokine receptor CXCR4. Mol. Cancer 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Subramaniya, B.R.; Srinivasan, G.; Sadullah, S.S.M.; Davis, N.; Subhadara, L.B.R.; Halagowder, D.; Sivasitambaram, N.D. Apoptosis-inducing effect of plumbagin on colonic cancer cells depends on the expression of COX-2. PLoS ONE 2011, 6, e18695. [Google Scholar] [CrossRef]
- Sameni, S.; Hande, M.P. Plumbagin triggers DNA damage response, telomere dysfunction and genome instability of human breast cancer cells. Biomed. Pharmacother. 2016, 82, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Yang, C.Y.; Rao, X.F.; Xiong, J.P. Plumbagin show anti-cancer activity in human breast cancer cells by the upregulation of p53 and p21 and suppression of G1 cell cycle regulators. Eur. J. Gynaecol. Oncol. 2016, 37, 30–35. [Google Scholar]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhor, D.E. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Induction and testing of hypoxia in cell culture. J. Vis. Exp. 2011, 54, e2899. [Google Scholar] [CrossRef] [PubMed]
- Chachami, G.; Simos, G.; Hatziefthimiou, A.; Bonanou, S.; Molyvdas, P.A.; Paraskeva, E. Cobalt induces hypoxia-inducible factor-1alpha expression in airway smooth muscle cells by a reactive oxygen species- and PI3K-dependent mechanism. Am. J. Respir. Cell Mol. Biol. 2004, 31, 544–551. [Google Scholar] [CrossRef]
- Ardyanto, T.D.; Osaki, M.; Tokuyasu, N.; Nagahama, Y.; Ito, H. CoCl2-induced HIF-1·expression correlates with proliferation and apoptosis in MKN-1 cells: A possible role for the PI3K/Akt pathway. Int. J. Oncol. 2006, 29, 549–555. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Liakos, P.; Tsakalof, A.; Georgatsou, E.; Simos, G.; Bonanou, S. Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic. Res. 2006, 40, 847–856. [Google Scholar] [CrossRef]
- Suzuki, E.; Matsunaga, T.; Aonuma, A.; Sasaki, T.; Nagata, K.; Ohmori, S. Effects of hypoxia-inducible factor-1a chemical stabilizer, CoCl2 and hypoxia on gene expression of CYP3As in human fetal liver cells. Drug Metab. Pharmacokinet. 2012, 27, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, Z.; Mao, S.; Chen, W.; Zeng, R.; Zhou, S.; Liu, J. Effect of hypoxia on hypoxia-inducible factor-1alpha, insulin-like growth factor I and vascular endothelial growth factor expression in hepatocellular carcinoma HepG2 cells. Oncol. Lett. 2015, 9, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. The role of hypoxia-induced factors in tumor progression. Oncologist 2004, 9, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Place, A.E.; Huh, S.J.; Polyak, K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011, 13, 227. [Google Scholar] [CrossRef]
- Hossain, C.F.; Kim, Y.P.; Baerson, S.R.; Zhang, L.; Bruick, R.K.; Mohammed, K.A.; Agarwal, A.K.; Nagle, D.G.; Zhou, Y.D. Saururus cernuus lignans—Potent small molecule inhibitors of hypoxia-inducible factor-1. Biochem. Biophys. Res. Commun. 2005, 333, 1026–1033. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Weng, Q.; Wu, R.; Yan, Y.; Jing, H.; Zhu, H.; He, Q.; Yang, B. Suppression of hypoxia-inducible factor 1alpha (HIF-1alpha) by tirapazamine is dependent on eIF2alpha phosphorylation rather than the mTORC1/4E-BP1 pathway. PLoS ONE 2010, 5, e13910. [Google Scholar]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.M.; Sehr, P.; Putzker, K.; Hentze, M.W.; Neumann, B.; Duncan, K.E.; Thoma, C. Automated high-throughput RNAi screening in human cells combined with reporter mRNA transfection to identify novel regulators of translation. PLoS ONE 2012, 7, e45943. [Google Scholar]
- Vries, S.D.; de Vries, I.S.N.; Urlaub, H.; Lue, H.; Bernhagen, J.; Ostareck, D.H.; Ostareck-Lederer, A. Identification of DEAD-box RNA helicase 6 (DDX6) as a cellular modulator of vascular endothelial growth factor expression under hypoxia. J. Biol. Chem. 2013, 288, 5815–5827. [Google Scholar] [CrossRef] [PubMed]
- Suárez, Y.; Sessa, W.C. MicroRNAs as novel regulators of angiogenesis. Circ. Res. 2009, 104, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J. The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. 2007, 5, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular Anti-VEGF therapy for diabetic retinopathy: The role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 2014, 37, 893–899. [Google Scholar] [CrossRef]
- Kumar, G.K.; Prabhakar, N.R. Post-translational modification of proteins during intermittent hypoxia. Respir. Physiol. Neurobiol. 2008, 164, 272–276. [Google Scholar] [CrossRef][Green Version]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, I.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia. Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Cho, C.Y. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol. Cancer Ther. 2006, 5, 3209–3221. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, O.J. Involvement of AMPK/mTOR/HIF-1α in anticancer control of quercetin in hypoxic MCF-7 cells. Food Sci. Biotechnol. 2011, 20, 371–375. [Google Scholar] [CrossRef]
- Li, G.; Shan, C.; Liu, L.; Zhou, T.; Zhou, J.; Hu, X.; Chen, Y.; Cui, H.; Gao, N. Tanshinone IIA inhibits HIF-1 alpha and VEGF expression in breast cancer cells via mTOR/p70S6K/ RPS6/4E-BP1 signaling pathway. PLoS ONE 2015, 10, e0117440. [Google Scholar]
- Yang, Y.; Cong, H.; Han, C.; Yue, L.; Dong, H.; Li, J. 12-Deoxyphorbol 13-palmitate inhibits the expression of VEGF and HIF-1alpha in MCF-7 cells by blocking the PI3K/Akt/mTOR signaling pathway. Oncol. Rep. 2015, 34, 1755–1760. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, M.; Wang, F.; Ouyang, X.; Jia, Y.; Du, H. Role and mechanism of hypoxia-inducible factor-1 in cell growth and apoptosis of breast cancer cell line MDA-MB-231. Oncol. Lett. 2010, 1, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Kilcar, A.Y.; Tekin, V.; Muftuler, F.Z.B.; Medine, E.I. 99mTc labeled plumbagin: Estrogen receptor dependent examination against breast cancer cells and comparison with PLGA encapsulated form. J. Radioanal. Nucl. Chem. 2016, 308, 13–22. [Google Scholar] [CrossRef]
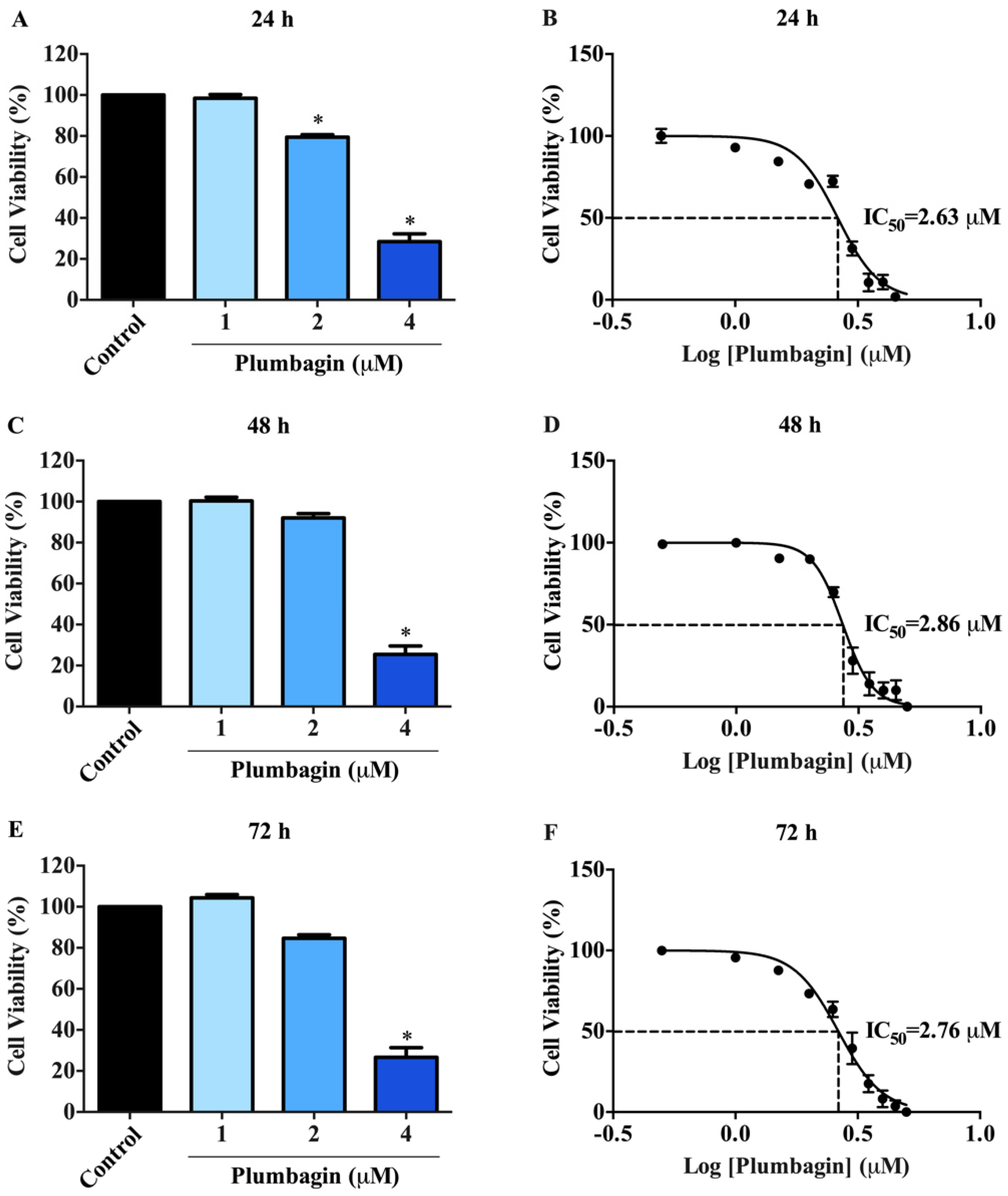

| Time (h) | IC50 a (µM) |
|---|---|
| 24 | 2.63 ± 0.01 |
| 48 | 2.86 ± 0.01 |
| 72 | 2.76 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampasri, S.; Reabroi, S.; Tungmunnithum, D.; Parichatikanond, W.; Pinthong, D. Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/mTOR Independent Pathway under Hypoxic Condition. Molecules 2022, 27, 5716. https://doi.org/10.3390/molecules27175716
Jampasri S, Reabroi S, Tungmunnithum D, Parichatikanond W, Pinthong D. Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/mTOR Independent Pathway under Hypoxic Condition. Molecules. 2022; 27(17):5716. https://doi.org/10.3390/molecules27175716
Chicago/Turabian StyleJampasri, Supawan, Somrudee Reabroi, Duangjai Tungmunnithum, Warisara Parichatikanond, and Darawan Pinthong. 2022. "Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/mTOR Independent Pathway under Hypoxic Condition" Molecules 27, no. 17: 5716. https://doi.org/10.3390/molecules27175716
APA StyleJampasri, S., Reabroi, S., Tungmunnithum, D., Parichatikanond, W., & Pinthong, D. (2022). Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/mTOR Independent Pathway under Hypoxic Condition. Molecules, 27(17), 5716. https://doi.org/10.3390/molecules27175716






